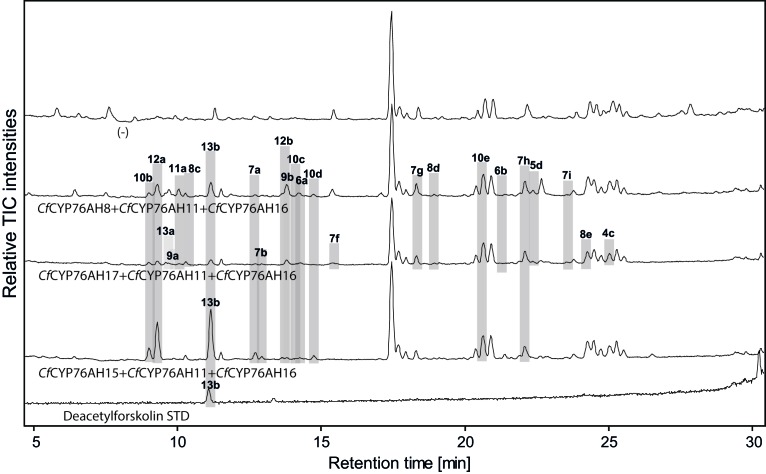
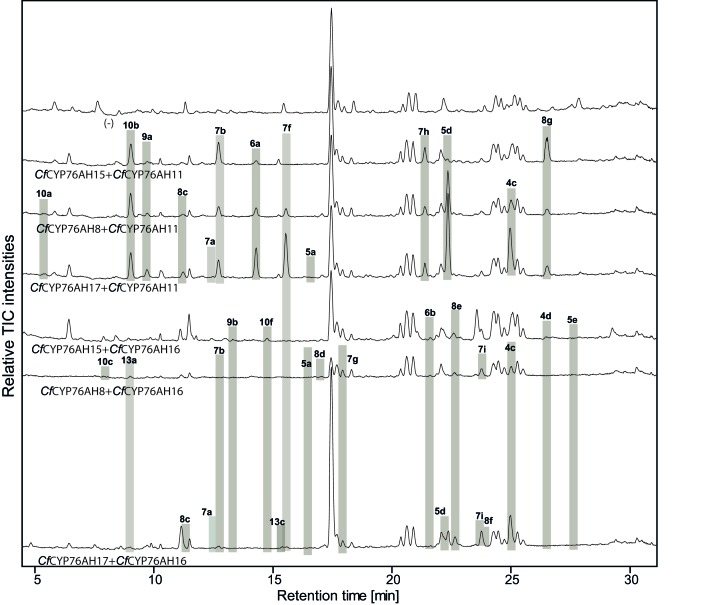
Figure 5. LC-qTOF-MS analysis of 13R-manoyl oxide-derived diterpenoids obtained by transient expression of combinations of C. forskohlii CYP encoding genes, together with genes encoding the required enzymes for biosynthesis of 13R-manoyl oxide in N. benthamiana leaves.
Total ion chromatograms (TIC) of extracts expressing the 13R-manoyl oxide biosynthesis genes (CfCXS, CfGGPPS, CfTPS2, CfTPS3), in combination with (from the top) water (-), CfCYP76AH8 + CfCYP76AH11 + CfCYP76AH16, CfCYP76AH17 + CfCYP76AH11 + CfCYP76AH16, and CfCYP76AH15 + CfCYP76AH11 + CfCYP76AH16 are shown. Hydroxylated 13R-manoyl oxide-derived diterpenoids (marked with grey bars) and their identity including their molecular formulas were confirmed by accurate mass (5 ppm tolerance, Supplementary file 1). Compounds present in trace amounts are not marked. The identity of 1,11-dihydroxy-13R-manoyl oxide (5d), 9-deoxydeacetylforskolin (10b) and 1,9-dideoxydeacetylforskolin (7h) was confirmed by NMR analysis (Figure 4 and Tables 1 and 2), whereas the identity of deacetylforskolin (13b) was confirmed by comparison to an authentic chemically synthesized standard. No 13R-manoyl oxide-derived diterpenoids were identified in the water control (-). For each combinaton, extracts from leaves of three different N. benthamiana plants have been analyzed and representative chromatograms are shown.