Summary
Feeding in most animals is confined to a defined period, leaving short periods of fasting that coincide with sleep. Fasting enables organisms to enter alternative metabolic phases, which rely less on glucose and more on ketone body-like carbon sources. Both intermittent and periodic fasting result in benefits ranging from prevention to the enhanced treatment of diseases. Similarly, time-restricted feeding (TRF), in which feeding time is restricted to certain hours of the day, allows the daily fasting period to last >12 h, thus imparting pleiotropic benefits in multiple organisms. Understanding the mechanistic link between nutrients and the fasting benefits is leading to the identification of fasting mimicking diets (FMDs) that achieve changes similar to those caused by fasting. Given the pleiotropic and sustained benefits of TRF and FMD, both basic science and translational research are warranted to develop fasting-associated interventions into effective and inexpensive treatments with the potential to improve healthspan.
Introduction
Life forms on our planet have evolved under the strong influence of a daily light/dark cycle. Sunlight being the primary source of energy for photosynthesis, the daily production of photosynthetic biomass has a predictable diurnal rhythm. The daily cyclical production of photosynthesized chemical energy is at the base of the food chain. Daily changes in light and darkness result in diurnal rhythms in other environmental parameters such as temperature and humidity. Such a predictable and robust daily rhythm in food availability and environmental factors has led to the evolution of a ~24 h internal timing mechanism or circadian rhythm to enable organisms to anticipate daily changes and to optimize fitness. Fundamental to this 24 h rhythms is the ability to acquire food when it is available and to store a portion of these resources for utilization during the rest of the day (i.e. the fasting period) without compromising fitness and vitality. The fasting period also serves as a time for standby and repair so that the organism is fit and competent to harvest energy when light (for photosynthetic organisms) or food becomes available. While many non-photosynthetic lifeforms with short lifespan (< a few days) may not derive profound benefit from a circadian timing system, they share fundamental biochemical mechanisms for acquiring and storing food when it is available and then utilizing this stored energy during a quiescent period of fasting for repair, stress resistance and vitality.
Inherent to this alternating cycle of feeding and fasting (irrespective of circadian rhythm -proficient or -deficient organisms), is the theory that “fasting physiology” (biochemical processes associated with fasting) is triggered once stored energy is being utilized and therefore does not occur during the feeding period. This theory also highlights the notion that certain aspects of repair and rejuvenation that are integral to fasting-re-feeding physiology may be associated only with fasting. Hence intermittent- and periodic- fasting may represent important factors in optimizing lifespan and healthspan. In circadian rhythm-deficient organisms, the optimal duration of fasting (i.e., one that avoids a low energy state that compromises viability) depends on the extent of stored nutrients and ambient conditions. These simple organisms have made tremendous contributions to the experimental dissection of molecules and mechanisms of cell autonomous fasting physiology that is conserved across species. In circadian rhythm-proficient organisms, the inherent circadian oscillator has programmed a natural cycle of feeding and fasting that occurs with ~24h periodicity. However, even oscillator proficient organisms have retained mechanisms to adapt to a few days of reduced or no energy intake without substantial loss of vitality. As a result, the oscillator proficient organisms can benefit from sustained daily rhythms as well as from periodic fasting of several hours. Reducing energy intake on a daily basis, as in caloric restriction, may allow the fasting physiology to be triggered sooner and to be sustained for longer periods of time than when consuming standard or excessive amounts of calories. Similarly, restricting the timing of food intake to a few hours without an overt attempt to reduce caloric intake, as in time-restricted feeding (TRF), may trigger the fasting physiology after a few hours of feeding cessation on a daily basis. In summary these arguments highlight the relevance of fasting physiology within the energy-restriction or time-restriction paradigms.
Modern humans face complex health challenges and solutions. While prevention, vaccination, and treatment for infectious diseases have prolonged lifespan, the presence of artificial light enables human activity throughout the 24h day. This disrupted activity-rest cycle indirectly disrupts the natural daily cycle of feeding and fasting, and facilitates excessive caloric intake. Such chronically disrupted temporal regulation contributes to metabolic diseases but may also accelerate the aging process. Treating for metabolic diseases has been challenging, as the traditional pharmacological approach to diseases management may not be sufficient. Long term chronic pharmacological interventions have been particularly successful when the pharmacological molecule is a replacement of an essential biochemical agent, such as insulin (for type 1 diabetes), thyroid hormone, vitamins or minerals which is deficient. These replacement agents often have multiple modes of actions and exert pleiotropic effects. If daily, alternate daily, or periodic fasting can promote healthy lifespan by exerting pleiotropic effects, restoring a fasting period or switching to a diet that mimics fasting may be an effective treatment strategy for several chronic diseases.
Historical and evolutionary arguments for the safety and potential efficacy of periodic fasting in health and longevity
The emergence at major university hospitals around the world of complimentary and integrative medicine centers that utilize nutrition, exercise, yoga, and acupuncture to prevent and treat disease is evidence that the medical field is sampling traditional interventions to discover ways of improving and replacing FDA-approved therapies involving peptides, antibodies, and pharmaceuticals (Rakel, 2012). Many of standard-of-care therapies are based on the discovery of enzymes, receptors, or other targets that mediate biological effects of interest, followed by the identification of specific drugs or biologicals that interfere with or enhance the activity of specific targets. Although processes by which the drug target and drug are identified are highly sophisticated, resulting intervention (e.g., the inhibition of cholesterol synthesis by statins or the damage of DNA by chemotherapy drugs) can be viewed as rather unsophisticated strategies. In the case of statins, the inhibition of cholesterol synthesis does not take into account long-term effects of the accumulation of cholesterol precursors or counteracting mechanism by which the human body is capable of synthesizing more cholesterol. In the case of chemotherapy drugs, the obvious collateral damage to non-cancerous cells is clear evidence of the lack of sophistication involved. It is therefore not surprising that years after a drug’s initial FDA approval, which is supposedly based on demonstrated efficacy, a wide range of side effects, as well as evidence for limited efficacy, often emerge. Undoubtedly, a more detailed understanding of the underlying causes of disease, and the undesirable short- and long-term consequences of standard therapies, could lead to more effective and safer drugs. One promising alternative to or complementary to pharmaceutical interventions is to identify dietary and traditional remedies that have been safely utilized for hundreds of years to trigger sophisticated physiological responses resulting from billions of years of evolution. These evolutionary and historical arguments are scientifically meaningless, unless accompanied by (1) insights into their molecular mechanisms of action, (2) extensive and positive cellular and animal data, (3) epidemiological data, and (4) randomized clinical trials.
Among alternative interventions for the prevention and treatment of chronic metabolic diseases, different forms of fasting and fasting mimicking diets have the greatest potential of being integrated into the standard medical care. These range from time restricted feeding (TRF), feeding every other day (alternate day fasting), adopting a reduced calorie regimen twice a week (5:2 fasting), or undergoing a periodic cycle of diets that provide a relatively high caloric content but able to mimic many of the effects of fasting (Fasting Mimicking Diets).
Here we will review the evolutionary origins of fasting-related health benefits by examining the response of microorganisms to starvation and the mechanisms by which nutrients affect cellular protection and longevity. We will then describe how different forms of fasting affect the health and/or longevity of rodents and humans. The focus will be both on efficacy and safety.
Model organisms and mechanisms – chronic and periodic fasting
The entry into fasting physiology that allow organisms to respond and adapt to starvation conditions first appeared in prokaryotes billions of years ago. This response represents one of the most potent examples of comprehensive cellular reprogramming and appears to be functioning in virtually all organisms (Longo and Mattson, 2014). Bacteria and baker’s yeast switched from rich medium to NaCl or water, respectively, survive several fold longer, and the removal of bacteria from the worm’s medium causes a major lifespan extension (Fabrizio and Longo, 2003; Kaeberlein et al., 2006; Wei et al., 2008). The network of genes and mechanisms responsible for these starvation responses are perhaps best understood in the unicellular S. cerevisiae, whose chronological lifespan is doubled when all nutrients are removed from the medium.
The two central dietary components that block the starvation response in yeast and whose removal results in lifespan extension and stress resistance are amino acids and glucose. In particular, serine activates Pkh/PDK signaling and threonine and valine activate Tor signaling. Both of these pathways converge on the serine/threonine kinase Sch9, the yeast ortholog of mammalian S6 kinase (Figure 1) (Mirisola et al., 2014). Other amino acids, including methionine, have been implicated in the reduced protection and longevity of S. cerevisiae (Ruckenstuhl et al., 2014). Glucose, on the other hand, activates the Ras, adenyalate cyclase, PKA pathway. Subsequently, the glucose and amino acid pathways converge to inactivate the serine/threonine kinase Rim15, which, in turn, positively regulates at least three major stress resistance transcription factors: Msn2, Msn4 and Gis1 (Figure 1). Therefore, when glucose and amino acids are removed from the medium as part of the switch from rich medium to water, these protective transcription factors are activated and regulate a wide range of stress resistance and metabolic genes responsible for protecting DNA against oxidative damage, switching from a glucose and mitochondria-dependent mode in which ethanol and acetic acid are accumulated to a mode in which acetic acid and ethanol are utilized for energy and glycerol plus trehalose are produced (Hu et al., 2014). This starvation-dependent reprogramming of the yeast metabolic pathways are reminiscent of the fasting-dependent switch from a glucose-driven oxidative phosphorylation to a ketone bodies and fatty acid-dependent metabolism in mammals, which is also associated with the generation of glycerol. The mechanisms of starvation-dependent lifespan extension in yeast are still not fully understood but, because they largely depended on the activity of transcription factors Msn2, Msn4 and Gis1, they are likely to be, in part, dependent on the increased expression of genes involved in the heat shock response as well as antioxidant genes SOD1 and SOD2, but also on the reduced generation and/or concentration of superoxide and other toxic molecules. One of the consequences of the inhibition of Tor-S6K signaling by fasting, is the activation of autophagy, which is known to play an important role in aging and lifespan (Eisenberg et al., 2009; Madeo et al., 2010).
Figure 1.
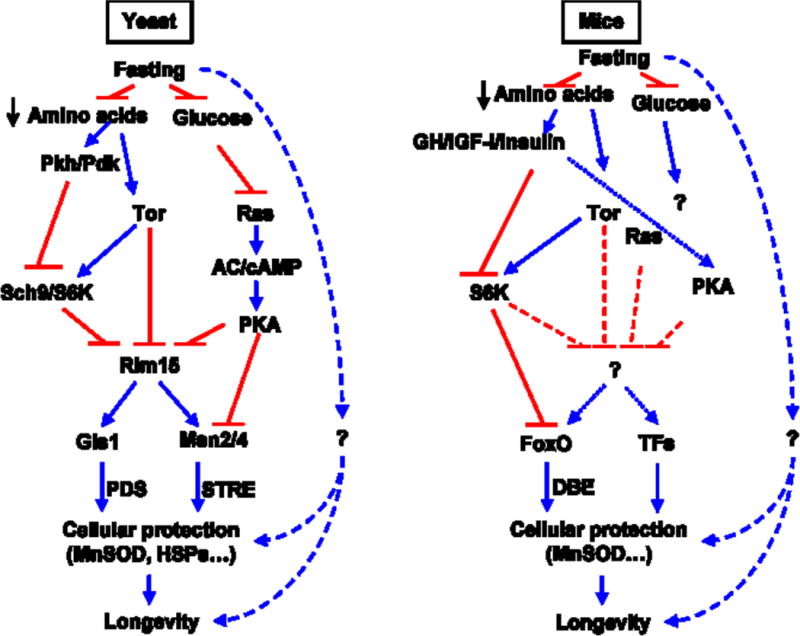
Conserved nutrient sensing pathways in yeast and mice.
In future studies, it will be important to understand how starvation conditions affect the ability of yeast to repair and replace damaged organelles and macromolecules. The simplicity of unicellular eukaryotes is likely to continue to yield fundamental new ideas and provide more in depth mechanistic insights that accelerate research in higher eukaryotes. Studies in worms and flies, will also be important to bridge the understanding the molecular biology of fasting in yeast and mammals.
Fasting and fasting mimicking diets in rodents diseases and longevity
There are two major forms of fasting studied in rodent models: intermittent fasting (IF), which usually refers to a water only or very low calorie period lasting less than 24 hours and followed by a normal feeding period of one to two days, or periodic fasting (PF) which lasts 2 or more days and is separated by the next cycle by at least one week (Longo and Mattson, 2014).
The role of IF on aging and diseases in rodents is still controversial. In rats, multiple studies indicate that every other day fasting consistently extends lifespan and that this effect is more pronounced than that caused by fasting for 1 day every 3–4 days (Carlson and Hoelzel, 1946; Kendrick, 1973). However mouse studies using different genetic backgrounds indicate that IF can have no effect on mean lifespan and may even reduce lifespan when started at 10 months of age (Goodrick et al., 1990). Even when the IF was started at 1.5 months, the effects on longevity were minor and not consistent (Goodrick et al., 1990).
However, in rodents IF enhances cognitive performance (Fontán-Lozano et al., 2007; Singh et al., 2012), which may be caused in part by its stimulatory effect on synaptic plasticity (Lee et al., 2006) and also improves insulin sensitivity, and reduces blood pressure and heart rate (Mager et al., 2006; Wan et al., 2003).
Whereas periodic and prolonged fasting has been studied extensively in bacteria, yeast and worms (Longo and Mattson, 2014), its role in the longevity and health of rodents is only beginning to being investigated. Because water only fasting leads to rapid weight loss in mice, Brandhorst et al developed a low protein and low sugar and a relatively high fat content Fasting Mimicking Diet (FMD) lasting 4 days and providing between 10 and 50% of the normal caloric, which achieved effects on markers for aging and diseases similar to those caused by a water-only fast lasting 2–3 days (Brandhorst et al., 2015; Brandhorst et al., 2013). When mice, starting at 16 months of age, were switched to the 4 day FMD twice a month, they lost body weight and particularly visceral fat even though they consumed the same level of calories per month as mice in the control diet group. Notably, this effect was not accompanied by a loss of lean body mass relative to total abdominal volume (Brandhorst et al., 2015). Neoplasms were reduced by approximately 45%, and their detection was delayed by approximately 4 months. Furthermore, the abnormal lesions were mostly detected in 2 or less organs, indicating the many of the neoplasms were either benign tumors or not aggressive cancers (Brandhorst et al., 2013). These results are in agreement with the effects of fasting in retarding the growth of a wide range of tumors and in enhancing the toxicity of chemotherapy drug to cancer cells (Lee and Longo, 2011; Safdie et al., 2012) while protecting normal cells (Raffaghello et al., 2008; Tinkum et al., 2015). Inflammation, and in particular dermatitis, was reduced by 50% when the entire lifespan period was considered. After 4 months of FMD treatment, white blood cell number in the FMD group mice was similar to that of 4 month old mice and nearly 3 times higher than in the age-matched mice on the control diet (Figure 2) (Brandhorst et al., 2015) in agreement with demonstrated effect of PF cycles in promoting an hematopoietic stem cell-dependent regeneration of immune cells, leading to a rejuvenated immune phenotype (Cheng et al., 2014).
Figure 2.
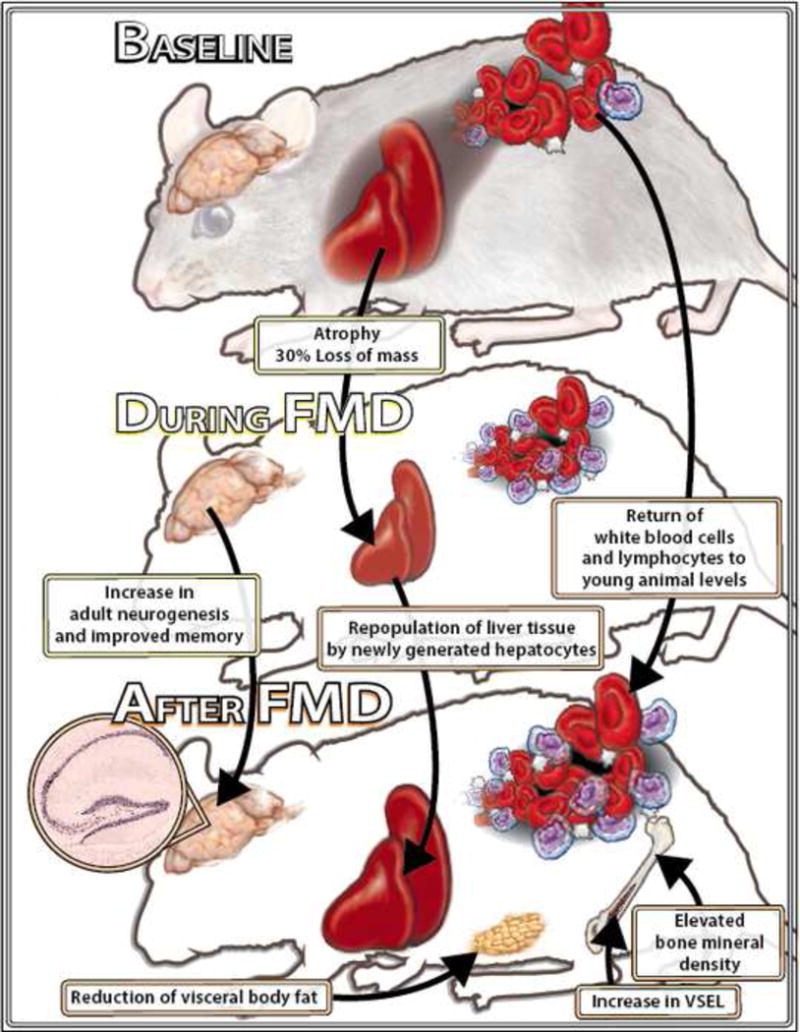
The multi-systemic effects of bi-monthly FMD feeding cycles in female C57Bl6 mice. Bi-monthly cycles of a low calorie Fasting Mimicking Diet (FMD) started at 16.5 months of age caused major decreases in the size of various organs, including the liver, heart and kidneys. However, these effects of the FMD were completely reversed after 7 days of refeeding and, in the liver, were accompanied by the repopulation of newly divided (Ki67+) cells. Additionally, the FMD lowered visceral fat without affecting lean body mass, delayed and reduced cancer incidence and inflammatory diseases, rejuvenated the immune system and either delayed or partially reversed bone mineral density loss. Chronic FMD cycles also caused a major increase in embryonic–like stem/progenitor cells, induced hippocampal neurogenesis and caused improvements in a wide range of cognitive tasks. Therefore, periodic FMD started even at a relatively old age may be utilized as a regenerative approach by causing cycles of a highly coordinated process in which various cell populations undergo a rapid depletion followed by regeneration which is likely to involve stem and progenitor cells, including MSPCs. We hypothesize that these multi-systemic effects of the FMD improve health span and reduce both inflammation and cancerogenesis, which in turn may contribute to the observed healthspan extension.
Seven months of FMD cycles also resulted in improvements in a battery of behavioral tests ranging from motor coordination, to long and short-term memory compared to mice on the standard control diet (Figure 2) (Brandhorst et al., 2015). The effect of the FMD on neurogenesis, possibly by the down-regulation of IGF-1 and PKA signaling, indicates that the generation of new and functional neurons may contribute to the enhanced cognitive performance in FMD-treated mice, in agreement with the effects of PF, IGF-1 and PKA on hematopoietic regeneration (Cheng et al., 2014).
Not surprisingly, the FMD cycles started at 16 month of age caused an 18% increase in the 75% survival point, and an 11% increase in the mean lifespan. Notably, after 24 months of age mice appeared to be negatively affected by the 4 day FMD, but not by an identical FMD reduced to 3 days, in agreement with results on protein restriction, indicating that young but not old mice can maintain a normal weight when protein intake contributes to less than 7% of total calorie intake (Figure 2) (Levine et al., 2014).
IF and PF in the prevention and treatment of chronic diseases in humans
IF and Diseases
IF can have a wide range of effects on metabolic markers and risk factors or diseases including body fat, blood pressure. In overweight subjects who consumed an approximately 500 kcalorie but relatively high protein diet for 2 days a week for 6 months, abdominal fat was reduced blood pressure was reduced and insulin sensitivity was increased (Harvie et al., 2011). Similar results were obtained after 2–3 weeks of every other day fasting (Halberg et al., 2005; Heilbronn et al., 2005). A review of findings for all relevant clinical trials assessing both chronic calorie restriction in the 20–50% range (CR) and intermittent fasting, concludes that CR is superior in causing loss of body weight compared to IF, but both interventions have similar effects on the reduction of visceral fat, insulin and insulin resistance (Barnosky et al., 2014). However, it was also concluded that neither CR nor IF had clinically significant effects on glucose levels raising the possibility that both of these interventions may have limited applications in the prevention and treatment of metabolic syndrome and diabetes, particularly considering the difficulties in achieving compliance to either severe and chronic CR or severe restrictions limiting calorie consumption to 500–600 kcal per day between 9 and 15 times per month, on average.
IF fasting may also have some effects on inflammatory diseases, since 2 months of alternate day fasting resulted in a significant reduction in inflammatory markers in patients suffering from asthma (Johnson et al., 2007).
PF and Diseases
One of the well-established clinical uses of PF is in the treatment of rheumatoid arthritis (RA). Four different controlled studies have indicated that fasting periods lasting from one to three weeks reduce the symptoms of RA although these effects are reversed by a return to the normal diet unless the PF is followed by a vegetarian diet (Müller et al., 2001).
PF may also be beneficial in the treatment of hypertension. In a study, 13 days of water only fasting reduced systolic blood pressure below 120 in 82% of subjects with mild hypertension (Goldhamer et al., 2002). PB remained significantly lower after subjects had returned to their normal diet for 6 days. In another study, 10–11 days of fasting decreased systolic blood pressure of hypertensive patients by 37–60 mm, but this study did not follow patients after they returned to their normal diet (Goldhamer et al., 2001). In summary, both IF and PF have potential applications for inflammatory and cardiovascular diseases, but additional, larger and randomized studies are needed before these strategies can be integrated in the standard of care by physicians.
Optimal methodology and public health
The Periodic Fasting Mimicking Diets (FMDs)
Several major obstacles may be responsible for the very limited contribution of PF to standard medical practice: 1) the lack or pre-clinical and clinical data supporting specific and consistent effects of fasting on the prevention and treatment of diseases, and the mechanisms involved, 2) the safety concerns related to the adoption of water only consumption or the frequently adopted very low calorie diets (approx. 200 kcal) outside of a clinic, 3) the difficulties associated with compliance to these extreme diets. Although, hundreds of thousands of people are likely to undergo some form of PF every year, healthcare professionals strongly recommend that water only or similar fasting interventions be limited to specialized clinics staffed with medical personnel.
FMDs, were developed first for mice and eventually for humans to address these concerns and help identify both the positive and potentially negative effects of fasting while minimizing safety and compliance concerns. The FMD, which was originally developed to replace water only fasting in mice, has now been tested in humans. The human FMD consists of a 5 day regimen providing between 725 and 1090 kcalories, with a macronutrient content selected to mimic water only fasting but a micronutrient content aimed at maximizing nourishment. 19 subjects were randomized to undergo 3 cycles of a monthly FMD whereas an additional 19 subjects were randomized to a control diet. After 3 FMD cycles, the average reported side effect caused by the diet was very low and below “mild” (<1 on a scale of 1–5) (Brandhorst et al., 2015). After they resumed their normal diet, subjects who completed 3 FMD cycles displayed a 5.9 % drop in fasting glucose, as well as a 15% reduction in the level of IGF-1, a factor associated with elevated cancer incidence (Chan et al., 2000; Giovannucci et al., 2000; Levine et al., 2014). Body weight was reduced by approximately 3% but abdominal fat loss appeared to represent a major portion of this loss, as it would be expected from the major and transient elevation in ketone bodies during the FMD, indicating a switch to a fatty acid catabolism mode. Remarkably, the relative lean body mass adjusted for body weight was increased after 3 FMD cycles (Brandhorst et al., 2015; Brandhorst et al., 2013).
In agreement with the anti-inflammatory effect of the FMD in mice, C-reactive protein, a marker of inflammation and risk factor cardiovascular disease, returned to the normal level in seven of the eight subjects with elevated baseline serum levels, whereas no changes were observed for subjects with normal baseline CRP levels. Also in agreement with the elevation of mesenchymal stem and progenitor cells (MSPC) in response to the FMD in mice, MSPC levels were transiently elevated during the FMD in human subjects (Brandhorst et al., 2015).
Taken together, these results indicate that PF, applied in the form of a moderate calorie FMD, can promote a rejuvenating effect on different systems leading to an improvement in markers/risk factors for disease particularly in subjects with elevated baseline levels for these markers. Although it is early to determine whether the multi-system generative effects caused by the FMD in mice can also occur in humans, the ability of the FMD to be more effective in at risk subjects together with the elevation of MSPC, indicate that PF may also promote regenerative effects in humans. Larger clinical trials, and ideally an FDA approval will be essential in moving the FMD and other PF type interventions from the few specialized clinic and a very limited number of patients to a much wider use in medicine.
Intermittent Fasting
Whereas a water only but also a 500–600 kcalories alternate day fasting is very unlikely to be feasible for the great majority of the population of most countries, the 5:2 diet described earlier requiring only 2 days of a 500–600 kcal intake has a much higher potential to be considered for medical interventions. However, additional rodent studies showing a more clear effect on longevity and healthspan are needed. Also, additional studies taking advantage of the new molecular understanding of the connection between specific nutrients and the activation of pro-aging and anti-regenerative genes exploited in the development of FMDs, is likely to enhance the efficacy of IF diets. In addition, larger clinical trials are necessary, including the investigation of safety concerns such as those related to the effects of the frequent but irregular changes in calorie consumption and eating pattern on sleep and metabolic disorders.
Time restricted feeding in health and longevity
Time-restricted feeding is a daily eating pattern in which all nutrient intake occurs within a few hours (usually ≤12h) everyday, with no overt attempt to alter nutrient quality or quantity. The concept of TRF arose within the context of circadian rhythms. Circadian rhythms are daily ~24h rhythms in metabolism, physiology and behavior that are sustained under constant light or dark condition. These rhythms are observed in diverse organisms from cyanobacteria to humans, but are absent in several bacteria and yeast. Although several features of 24h rhythms in cellular metabolism, redox, DNA replication, DNA repair, and cellular growth are conserved across species, the core circadian oscillator components are not conserved across phylogenetic kingdoms. We will discuss the molecular mechanism of circadian rhythms in mammals and use a few examples from other species to highlight the adaptive advantages of having a robust circadian clock.
At the molecular level, circadian rhythms in mammals are generated and sustained by cell-autonomous interlocked transcription-translation feedback loops. In this molecular circuit, the transcriptional activators BMAL1 (MOP3 or ARNTL) and CLOCK or NPAS2 bind to the cis-acting E-box elements in Period 1 (Per1), Per2, Cryptochrome 1 (Cry1), and Cry2 genes, thereby driving their transcription. As PER and CRY protein levels rise, they repress CLOCK/NPAS2/BMAL1 transcriptional activity, inhibiting their own expression. PER and CRY are subsequently degraded, thus closing the loop (reviewed in (Hardin and Panda, 2013)). In an interlocked loop, CLOCK and BMAL1 proteins also drive transcription of the retinoid-related orphan receptor (ROR) and the REV-ERB family of transcription factors (Cho et al., 2012; Preitner et al., 2002; Sato et al., 2004; Ueda et al., 2002). ROR proteins activate transcription of the Bmal1 gene by binding to the ROR element (RORE), whereas REV-ERB proteins competitively bind to the same site and negate the function of ROR proteins, thus producing rhythmic levels of Bmal1 gene products (Sato et al., 2004).
Although the mammalian circadian oscillator is cell autonomous, there is a hierarchical architecture to the organization of these oscillators. A central oscillator in the hypothalamic suprachiasmatic nucleus (SCN) generates daily rhythms in activity-rest and associated rhythms in core body temperature, feeding-fasting, and rhythms in several hormones, such as melatonin, growth hormone, and cortisol. The SCN oscillator is entrained to the ambient light in order to be synchronized with the 24h light/dark cycle. This ensures adaptation of the organism to changing daylength under natural condition. Peripheral oscillators in individual organs indirectly receive daily timing cues through systemic signals such as body temperature and hormones (glucagon, cortisol). The daily acts of feeding and fasting also affect these systemic signals as well as cell autonomous energy sensing pathways that interact with the cellular clocks. Therefore, the light/dark cycle and the feeding/fasting cycle both profoundly the circadian system.
In different organs the cell autonomous oscillator modulates daily rhythms in transcription and protein translation of a large number of genes, which in turn generate daily rhythms in cellular metabolism, repair, cell division, and growth in a tissue-specific manner (Mohawk et al., 2012). Such temporal coordination from individual cells to the whole organism optimizes fitness. The role of circadian oscillator in fitness and lifespan is conserved across species and has been demonstrated in both laboratory and natural conditions.
The circadian clock intimately interacts with nutrient sensing pathways. Frequent eating and the absence of a defined fasting period likely sustain modestly elevated levels of fed-state physiology and disturb the normal counter-regulatory metabolic state that occurs during fasting. During feeding, activation of the insulin-pAKT-mTOR pathway drives downstream gene activities that promote anabolic processes. In contrast, a few hours of fasting activate AMPK, which triggers repair and catabolic processes. In parallel, AMPK-mediated inhibition of mTOR activity (Inoki et al., 2011) and downstream anabolic pathways ensures separation of catabolic and anabolic processes. In addition to well characterized anabolic targets, the mTOR pathway also phosphorylates casein kinase 1 (CK1) and glycogen synthase kinase 3 (GSK3), both of which phosphorylate the circadian clock component PER, altering its stability (Zheng and Sehgal, 2010). This is one mechanism by which feeding affects the clock. However, fasting signals also affect the circadian clock, as AMPK phosphorylates CRY and promotes its degradation (Lamia et al., 2009). Additionally, nicotinamide adenine dinucleotide (NAD) and Sirtuins, whose levels or activity fluctuate with the cellular energy state also affect the circadian clock (Asher et al., 2008; Masri and Sassone-Corsi, 2013; Nakahata et al., 2008; Nakahata et al., 2009; Ramsey et al., 2009). Therefore, feeding/fasting rhythms enhance the robustness or amplitude of the oscillation of circadian activator and repressor components (Figure. 3). Components of the circadian clock bind to transcriptional regulatory regions of thousands of genes and drive their rhythmic transcription in a largely tissue-specific manner (Koike et al., 2012; Vollmers et al., 2012). The mTOR and AMPK pathways also modulate activities of downstream proteins, including the transcription regulators CREB, PPAR, FOXO, Hsf1, HNF, and PGC1 (Inoki et al., 2011). However, the regulatory regions of most genes are targeted by multiple transcription factors (Hager et al., 2009). Accordingly, many transcripts regulated in a circadian fashion that are downstream targets of clock components are also targeted by transcriptional regulators whose activities are modulated by feeding and fasting (Bugge et al., 2012; Feng et al., 2011; Koike et al., 2012; Vollmers et al., 2009). Such convergent regulation by circadian clock and feeding/fasting sensors offer adaptive advantages to the organism.
Figure 3.
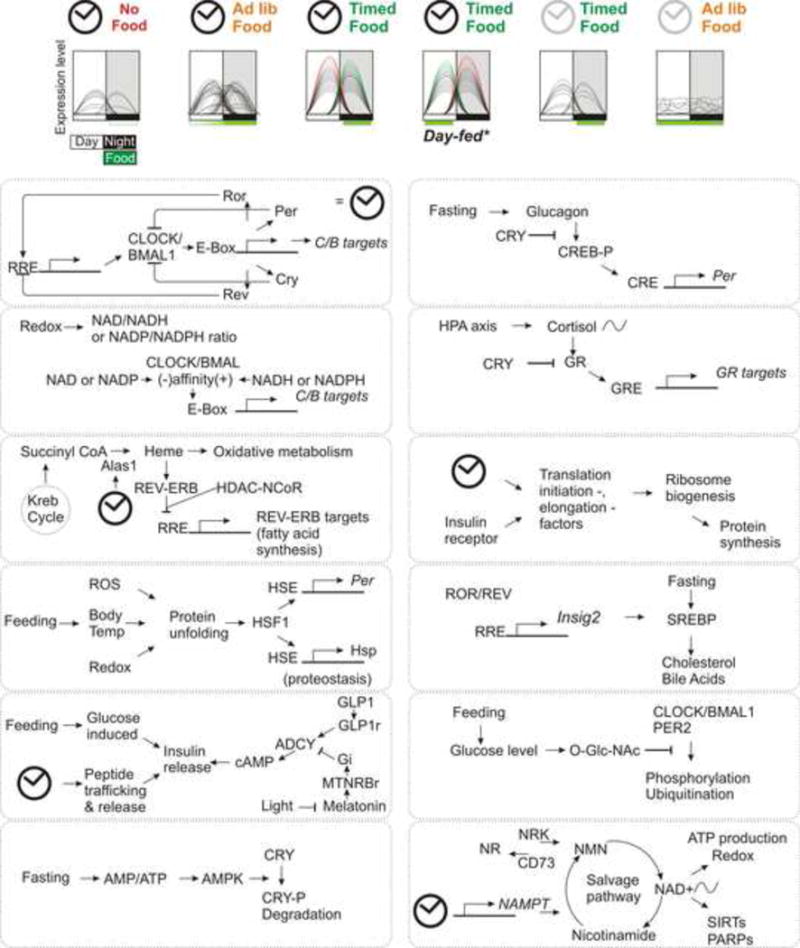
Eating pattern and circadian clock synergistically regulate temporal expression patterns of a large number of genes. (a) Summary hepatic circadian gene expression profile of C57/B6 adult mice without or with access to food ad lib or within a defined time interval. In the absence of food, very few transcripts show oscillation, while under ad lib access to standard diet mice eat a larger portion of their daily food intake during the night and show somewhat increased number of rhythmic transcripts. Restricting food access to night time only does not reduce food intake, but increases the number of rhythmic transcripts, improves amplitude and synchronizes the phases of oscillations of rhythmic transcripts. Daytime feeding of the same diet reverses the phases of oscillation of nearly all hepatic transcripts, thus illustrating the dominant role of eating time on the peripheral circadian oscillations. The absence of a circadian clock, as in Cry1−/−;Cry2−/− mutant mice, disrupts eating pattern and their liver shows no significant rhythm in gene expression. Subjecting mutant mice to TRF restores oscillation of some, not all, genes that normally oscillate in the wild type mice. (b) A summary sampling of some of the pathways underlying interaction among circadian oscillator (illustrated in the top left panel and represented as a clock), nutrient sensing pathways and systemic signals that affect daily rhythms in physiology.
In the absence of food intake, the self-sustaining circadian oscillator drives rudimentary oscillation of a handful of transcripts in the liver (Figure 3), thereby offering an anticipatory drive for feeding or fasting. Daily feeding/fasting rhythms drive signaling pathways that interact with the circadian oscillator to increase the robustness or peak-to-trough differences of these transcriptional oscillations. These transcripts then mediate anabolic and catabolic processes that are appropriate for specific phases of the feeding/fasting cycle. In the absence of a functional circadian clock, feeding- and fasting-driven pathways can drive some oscillations in transcription (Vollmers et al., 2009), downstream metabolites (Adamovich et al., 2014), and even the gut microbiota (Thaiss et al., 2014), but these signals cannot fully compensate for the loss of the circadian clock. Therefore, synergistic interactions between the circadian oscillator and feeding/fasting signals ensure that anabolic and catabolic types of metabolism are coordinately regulated in harmony with the animal’s activity/rest cycle.
Epidemiology of circadian disruption
Susceptibility of the SCN circadian clock to light and the peripheral clocks to the time of food intake help synchronize the internal timing system with ambient conditions. Such plasticity of the circadian system has evolved to adapt to seasonal changes in day length and associated changes in the time of food availability. However, circadian plasticity has become a liability in modern days, as our human daily patterns are no longer constrained by the day/night cycle. The use of artificial light during the night has enabled shiftwork for industrial production, public safety, health care, transportation, entertainment, information technology, food preparation, and the hospitality industry. These industries and services, in turn, enable the larger public to be awake, active, and hungry at any time of the day. Such an erratic lifestyle can cause chronic disruption to the circadian system. Nutrition quality also plays a role in maintaining robustness of the circadian system. In rodents, ad libitum access to a high fat diet (HFD) has been used extensively to induce obesity and the associated metabolic diseases. However, the HFD eating paradigm also blunts the daily cycle of nocturnal eating and daytime fasting in these animals (Kohsaka et al., 2007). Frequent eating in this model causes chronic disruption of the circadian clock and dampens molecular circadian rhythms (Hatori et al., 2012).
Finally, age is also a risk for dampening the endogenous circadian clock. Fibroblasts from older individuals have a dampened circadian clock, and some of this dampening is mediated by serum factors (Pagani et al., 2011). With increased human longevity, age-related dampening of the circadian clock is becoming a risk for chronic circadian disruption.
Lifestyles that chronically disrupt circadian rhythms are endemic to modern society and may include bona fide shift-workers, individuals with a lifestyle that resembles a shift-work, and individuals with underlying sleep disruption. With the emerging evidence that eating patterns also determine the robustness of circadian rhythms, it is conceivable that individuals with night-eating syndrome or binge-eating habits may also have underlying circadian disruption. Shift workers who work in the morning (starting at or before 6am), evening (ending after 6pm) or overnight comprise 15–20% of the work force population in the United States (McMenamin, 2007). Given a defined nature of their work and disruptive circadian lifestyle, they have been extensively studied to determine the health impact of shiftwork. There are strong correlations between shiftwork and predispositions to cancer, obesity, and metabolic syndrome (Arble et al., 2015; Knutson et al., 2007; Spiegel et al., 2005). In addition to the disruption of cell-autonomous circadian rhythms, shiftwork may also cause the desynchronization of tissue-specific clocks via disruption of the hypothalamus-pituitary-adrenal (HPA) signaling axis (Nicolaides et al., 2014; Sookoian et al., 2007). Interestingly, other factors that affect the normal cyclical oscillation of this axis (e.g., chronic stress or treatment with high doses of corticosteroids) lead to metabolic syndrome. Some of this effect may be related to circadian dyssynchrony.
Causal effect of shiftwork on metabolic diseases is increasingly tested in both animals and humans under controlled laboratory condition. Simulated night-shift work reduced daily energy expenditure in human volunteers (by ~12–16%) and in response to meals (McHill et al., 2014). This was associated with decreased levels of the satiety hormones leptin and peptide YY. Individuals subjected to circadian misalignment for 10 days develop elevated post-prandial glucose, elevated insulin (insulin resistance), and increased mean arterial pressure (Scheer et al., 2009).
Clinical Applications
Evidence for the physiological importance of a robust circadian eating pattern comes from rodents. Mice fed a HFD exhibit a severely dampened circadian eating rhythm and develop metabolic diseases (Kohsaka et al., 2007). Restricting access to high-fat food to only 8–12 hours per day does not reduce overall caloric intake (compared to animals fed ad libitum), but improves circadian rhythms and helps prevent or reverse metabolic diseases (Chaix et al., 2014; Hatori et al., 2012; Zarrinpar et al., 2014) (Figure 4). The benefits associated with TRF or a robust feeding/fasting cycle against metabolic disease involves multiple temporal changes in physiology and metabolism in different organs. In the liver, TRF reprograms metabolic flux through gluconeogenesis by redirecting pyruvate metabolism to the TCA cycle and glucose-6P metabolism through the pentose phosphate pathway. These two pathways contribute to increased production of nucleotides. TRF also improves the expression of Cyp7A, which redirects cholesterol to bile acid production. TRF also increases activity levels of brown adipose tissue (thereby increasing metabolic rate), increases fatty acid β–oxidation, and reduces hepatic glucose production (Froy, 2011; Froy et al., 2006; Froy and Miskin, 2010). In white adipose tissue, TRF reduces macrophage infiltration and resulting inflammation. In Drosophila, TRF has significant beneficial effect on cardiac health and also improves sleep (Gill et al., 2015) (Figure 4).
Figure 4.
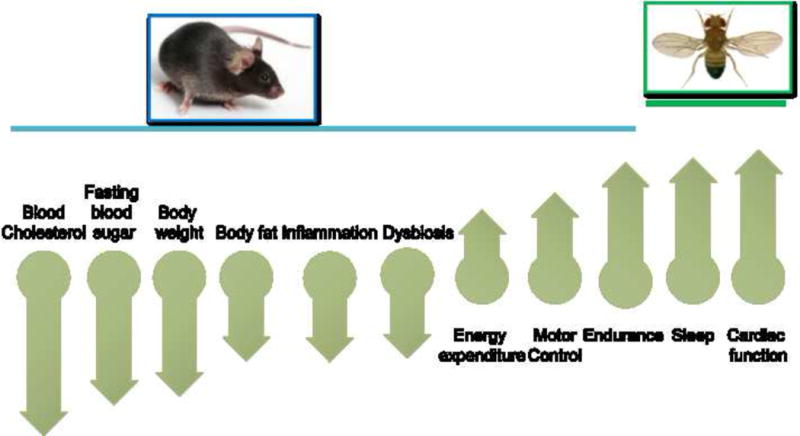
Benefits of TRF in rodents and Drosophila. TRF of 8–12 h during the night in rodents or 12 h during the day for Drosophila imparts pleiotropic benefits that involve multiple organ systems. The benefits and the direction of change imparted by TRF relative to ad lib feeding of a similar obesogenic or high sugar diet.
These observations in animals beg the question of whether the temporal pattern of energy intake (i.e., the duration of feeding) is a determinant of metabolic health in humans. The daily eating pattern in human has not been well characterized in an evidence-based manner. The traditional 24-hour recall or food frequency questionnaires are relatively weak in extracting meal timing and variability in meal timing from day to day. In a simplified and evidence-based approach, a smartphone-based method to longitudinally monitor what, when, and how much individuals eat on a daily basis has begun to shed new light on eating patterns (Gill and Panda, 2015). Participants logged food for 3 weeks to account for day-to-day or weekday-weekend variations. Average estimated caloric intake was 122% of their calculated maintenance calories, thus validating the method does not underestimate caloric intake. Surprisingly, among a cohort of 156 adults (age 21–56 years, both males and females, BMI 19–36, no shift-worker), nearly 50% were likely to eat during a prolonged period every day (>15h) and only 20% ate 3 meals/day. Nearly 25% of adults changed their breakfast time by ≥ 2h during the weekend. Overall 80% of adults consumed a non-water food or beverage within an hour of waking up and 50% of adult consumed food or beverage ≤2h before bed time. This implies that a sizeable portion of daily food intake is in the form of frequent snacks between major meals and after-dinner snacks or beverages. Extension of daily eating duration (from first caloric intake to last caloric intake) may also account for excessive caloric intake.
The feasibility of humans adopting a TRF protocol has shown some promise. The same study (Gill and Panda, 2015) tested whether altering the daily eating duration by allowing participants to eat their daily caloric intake within a self-selected 10–11h period would impart health benefits to overweight individuals. Eight overweight participants ate their entire daily caloric intake within self-selected 10–11h window. For half of them the eating window ended past 8pm so that they could have dinner with family. However, unlike rodents who consume the same number of calories when TRF of 8–15h is imposed, reducing eating duration in humans also reduced their daily caloric intake by up to 20% (some of this reduction came from reduction in late night alcohol and snacks). They lost up to 4% body weight in 16 weeks and retained this weight loss for up to 1 year. They also reported improved sleep at night and elevated alertness during the day.
Additional studies have also suggested that TRF confers potential benefits to human health. In a retrospective study examining the self-reported duration of overnight fasting and breast cancer incidence, a prolonged overnight fasting period of ≥13 h correlated with reduced breast cancer risk (Marinac et al., 2015a; Marinac et al., 2015b). While these reports show feasibility of TRF as an intervention or voluntary adoption of TRF as a lifestyle, more focused studies on the impact of TRF on both prevention and prognosis of chronic diseases is warranted. Nearly 50% of US adults have an existing chronic disease condition for which they may be taking a medication or supplement. Circadian transcriptome and proteome studies have identified several proteins that are the target or that impact absorption and clearance of drugs are also under circadian modulation (Neufeld-Cohen et al., 2016; Robles et al., 2014; Zhang et al., 2014). Since eating pattern determines the phases of circadian rhythms in peripheral organs, timing of medication relative to the timing of food intake will likely impact prognosis.
Age-specific Nutrition
PF as well as TRF and IF can have profound beneficial effects on the health of rodents and humans. However, most of the studies testing these interventions have been performed in young organisms or have not compared their effects in young and old. Understanding the age-specific effects of these interventions is particularly important considering the major changes in weight, growth hormones, and steroid hormones that occur at different ages and the studies indicating that certain restriction can be beneficial or have no negative effects in young but not old organisms. For example, severe protein restriction causes weight loss in old but not young mice and low protein intake is associated with protection from mortality in 65 and younger but not 66 and older individuals (Levine et al., 2014). Similarly, FMD cycles were highly protective in middle age and old mice, but 4 but not 3 days of the FMD appeared to be detrimental in very old mice (Brandhorst et al., 2015). These studies indicate that dietary interventions in rodents and humans must be modified to optimize efficacy in at least two but possibly more adult age ranges, with results indicating that the 65 to 70 age range represents a key transition point. This is clearly an area of longevity research in need of a major expansion of both basic and clinical investigation.
Conclusions
Obesity in the US, which has nearly tripled in the last 50 years, has been accompanied by an approximately 7-fold increase in diabetes prevalence but a much more modest increase in cancer incidence (DeSantis et al., 2014). Although everyday dietary choices can clearly accelerate aging, increase the incidence of age-related diseases, and anticipate their onset, we are far from a consensus on what constitutes a diet that optimizes healthspan. Even if a consensus could be reached, its implementation could take decades. Pharmacological interventions can have potent effects on risk factors for diseases, but they are often accompanied by considerable side effects and the lowering of a specific risk factor often does not lower mortality. Recently, IF and PF, as well as TRF have emerged as potential strategies for avoiding major dietary changes while achieving strong effects not just for one diseases risk factor but for an array of factors that constitutes the foundation for metabolic syndrome, cardiovascular disease, cancer, and possibly neurodegenerative diseases (Mattson et al., 2014). Although their mechanisms of action are still poorly understood, they appear to promote coordinated effects on the aging process and do not simply inhibit specific enzymes, as it is often the case for drugs. To allow these interventions to be implemented by a large portion of the population, it will be necessary to test and standardize them by methodologies similar to those carried out for the approval of drugs by the FDA. Thus, extensive and conclusive experiments should be performed in model organisms to understand molecular mechanisms of actions and demonstrate efficacy, which will serve as the foundation for randomized clinical trials. Because rodent models have had limited success in helping identify effective therapies particularly for cardiovascular disease and Alzheimer’s, it is important to take advantage of organisms in which diseases of interest develop spontaneously and have strong similarities to the human counterpart. For example, dogs and monkeys may represent valuable model organisms for testing PF and TRF and their effect on diseases, particularly since these interventions will not require chronic dietary restrictions and can be adjusted to only cause a minor weight loss. Undoubtedly, FDA approval would be important for these dietary interventions to become standard of care, or at least viable and safe option to pharmacological therapies. In addition these dietary interventions have the potential to have additive and possibly synergistic effects when combined with drugs, as it is clearly emerging in both rodent and human studies in which FMD are combined with standard cancer therapies. Because they require a more in-depth understanding of what they do, how they can be combined, and the type of condition or disease they can prevent or treat, it will be necessary to involve and train medical doctors, registered dietitians, and other healthcare professionals on how to safely and effectively implement their use.
Acknowledgments
This work was supported, in part, by the NIH grants AG20642 and AG034906 to V.D.L. and EY016807 to S.P. We thank Dr. Min Wei for the careful reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Information
VDL has equity interest in L-Nutra, a company that develops medical food.
References
- Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J, Wang M, Han X, Asher G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell metabolism. 2014;19:319–330. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble DM, Bass J, Behn CD, Butler MP, Challet E, Czeisler C, Depner CM, Elmquist J, Franken P, Grandner MA, et al. Impact of Sleep and Circadian Disruption on Energy Balance and Diabetes: A Summary of Workshop Discussions. Sleep. 2015 doi: 10.5665/sleep.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Translational research : the journal of laboratory and clinical medicine. 2014;164:302–311. doi: 10.1016/j.trsl.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell metabolism. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Wei M, Hwang S, Morgan TE, Longo VD. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Experimental gerontology. 2013;48:1120–1128. doi: 10.1016/j.exger.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA. Rev-erbalpha and Rev-erbbeta coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AJ, Hoelzel F. Apparent prolongation of the life span of rats by intermittent fasting. J Nutr. 1946;31:363–375. doi: 10.1093/jn/31.3.363. [DOI] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell metabolism. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci E, Ma J, Pollak M. Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and prostate cancer risk: epidemiological studies. Growth Hormone & IGF Research. 2000;10:S32–S33. doi: 10.1016/s1096-6374(00)90015-7. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Da Sacco S, Mirisola M, Quinn DI, Dorff TB, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell stem cell. 2014;14:810–823. doi: 10.1016/j.stem.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA: a cancer journal for clinicians. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L. Induction of autophagy by spermidine promotes longevity. Nature cell biology. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Longo VD. The chronological life span of Saccharomyces cerevisiae. Aging Cell. 2003;2:73–81. doi: 10.1046/j.1474-9728.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- Feng D, Liu T, Sun Z, Bugge A, Mullican SE, Alenghat T, Liu XS, Lazar MA. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontán-Lozano Á, Sáez-Cassanelli JL, Inda MC, de los Santos-Arteaga M, Sierra-Domínguez SA, López-Lluch G, Delgado-García JM, Carrión ÁM. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. The Journal of Neuroscience. 2007;27:10185–10195. doi: 10.1523/JNEUROSCI.2757-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froy O. Circadian rhythms, aging, and life span in mammals. Physiology (Bethesda) 2011;26:225–235. doi: 10.1152/physiol.00012.2011. [DOI] [PubMed] [Google Scholar]
- Froy O, Chapnik N, Miskin R. Long-lived alphaMUPA transgenic mice exhibit pronounced circadian rhythms. Am J Physiol Endocrinol Metab. 2006;291:E1017–1024. doi: 10.1152/ajpendo.00140.2006. [DOI] [PubMed] [Google Scholar]
- Froy O, Miskin R. Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging (Albany NY) 2010;2:7–27. doi: 10.18632/aging.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Le HD, Melkani GC, Panda S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science. 2015;347:1265–1269. doi: 10.1126/science.1256682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell metabolism. 2015 doi: 10.1016/j.cmet.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Pollak M, Platz EA, Willett WC, Stampfer MJ, Majeed N, Colditz GA, Speizer FE, Hankinson SE. Insulin-like growth factor I (IGF-I), IGF-binding protein-3 and the risk of colorectal adenoma and cancer in the Nurses’ Health Study. Growth Hormone & IGF Research. 2000;10:S30–S31. doi: 10.1016/s1096-6374(00)90014-5. [DOI] [PubMed] [Google Scholar]
- Goldhamer A, Lisle D, Parpia B, Anderson SV, Campbell TC. Medically supervised water-only fasting in the treatment of hypertension. Journal of manipulative and physiological therapeutics. 2001;24:335–339. doi: 10.1067/mmt.2001.115263. [DOI] [PubMed] [Google Scholar]
- Goldhamer AC, Lisle DJ, Sultana P, Anderson SV, Parpia B, Hughes B, Campbell TC. Medically supervised water-only fasting in the treatment of borderline hypertension. The Journal of Alternative & Complementary Medicine. 2002;8:643–650. doi: 10.1089/107555302320825165. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Hager GL, McNally JG, Misteli T. Transcription dynamics. Mol Cell. 2009;35:741–753. doi: 10.1016/j.molcel.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N, Henriksen M, Soderhamn N, Stallknecht B, Ploug T, Schjerling P, Dela F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol (1985) 2005;99:2128–2136. doi: 10.1152/japplphysiol.00683.2005. [DOI] [PubMed] [Google Scholar]
- Hardin PE, Panda S. Circadian timekeeping and output mechanisms in animals. Curr Opin Neurobiol. 2013;23:724–731. doi: 10.1016/j.conb.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, Ditacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell metabolism. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. The American journal of clinical nutrition. 2005;81:69–73. doi: 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- Hu J, Wei M, Mirzaei H, Madia F, Mirisola M, Amparo C, Chagoury S, Kennedy B, Longo VD. Tor-Sch9 deficiency activates catabolism of the ketone body-like acetic acid to promote trehalose accumulation and longevity. Aging Cell. 2014;13:457–467. doi: 10.1111/acel.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Kim J, Guan KL. AMPK and mTOR in Cellular Energy Homeostasis and Drug Targets. Annu Rev Pharmacol Toxicol. 2011 doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Tellejohan R, Maudsley S. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radical Biology and Medicine. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, Fields S, Kennedy BK, Kaeberlein M. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5:487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- Kendrick DC. The effects of infantile stimulation and intermittent fasting and feeding on life span in the black-hooded rat. Dev Psychobiol. 1973;6:225–234. doi: 10.1002/dev.420060307. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell metabolism. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional Architecture and Chromatin Landscape of the Core Circadian Clock in Mammals. Science. 2012 doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- Lee GD, Longo DL, Wang Y, Rifkind JM, Abdul-Raman L, Mamczarz JA, Duffy KB, Spangler EL, Taub DD, Mattson MP, et al. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:198–205. doi: 10.1158/1078-0432.CCR-05-1286. [DOI] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell metabolism. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: Molecular Mechanisms and Clinical Applications. Cell metabolism. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nature cell biology. 2010;12:842–846. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Marinac CR, Natarajan L, Sears DD, Gallo LC, Hartman SJ, Arredondo E, Patterson RE. Prolonged Nightly Fasting and Breast Cancer Risk: Findings from NHANES (2009–2010) Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015a;24:783–789. doi: 10.1158/1055-9965.EPI-14-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinac CR, Sears DD, Natarajan L, Gallo LC, Breen CI, Patterson RE. Frequency and Circadian Timing of Eating May Influence Biomarkers of Inflammation and Insulin Resistance Associated with Breast Cancer Risk. PLoS ONE. 2015b;10:e0136240. doi: 10.1371/journal.pone.0136240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri S, Sassone-Corsi P. The circadian clock: a framework linking metabolism, epigenetics and neuronal function. Nat Rev Neurosci. 2013;14:69–75. doi: 10.1038/nrn3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. 2014;111:16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, Wright KP. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proceedings of the National Academy of Sciences. 2014;111:17302–17307. doi: 10.1073/pnas.1412021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin TM. Time to work: recent trends in shift work and flexible schedules, A. Monthly Lab Rev. 2007;130:3. [Google Scholar]
- Mirisola MG, Braun RJ, Petranovic D. Approaches to study yeast cell aging and death. FEMS yeast research. 2014;14:109–118. doi: 10.1111/1567-1364.12112. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H, de Toledo FW, Resch KL. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: a systematic review. Scandinavian journal of rheumatology. 2001;30:1–10. doi: 10.1080/030097401750065256. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, Nir D, Rousso-Noori L, Kuperman Y, Golik M, et al. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1519650113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides NC, Charmandari E, Chrousos GP, Kino T. Circadian endocrine rhythms: the hypothalamic–pituitary–adrenal axis and its actions. Annals of the New York Academy of Sciences. 2014;1318:71–80. doi: 10.1111/nyas.12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani L, Schmitt K, Meier F, Izakovic J, Roemer K, Viola A, Cajochen C, Wirz-Justice A, Brown SA, Eckert A. Serum factors in older individuals change cellular clock properties. Proc Natl Acad Sci U S A. 2011;108:7218–7223. doi: 10.1073/pnas.1008882108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakel D. Integrative medicine. Elsevier Health Sciences; 2012. [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles MS, Cox J, Mann M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014;10:e1004047. doi: 10.1371/journal.pgen.1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckenstuhl C, Netzberger C, Entfellner I, Carmona-Gutierrez D, Kickenweiz T, Stekovic S, Gleixner C, Schmid C, Klug L, Sorgo AG, et al. Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet. 2014;10:e1004347. doi: 10.1371/journal.pgen.1004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, Conti PS, Chen TC, Longo VD. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS One. 2012;7:e44603. doi: 10.1371/journal.pone.0044603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, Kaur G. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordr) 2012;34:917–933. doi: 10.1007/s11357-011-9289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian S, Gemma C, Fernandez Gianotti T, Burgueno A, Alvarez A, Gonzalez C, Pirola C. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. Journal of internal medicine. 2007;261:285–292. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol (1985) 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- Tinkum KL, Stemler KM, White LS, Loza AJ, Jeter-Jones S, Michalski BM, Kuzmicki C, Pless R, Stappenbeck TS, Piwnica-Worms D, et al. Fasting protects mice from lethal DNA damage by promoting small intestinal epithelial stem cell survival. Proc Natl Acad Sci U S A. 2015;112:E7148–7154. doi: 10.1073/pnas.1509249112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, et al. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmers C, Schmitz RJ, Nathanson J, Yeo G, Ecker JR, Panda S. Circadian Oscillations of Protein-Coding and Regulatory RNAs in a Highly Dynamic Mammalian Liver Epigenome. Cell metabolism. 2012;16:833–845. doi: 10.1016/j.cmet.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 2003;17:1133–1134. doi: 10.1096/fj.02-0996fje. [DOI] [PubMed] [Google Scholar]
- Wei M, Fabrizio P, Hu J, Ge H, Cheng C, Li L, Longo VD. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor, and Sch9. PLoS Genet. 2008;4:e13. doi: 10.1371/journal.pgen.0040013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell metabolism. 2014;20:1006–1017. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Sehgal A. AKT and TOR signaling set the pace of the circadian pacemaker. Curr Biol. 2010;20:1203–1208. doi: 10.1016/j.cub.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


