Abstract
Regulation of the formation and rewiring of neural circuits by neuropeptides may require coordinated production of these signaling molecules and their receptors that may be established at the transcriptional level. Here, we address this hypothesis by comparing absolute expression levels of opioid peptides with their receptors, the largest neuropeptide family, and by characterizing coexpression (transcriptionally coordinated) patterns of these genes. We demonstrated that expression patterns of opioid genes highly correlate within and across functionally and anatomically different areas. Opioid peptide genes, compared with their receptor genes, are transcribed at much greater absolute levels, which suggests formation of a neuropeptide cloud that covers the receptor-expressed circuits. Surprisingly, we found that both expression levels and the proportion of opioid receptors are strongly lateralized in the spinal cord, interregional coexpression patterns are side specific, and intraregional coexpression profiles are affected differently by left- and right-side unilateral body injury. We propose that opioid genes are regulated as interconnected components of the same molecular system distributed between distinct anatomic regions. The striking feature of this system is its asymmetric coexpression patterns, which suggest side-specific regulation of selective neural circuits by opioid neurohormones.—Kononenko, O., Galatenko, V., Andersson, M., Bazov, I., Watanabe, H., Zhou, X. W., Iatsyshyna, A., Mityakina, I., Yakovleva, T., Sarkisyan, D., Ponomarev, I., Krishtal, O., Marklund, N., Tonevitsky, A., Adkins, D. L., Bakalkin, G. Intra- and interregional coregulation of opioid genes: broken symmetry in spinal circuits.
Keywords: neuropeptides, spinal cord, lateralization
Neuropeptides exert specific, coherent effects on formation and rewiring of neural circuits and, consequently, on behavior (1–6). Most neuropeptides are expressed in tiny neuronal subpopulations that are characterized by specific distribution patterns. After release, neuropeptides diffuse through the extracellular space to their cognate receptors on proximally or distantly located cells (7, 8). In many CNS loci, a particular neuropeptide and its receptor are localized in different brain regions (9, 10). This mismatch suggests that neuropeptides form the dynamic neuropeptide cloud that spreads over the extracellular space to cover local neural circuits via volume transmission (8). Both the wide-ranging coverage of neural circuits by the neuropeptide cloud and the maintenance of the suprathreshold concentration of neuropeptides may be accomplished if neuropeptides are generated in excess of their receptors in the same or adjacent areas. We hypothesize that coregulated neuropeptide-receptor production within and across CNS regions may be established already at the transcriptional level.
Here, we addressed this hypothesis by comparing absolute expression levels of opioid peptides with their receptors and by characterizing coexpression (transcriptionally coordinated) patterns of these genes. The endogenous opioid system (EOS) includes the proenkephalin (Penk), prodynorphin (Pdyn), and proopiomelanocortin genes that give rise to enkephalins, dynorphins, and endorphins that act via μ-, κ-, and δ-opioid receptors encoded by the Oprm1, Oprk1, and Oprd1 genes, respectively.
Absolute expression levels and coregulation patterns of EOS genes were examined in the striatum and the dorsal spinal cord (DSC) and ventral spinal cord (VSC) where these genes are expressed in different neuronal subtypes and at high and moderate levels. In striatum, medium spiny projection neurons that form 2 major efferent pathways differ in expression of Penk and Pdyn (11). In the spinal cord, enkephalins and dynorphins are mainly located in the dorsal laminae, whereas receptors are evenly distributed between the dorsal and ventral parts where they are involved in processing of sensory information and control of motor reflexes (12–15).
The EOS coexpression pattern may be specific for each region, vary between the regions, and correlate between the left and right halves of each region that are anatomically and functionally similar. On the top of intraregional mechanisms, expression of EOS genes may be coregulated across CNS regions; therefore, both intra- and inter-regional coexpression patterns were analyzed. We also examined whether these patterns are flexible and may be changed upon transition to a new physiologic state. For this purpose, a unilateral traumatic brain injury (TBI) and sham operation (SO) as a control were applied to perturb EOS brain and spinal circuits. TBI produces generalized effects that result in impairment of multiple processes, including motor and sensory functions, emotions, and cognition, all of which are regulated by the EOS (12, 16–20). The SO, which includes a craniotomy, produces proinflammatory and morphologic damage (21) that may, by itself, affect EOS expression patterns, and therefore, was controlled by analysis of a naive animal group. Neurochemical and behavioral effects of TBI are often lateralized (22–26); however, the EOS has not yet been studied in this regard other than in a single work that demonstrated differential effects of left and right brain trauma on neural circuits that expressed the dynorphin opioid peptide (26). Therefore, effects of both the left- and right-side TBI and SO were analyzed.
MATERIALS AND METHODS
Animals
Fifty Long-Evans hooded male rats, age ∼3 mo, were divided into 5 groups (10 rats per each naive, left-SO, right-SO, left-TBI, and right-TBI groups). All animal protocols followed the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Medical University of South Carolina Animal Care and Use Committee.
Surgery
TBI was induced in the forelimb representation area of the motor cortex by using the controlled cortical impact (CCI) method (27–29). This injury model produces a focal contusion injury and results in forelimb motor impairments. Animals were anesthetized using 110 mg/kg ketamine and 9 mg/kg xylazine, i.p.; CCI and SO groups received a 4-mm-diameter craniotomy centered around 0.5 mm anterior and 4.0 mm lateral to bregma, directly over the forelimb area of the sensorimotor cortex either on the left or right hemisphere. SO animals underwent all procedures, except that the impact was not delivered. CCI was delivered by a small-bore electromagnetically controlled device (Benchmark Stereotaxic Impactor; Cortech Holdings, St. Louis, MO, USA). The electromagnetic controlled probe tip was angled 18° away from vertical, placing the flat impactor tip (3.0 mm in diameter) perpendicular to the surface of the brain. The tip was connected to a sensor that signals when the tip comes into contact with dura. The impactor then penetrated the exposed brain at 0.3 m/s at a depth of 1.7 mm below the cortical surface for 250 ms. After impact, gel film was used to cover exposed cortex and was held in place using dental acrylic.
Tissue harvesting
On postsurgery d 7, animals were anesthetized with isoflurane/O2, decapitated, and the brain and spinal cord were quickly removed. Cervical (C1–8) spinal cord and left and right striatum dissected on ice were frozen at –80°C. Frozen spinal cord was dissected into left and right dorsal [tissue weight: 47 ± 10% of total tissue samples (mean ± sd)] and ventral domains (53 ± 11%).
RNA purification and cDNA synthesis
Total RNA was purified by using RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA, USA). RNA concentrations were measured with Nanodrop (Nanodrop Technologies, Wilmington, DE, USA). RNA quality indicator was measured by using Bio-Rad Experion (Bio-Rad, Hercules, CA, USA) with Eukaryote total RNA StdSens assay according to manufacturer's protocol. Average RNA quality indicator value was 9.01 ± 0.27 (mean ± sd), which demonstrated high RNA quality. RNA (500 ng) was reverse-transcribed to cDNA with the cDNA iScript kit (Bio-Rad) according to manufacturer's protocol. cDNA samples were aliquoted and stored at −20°C.
Droplet digital PCR
Assay was described elsewhere (30). Reaction mixture contained droplet digital PCR (ddPCR) supermix for probes (Bio-Rad), TaqMan Assay (Penk, Rn00567566_m1; Pdyn, Rn00571351_m1; Oprm1, Rn01430371_m1; Oprd1, Rn00561699_m1; Oprk1, Rn01448892_m1; Applied Biosystems, Foster City, CA, USA), and cDNA corresponding to 8 ng transcribed RNA. The mixture was supplemented with droplet generation oil (Bio-Rad) and partitioned into 14,000–17,000 droplets in QX200 droplet generator (Bio-Rad). PCR was performed in a T100 Thermal Cycler (Bio-Rad) under the following conditions: 10 min at 95°C, 40 cycles for 30 s at 94°C followed by incubation for 60 s at 60°C and for 10 min at 98°C. Fluorescence intensity of the droplets was measured by using the QX200 droplet reader (Bio-Rad). Data analysis was performed with QuantaSoft droplet reader software (Bio-Rad). Positive and negative droplet populations were automatically detected.
Absolute transcript levels of the 5 EOS mRNAs were quantified in 3 areas (striatum and VSC, n = 8; DSC, n = 9) by ddPCR. Six technical outliers were excluded. Samples prepared from the left and right halves of each area were combined in equal amounts. Absolute mRNA amount was calculated by using Poisson statistics (31), and background was corrected on the basis of the no template control data. Absolute transcript levels were expressed in RNA copy numbers per nanogram of total RNA.
Quantitative RT-PCR
Quantitative RT-PCR (qRT-PCR) was performed on a CFX96 Real-Time PCR Detection System (Bio-Rad) to study the effects of perturbation of the EOS expression by unilateral injury (n = 50 rats). Primers for Oprk1 and Pdyn were designed by using Primer3 (SourceForge; https://sourceforge.net/) and Oligo Explorer (Teemu Kuulasmaa, University of Eastern Finland, Kuopio, Finland) software. Primers for Oprd1, Oprm1, and Penk genes are described elsewhere (Supplemental Table 1) (32–34). Housekeeping gene primer set was purchased from Biomol (Hamburg, Germany). mRNA levels were calculated by using a normalization factor (geometric mean of 2 reference genes selected from 10 genes using geNorm program; https://genorm.cmgg.be/) (35). Gapdh and Pgk genes were used as reference genes for analysis of striatum, and Tfrc and Ppia genes for analysis of spinal cord.
Reaction mixture consisted of cDNA (corresponding to 21 and 18 ng transcribed RNA from striatum and spinal cord), iQ SYBR Green Supermix (Bio-Rad), and forward and reverse primers. The following conditions were applied for the 2-step real-time PCR reaction: 3 min at 95°C, then 40 cycles for 5 s at 95°C, followed by incubation for 30 s at 60°C. No template control reactions were run in parallel. Melting curve analysis of PCR products was performed to ensure primer specificity and lack of primer dimers. To ensure correct amplification, PCR products were separated on agarose gel and sequenced in both directions.
Statistical analysis
We analyzed expression levels of opioid genes (Penk, Pdyn, Oprm1, Oprd1, and Oprk1), their asymmetry index, pairwise log-scaled ratio of opioid receptor genes (Oprk1/Oprm1, Oprk1/Oprd1, Oprm1/Oprd1), and correlations of expression levels within and between CNS areas. We processed the data obtained for the left and right halves of 3 CNS regions, namely striatum, and the dorsal and ventral halves of the cervical spinal cord. Asymmetry index (AI) was defined as (L − R)/(L + R), where L and R are expression levels in the left and right halves, respectively (18). Normality of data distribution was tested using the Shapiro-Wilks test, and homogeneity of variances by Levene’s test. Differences between animal groups were assessed by 1-way ANOVA (normally distributed data) or Kruskal-Wallis test of ranks (data with non-normal distribution). Expression levels for each gene in each part of each region were analyzed with 30 ANOVAs with group (naive, L-SO, R-SO, L-CCI and R-CCI) as a factor. AI and log-scaled ratio of opioid receptor expression levels were analyzed by 15 and 18 ANOVAs, respectively. Post hoc analysis was performed by using Tukey’s HSD test. In the absence of significant differences between animal groups identified by ANOVA, these groups were combined into a pooled sample. Pooled data set was used for analysis of AI, and the lateralization of both expression and log-scaled ratio of opioid receptors by paired Student’s t test (normally distributed data) or Wilcoxon signed-rank test (data with non-normal distribution). Spearman’s rank correlations were computed to assess interactions between gene expression within and between anatomic regions. Comparison of correlation sets was performed by Wilcoxon signed-rank test. The sum of correlation squares was used to characterize the aggregated level of intra- or interarea coregulation of expression, or, in other words, the level of coordination; direction was assigned depending on whether correlations were positive or negative. The significance level was set to P ≤ 0.01. Values of P < 0.05 and P > 0.01 were regarded as borderline significant. The Benjamini-Yekutieli procedure (36) was used for multiple testing adjustments.
Gene network analysis
A sparse inverse covariance matrix for opioid gene mRNA levels was estimated by using lasso (L1) penalty by R package glasso (37). glasso cycles through the opioid genes, fitting a modified lasso regression to each gene in turn, solving the resulting individual lasso problems by coordinate descent. The estimate of sparse inverse covariance matrix was used to construct a sparse undirected weighted network by R package igraph (38), which was finally visualized by VisANT (39, 40).
RESULTS
In the first part of the study, absolute expression levels of opioid peptide genes were compared with those of the receptor genes within each striatum and DSCs and VSCs naive animals by ddPCR. Next, effects of perturbation of EOS expression patterns in spinal and brain circuits by unilateral injury were measured by qRT-PCR. Unilateral SO was performed to control for CCI-induced brain injury. The SO that causes itself tissue injury (21) was controlled by analysis of naive rats. Expression levels in the left and right halves of the 3 regions were analyzed after the left and right side CCI or SO and in the naive group. We assumed that significant differences in expression levels of opioid genes (Penk, Pdyn, Oprm1, Oprd1, and Oprk1) between the naive group and both the SO and CCI groups and the AI between the SO and CCI groups may justify the effect of body injury and CCI, respectively. In the second part of the study, coexpression patterns within and between each of 6 CNS domains (3 regions × 2 halves), including interactions between left and right domains of each of 3 regions, were assessed by analysis of correlations.
Absolute quantification of EOS mRNA molecules by ddPCR
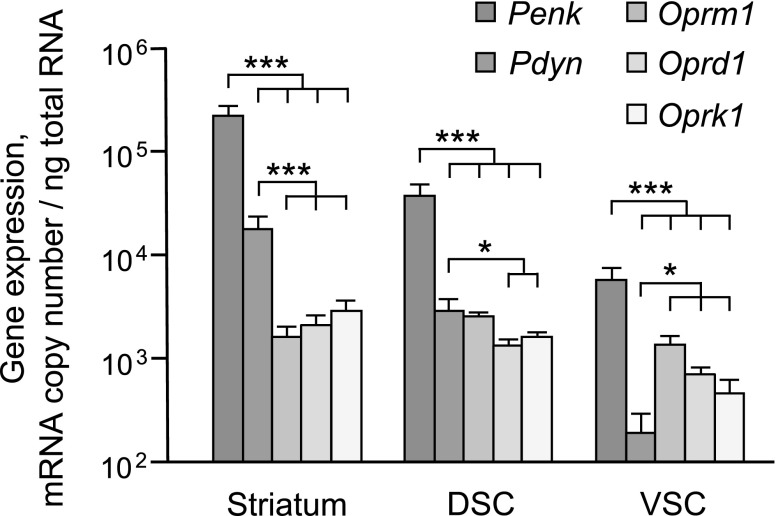
Levels of Penk, Pdyn, Oprm1, Oprd1, and Oprk1 mRNAs were analyzed in striatum and DSC and VSC (Fig. 1). The proopiomelanocortin gene was expressed at a low level and was excluded from further analysis. One-way ANOVA with gene as a factor was performed separately for each brain area and demonstrated significant gene effect (striatum, F4,31 = 174.5; DSC, F4,38 = 455.1; VSC, F4,35 = 9.9; P < 0.001 for each area). Penk mRNA levels were dramatically higher—from 4- to 138-fold higher than those of the receptor and Pdyn genes in striatum and spinal cord (post hoc Student's t test: P < 0.001 for all comparisons; Fig. 1). Compared with opioid receptor mRNA levels, Pdyn mRNA levels were substantially higher—from 2- to 11-fold greater in the striatum (P < 0.0001 for each comparison) and in the DSC (P = 0.001); however, mRNA levels were lower—from 2- to 7-fold—in VSC (Oprk1 and Oprd1, P = 0.05; Oprm1, P < 0.0001). No significant differences between the expression of 3 opioid receptors within each region and across 3 regions were evident. Thus, neuropeptide genes are expressed in excess compared with their receptor genes, with the exception of Pdyn in the VSC.
Figure 1.
Absolute expression levels of EOS genes in striatum and DSC and VSC of naïve animals measured by ddPCR. Data are shown as means ± sd of mRNA copy numbers per nanogram of total RNA (striatum and VSC, n = 8; DSC, n = 9 rats). *P < 0.05, ***P < 0.001; post hoc Student's t test.
Effects of CCI and SO on EOS expression levels and AI
In this and following sections, mRNA levels were analyzed by qRT-PCR. Analysis of the dorsal and ventral parts of the cervical spinal cord revealed no significant differences in expression levels, AI, and the opioid receptor ratio between naive, left, and right SO (L- and R-SO), and left and right CCI groups (L- and R-CCI). Analysis of striatum revealed the difference in AI at the borderline significance level after adjustment for multiple testing between animal groups for Oprd1 (F4,45 = 6.42; P = 0.02) and Oprm1 (F4,45 = 5.31; P = 0.035; Supplemental Fig. 1A, B; n = 10 rats/group). Tukey’s HSD test indicated differences in Oprd1 AI between naive and L-SO groups (P = 0.004), and in Oprm1 AI between naive and L-SO groups (P = 0.015), and naive and L-CCI groups (P = 0.002). Thus, left-side tissue and brain injury resulted in the decrease in Oprd1 and Oprm1 AI that is consistent with the lower expression of these genes in the left vs. the right striatum.
Comparison of expression in the left and right spinal domains
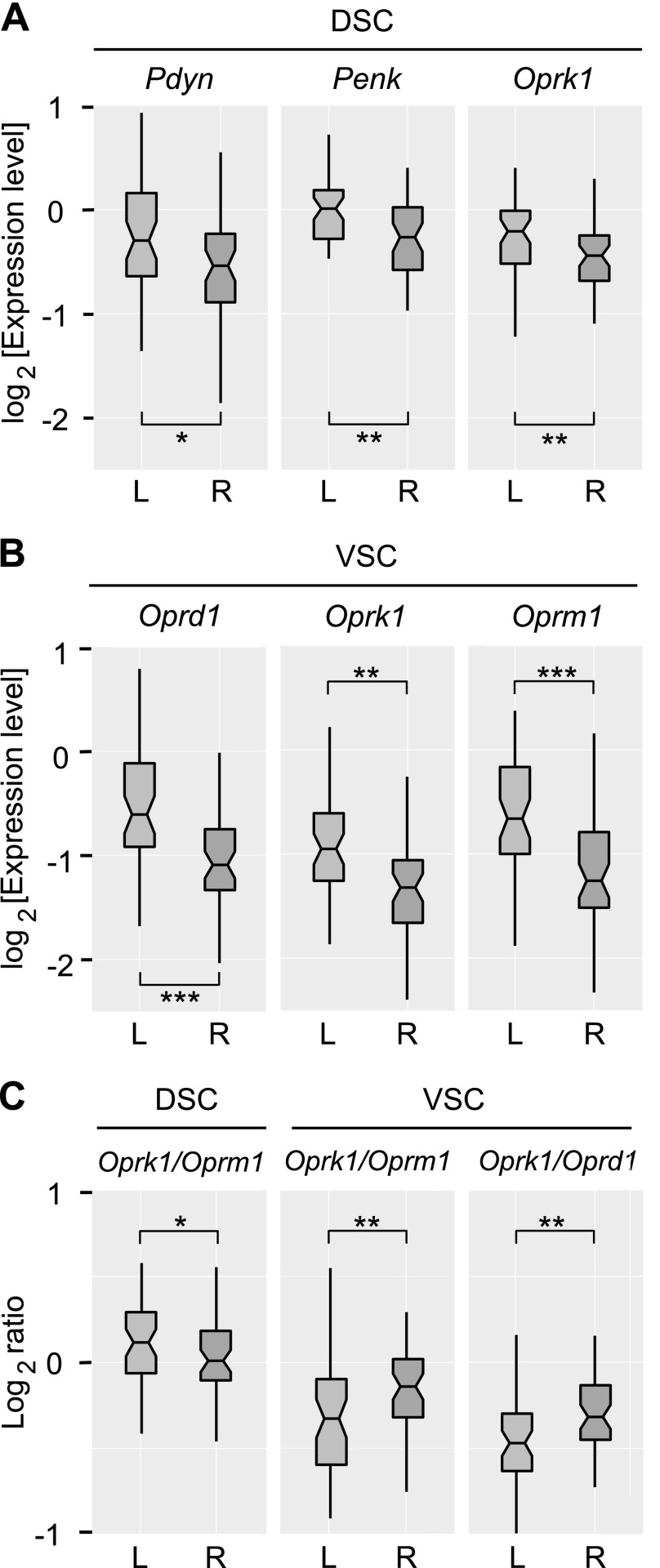
Because no significant differences between animal groups were identified for the spinal cord, data from 5 groups (n = 50 rats) were combined, and the pooled sample was used to compare expression levels between the left and right halves of each dorsal and ventral spinal part. This analysis revealed significantly higher expression of Pdyn (adjusted P = 0.027), Penk (adjusted P = 0.009), and Oprk1 (adjusted P = 0.009) in left-DCS (L-DSC) compared with right-DSC (R-DSC; Fig. 2A); and Oprd1 (adjusted P = 5.5 × 10−6), Oprk1 (adjusted P = 0.002), and Oprm1 (adjusted P = 3.4 × 10−4) in left-VSC (L-VSC) compared with right-VSC (R-VSC; Fig. 2B).
Figure 2.
A, B) Lateralization of opioid gene expression in the DSC (A) and VSC (B) for the pooled sample (n = 50 rats) measured by qRT-PCR. Light and dark gray denote the left (L) and right (R) areas. C) Lateralization of the Oprk1/Oprm1 and Oprk1/Oprd1 ratio (log scaled) in the DSC and VSC. The horizontal line in the box represents the median; the box hinges represent the first (Q1) and third quartiles (Q3). Upper and lower whiskers extend from the hinge to the highest/lowest value that lies within the 1.5× interquartile range (IQR) of the hinge. Notches extend from median to ± 1.58 [IQR/sqrt(n)], where n represents sample size. *P < 0.05, **P < 0.01, ***P < 0.001; post hoc Student's t test.
Concordant results were obtained for AI (Supplemental Fig. 1C–E); left-right asymmetry was evident for Pdyn (adjusted P = 0.005), Penk (adjusted P = 0.003), and Oprk1 (adjusted P = 0.002) genes in the DSC, and for Oprk1 (adjusted P = 0.0007), Oprd1 (adjusted P = 1.3 × 10−6), and Oprm1 (adjusted P = 9.8 × 10−5) genes in the VSC.
Analysis of the log-scaled Oprk1/Oprm1, Oprk1/Oprd1, and Oprm1/Oprd1 ratios revealed significant left-right side differences in the Oprk1/Oprm1 ratio in both DSC (adjusted P = 0.041) and VSC (adjusted P = 0.004) as well as in the Oprk1/Oprd1 ratio in the VSC (adjusted P = 0.001; Fig. 2C). Oprk1 mRNA dominated over Oprm1 mRNA in the L-DSC and R-VSC, which demonstrated left-right inversion of expression patterns between dorsal and ventral parts.
Coexpression patterns of opioid genes
Functionally related genes may be coregulated and therefore represent molecular networks that are characterized by high correlations between expression levels (41–43). We examined whether opioid genes are coexpressed within the left and right halves of the striatum, DSC, and VSC, and between all 6 domains of these 3 regions. Intraregional Spearman’s rank correlations between expression levels of 5 genes (10 correlations corresponding to 10 gene pairs) were calculated for each animal group (n = 10 rats) and for pooled sample (n = 50 rats). Interregional analysis examined correlations between 15 pairs formed by 6 domains that were left and right domains of each striatum, DSC, and VSC in the pooled sample. For each pair, Spearman’s rank correlations for all 25 gene pairs were measured; thus, altogether 15 × 25 = 375 inter-regional correlations were analyzed.
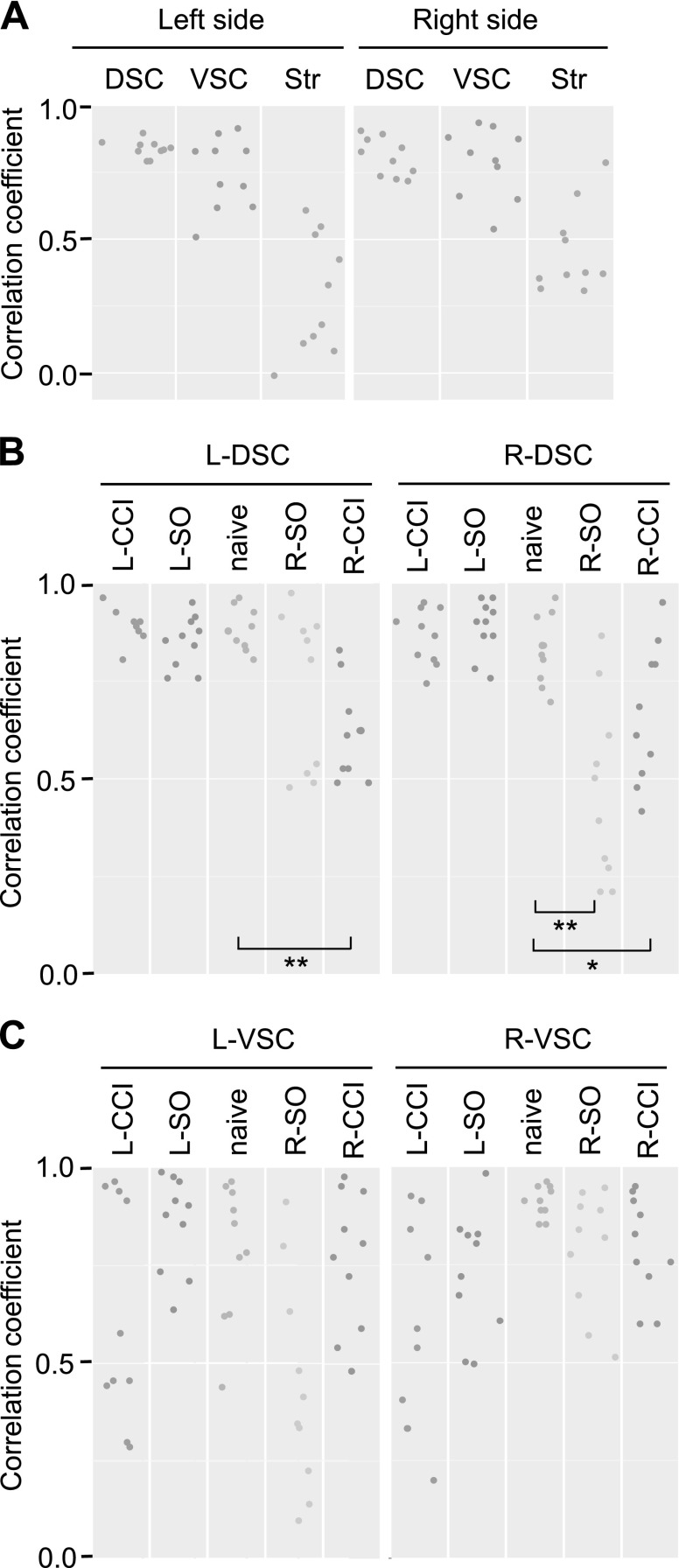
Intraregional analysis identified strong and significant pairwise correlations between expression of the 5 EOS genes within each left and right dorsal and ventral areas of spinal cord in both the pooled sample that consisted of the 5 animal groups and the naive group separately (Fig. 3A–C and Supplemental Tables 2 and 3 and additional data are available upon request). Correlation strength and significance level were much weaker in the striatum; only few correlations remained significant after correction for multiple testing. Comparison of intraregional correlations between animal groups revealed no significant differences between each L-SO and L-CCI group and naive group for all 3 regions. In contrast, both R-SO and R-CCI substantially reduced the strength and significance of intraregional correlations in the DSC (Wilcoxon test; naive vs. R-SO: R-DSC, P = 0.006; naive vs. R-CCI: L-DSC, P = 0.006, and R-DSC, P = 0.025; Fig. 3B). Differences between the group with right-side injury (R-SO and R-CCI groups pooled together; n = 20 rats) and the group with left-side injury (L-SO and L-CCI groups pooled together; n = 20 rats) were significant for L-DSC (Wilcoxon test; P = 0.0012) and R-DSC (Wilcoxon test; P = 0.00023). No significant patterns were evident in the VSC and striatum.
Figure 3.
A) Intraregional Spearman’s rank correlations between expression levels of 5 genes in the left and right areas of DSC and VSC and striatum (Str) for the pooled sample (n = 50 rats). B, C) Intraregional correlations in the left and right areas of DSC (B) and VSC (C) for the naive animal group and groups received left (L) or right (R) side unilateral controlled cortical impact (L- or R-CCI), or the L or R unilateral sham operation (L-SO or R-SO; n = 10 rats/group). Dots show the Spearman’s rank correlation coefficient (ρ) for each of 10 gene pairs. Vertical axis shows Spearman's ρ values. *P < 0.05, **P < 0.01; Wilcoxon test (B).
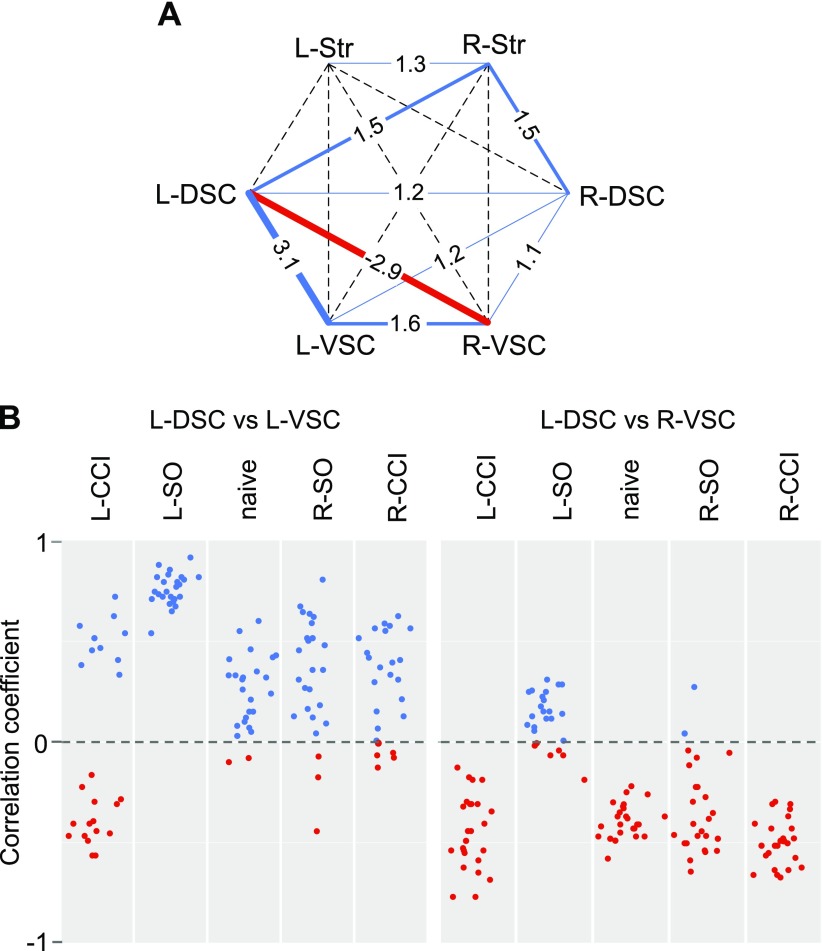
Analysis of the pooled sample detected robust interarea correlations between L-DSC and L-VSC, and between the L-DSC and R-VSC (Fig. 4A and Supplemental Table 4). All L-DSC–L-VSC correlations were positive, whereas L-DSC–R-VSC correlations were negative. The level of coordination or the aggregated level of coexpression, defined as the sum of correlation squares, was high between L-DSC and L-VSC areas in 4 groups, but not in the L-CCI group, and between L-DSC and R-VSC areas in 4 groups, with the exception of the L-SO group (Fig. 4B). Coordination levels between L-DSC and R-VSC, as well as between L-DSC and L-VSC, were significantly greater than those between the respective symmetric pairs of areas (Wilcoxon test; L-DSC–R-VSC vs. R-DSC–L-VSC, adjusted P = 0.012; L-DSC–L-VSC vs. R-DSC–R-VSC, adjusted P = 0.002; Fig. 4A). L-DSC–R-VSC and L-DSC–L-VSC coordination levels were high because of strong correlations that constituted these aggregated levels, including those between both the same (e.g., x-x) and different (e.g., x-y) genes (Fig. 4B and Supplemental Data are available upon request). L-DSC–L-VSC correlations were significant between Pdyn-Oprd1 (ρ = 0.60), Pdyn-Oprm1 (ρ = 0.59), Oprd1-Oprd1 (ρ = 0.51), Oprm1-Oprm1 (ρ = 0.51), and Oprm1-Oprd1 (ρ = 0.50) genes. L-DSC–R-VSC correlations were also significant between Oprm1-Oprk1 (ρ = −0.45), Penk-Oprk1 (ρ = −0.43), Oprd1-Oprk1 (ρ = −0.43), Penk-Oprm1 (ρ = −0.42), and Oprm1-Oprm1 (ρ = −0.40) genes. Adjusted value of P < 0.05 for each correlation.
Figure 4.
A) Interarea correlation levels for the pooled sample (n = 50 rats) between the left (L) and right (R) areas of DSC and VSC and striatum (Str). Vertices correspond to analyzed regions. Values (sums of correlation squares) and line widths indicate coordination level between regions; dashed lines correspond to low correlation levels. Blue and red colors show positive and negative correlations, respectively. B) Interregional correlations between the L-DSC and L-VSC areas (left) and between the L-DSC and R-VSC areas (right) for the naive animal group and groups received the L- or R-CCI, or the L- or R-SO (n = 10 rats/group). Dots show the Spearman’s rank correlation coefficient for each of 10 gene pairs. Vertical axis shows Spearman's ρ values. Blue and red colors show positive and negative correlations, respectively.
Undirected weighted network for opioid genes
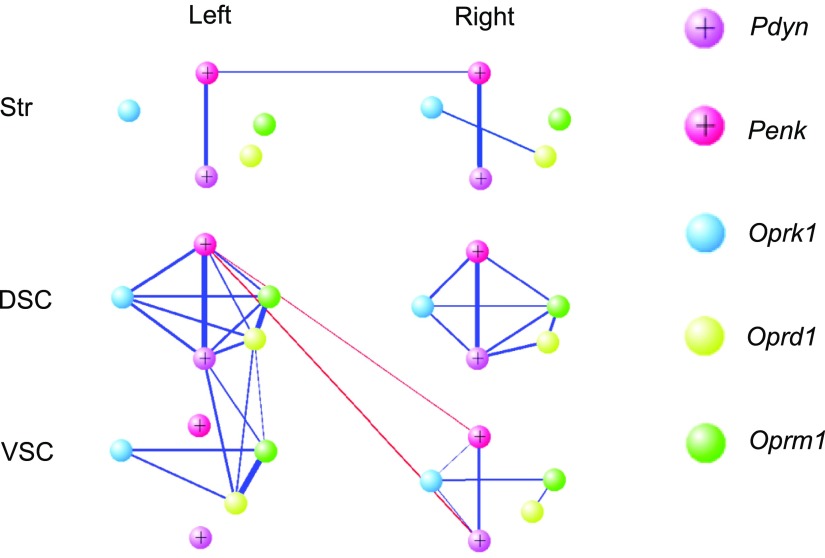
Results obtained by canonical correlation analysis were validated by using an undirected weighted network constructed by R packages glasso and igraph. The network of opioid genes was visualized by using VisAnt (39). Processing of the pooled data (n = 50 rats) resulted in the construction of the opioid genes network with strong positive correlations within each spinal area and weak correlations within the striatal halves (Fig. 5A). Consistent with a previous analysis, positive correlations were identified between L-DSC and L-VSC, whereas negative correlations characterized interactions between L-DSC and R-VSC. Pearson correlations matrices (5 × 5) for opioid genes in each spinal (L-DSC, R-DSC, L-VSC, and R-VSC) and striatal (L-Str and R-Str) domain were estimated. The equality of the matrices was compared by the Jennrich method (Table 1) (44). The left and right striatal domains were significantly different between each other and from 4 spinal areas. In the spinal cord, L-DSC area was significantly different from L-VSC and R-VSC, which was in agreement with both canonical correlation and network analyses visualized in Figs. 4A and 5, respectively.
Figure 5.
Network of opioid genes within and between left- and right-side domains of striatum (Str) and DSC and VSC. The width of the lines is proportional to the partial correlation coefficients. The blue and red lines denote positive and negative correlations, respectively.
TABLE 1.
Differences in the equality of the correlation matrices between each pair of the spinal (L-DSC, R-DSC, L-VSC, and R-VSC) and striatal (L-Str and R-Str) areas for the pooled sample (n = 50 rats)
| Brain area | L-DSC | R-DSC | L-VSC | R-VSC | L-Str | R-Str |
|---|---|---|---|---|---|---|
| L-DSC | — | 1.00 | 9.09−03 | 5.70−06 | 1.09−13 | 3.23−07 |
| R-DSC | — | 0.237 | 1.00 | 1.19−14 | 3.93−09 | |
| L-VSC | — | 1.00 | 1.27−21 | 3.48−14 | ||
| R-VSC | — | 3.76−23 | 3.14−16 | |||
| L-Str | — | 8.34−03 | ||||
| R-Str | — |
Data are Bonferroni corrected P values. Str, striatum.
DISCUSSION
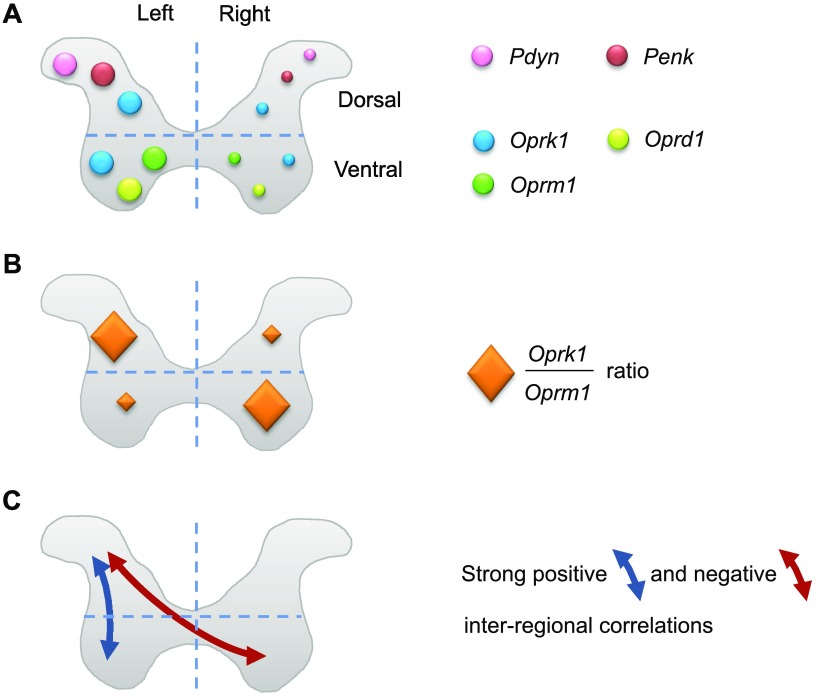
EOS gene transcription may be regulated at the level of individual genes (a), gene coexpression networks in the same cell types/subtypes (b), gene coexpression networks between different cell types/subtypes in the same area or tissue (c), and gene coexpression networks between areas or tissues (d). If level a has been relatively well studied, b, c, and d patterns have not been addressed for EOS genes. This study is the first, to our knowledge, that uncovers the novel levels in regulation of the opioid system that are the coexpression patterns within and between CNS areas. The principal findings of this study are: 1) remarkably high expression of Penk and Pdyn in the striatum and DSC and Penk in VSC compared with that of the receptor genes; 2) strong correlations in expression of opioid genes within and between spinal areas; and 3) lateralized expression of these genes and asymmetry of the interregional interactions in the spinal cord that were unanticipated and that are likely interconnected phenomena (Fig. 6).
Figure 6.
Schematic representation of lateralized expression of the opioid genes (A, B) and interregional correlations in their expression levels in the DSC and VSC (C). Findings for the pooled sample are presented. The left/right differences in the size of circles or diamonds denote the direction of asymmetry in opioid gene expression (circles in panel A) and the Oprk1/Oprm1 expression ratio (diamonds in panel B); the size is not proportional to the real expression values. For example, the pink Pdyn circle is larger on the left side than on the right side, showing higher Pdyn expression in the left compared with the right dorsal horn.
Penk (from 4- to 138-fold) and Pdyn (from 12- to 30-fold) were found to be expressed at much higher levels compared with those of opioid receptors in the striatum and DSC. Low Pdyn expression in the VSC is an example of neuropeptide-receptor mismatch (9, 10), suggesting that dynorphins synthesized in the dorsal part reach κ-opioid receptor in the ventral laminae by volume transmission through extracellular space. Penk and Pdyn protein molecules give rise to 7 and 3 opioid peptide copies, respectively, that further increase the molar proportion of opioid peptides over receptors. These features and expression patterns may compensate for extensive degradation of enkephalins and dynorphins and their adsorption on cellular components, thereby generating suprathreshold neuropeptide concentrations over distributed opioid neural circuits.
Coregulation by the same transcription programs may underlie EOS coexpression patterns in the same or different cells. Preliminary in silico analysis of cis-elements identified binding sites for 6 transcription factors (TFs) in promoters of 5 analyzed EOS genes, namely for nuclear factors of activated T cells (NFAT1 and NFAT4), glucocorticoid receptor, signal transducers and activators of transcription (STAT1 and STAT3), and TF-AP2A (the target site was absent in Oprm1). These TFs are expressed and regulate gene transcription in the spinal cord (45–47). Furthermore, EOS genes may be regulated by glucocorticoids via the classical nuclear receptor pathway and nonclassical membrane mechanism (48–50). Enkephalins and dynorphins are differentially susceptible to the effects of glucocorticoids, and Pdyn expression has a selective, glucocorticoid-permissive component (48). STAT may physically interact with and be regulated via μ-opioid receptor (51, 52). TF-AP-2 has a role in the regulation of κ-opioid receptor gene (53). It is important to establish experimentally a role for these TFs in the coregulation and lateralization of EOS gene transcription in the spinal cord.
Interactions between cells mediated by extracellular signaling molecules or neural mechanisms may be a prerequisite for coherent regulation of transcription of EOS genes in different neurons and neural circuits. Steroid hormones have a role in the modulation of neural circuits: they are produced within the circuits and help to stabilize them, which results in behavioral transitions (54). Regulation of EOS genes by steroid hormones (48–50, 55) is intriguing considering the selective effects of steroids on the left or right brain areas. Secreted cortisol may have lateralized effects on hippocampal microstructure (56), whereas interhemispheric communication is, in part, modulated by gonadal steroid hormones (57). In development, testosterone regulates cortical thickness and the associated lateralization of hemispheric function (58).
Specialization of the left and right hemisphere is an organizing principle of the brain. The computational advantages of functional specialization with distinct processes in and encoding of different functions between the hemispheres may facilitate processing of information and improve cognitive performance (59–62). For benefit of lateralization, specialized functions of the left and right hemispheres may be differentially regulated by specific neurotransmitter systems. Several rodent and human studies support this hypothesis (e.g., oxytocin may enable pup retrieval behavior in female mice by enhancing responses of the left, but not right, auditory cortex via its receptors expressed on the left side) (63). The structure of the oxytocin circuitries may represent a basis for the lateralized processing of auditory signals. In the human brain, asymmetric distribution of opioid peptides, which when acting via μ- and κ-opioid receptors elicit euphoria and dysphoria, respectively, may provide a biologic basis for the lateralized processing of positive and negative emotions (18).
The phenomenon of fixation of hind-limb postural asymmetry induced by a unilateral cerebellar lesion was discovered by Anna DiGiorgio 90 yr ago (64, 65) and has since been regarded as the first evidence of neuronal plasticity and as the model of pathologic spinal memory. DiGiorgio (64, 65) and others (66) demonstrated that abnormal descending activity produced by a hemicerebellar lesion causes a lasting change in spinal cord function manifested as flexion of the ipsilesional hind limb in dogs, rabbits, guinea pigs, and rats. Asymmetry in the hind-limb position remained after complete midthoracic spinal section if the interval between the cerebellar lesion and spinal cord section exceeds a critical period (45 min in rats). Searching for endogenous substances that are involved in hind-limb asymmetry formation, we unexpectedly found that selective opioid agonists upon symmetric route of administration (intrathecal or intravenously) in naive anesthetized animals may induce postural asymmetry, which was evident after spinal cord transection (67–70). When the side of the flexed limb was taken into account, it became apparent that κ-agonists, dynorphin and bremazocine, induce predominant flexion of the left hind limb, whereas δ-/μ-agonist, Leu-enkephalin, causes the right hind limb to flex. These unanticipated effects were robust, statistically significant, and well replicated in multiple blind experiments performed by different researches.
The present study revealed the lateralization of the entire EOS system, including 3 opioid receptors and/or their ligands, to the left with specific patterns in the dorsal and ventral spinal horns (Fig 6A). Not only the expression levels, but also the proportion of κ-, μ-, and δ-opioid receptors was different between the left and right ventral areas (Fig 6A). The only known physiologic phenomenon that may develop as a result of spinal EOS lateralization is the aforementioned side-specific effects of selective opioid agonists on motor responses of the left and right hind limbs that underlie the formation of postural asymmetry (67–70). Although a functional role has not yet been established, the phenomenon suggests that the neurohormonal opioid system in the spinal cord has the side-specific, receptor-selective component that differentially regulates the excitability of motor neurons that innervate the left or right limbs. In general terms, neuropeptide mechanisms that selectively regulate the left- or right-side neural circuits in the CNS may be proposed. Several lines of evidence support this hypothesis (18, 63, 71, 72). As already mentioned, oxytocin modulates the salience of social stimuli via the left-side–specific mechanism (63). Vasopressin, a key neurohormonal mediator of social behavior, modulates social recognition–related activity in the left temporoparietal junction in humans (72). Examples of neuropeptide lateralization in development include the left-side–specific expression of galanin in the heart tube of the mouse embryo that is independent of the nodal signaling (71) and the lateralized effects of selective opioid agonists on cell division in the germinal zone of embryonic neocortex (73). Electrophysiologic and biochemical analyses demonstrated lateralization of opioid receptors in the visual cortex of the turtle; evoked electrical activity of the left or right visual cortex was controlled via κ- and δ-opioid receptors, respectively, that may be relevant for side-specific neurohormonal regulation of plasticity in the visual system (74).
Neurochemical and behavioral responses to unilateral brain injury are often lateralized (22–25). Region-specific differences in norepinephrine turnover were dependent on the injury side in rats. Right, but not left, lesions resulted in behavioral hyperactivity and decreased concentrations of norepinephrine in both hemispheres (23). In humans, damage of the left hemisphere produced deficits in controlling the trajectory of arm movement, but not in achieving final position. In contrast, right hemisphere damage led to deficits in the accuracy of final position, but not in the ability to coordinate complex movement of a limb with multiple joints (25). In the mouse TBI model, injury to the right hemisphere affected the striatal and cortical levels of the dynorphin opioid peptide to a greater extent than injury to the left hemisphere (26). Asymmetric response of the dynorphin-expressing neural circuits may mediate effects of right-side, but not left-side, brain injury on lateralized brain functions, including emotions (18, 26). Consistent with these observations, right-side body injury decreased the strength of intraregional coexpression patterns of EOS genes in the dorsal spinal cord, whereas effects of left-side body injury were negligible (the present study).
The initial hypothesis on the perturbation of EOS expression patterns by unilateral brain injury did not gain experimental support in this study. No significant differences between the CCI and SO groups were found in all areas analyzed; however, the applied design has been productive. First, in the absence of significant differences, analysis of the large pooled sample (n = 50 rats) consisting of the combined 5 animal groups allowed identification of the highly significant interactions at the level of intra- and interregional correlations as well as of the lateralization pattern that are novel EOS transcription features. Second, side-specific effects of unilateral body injury similar to SO and CCI on the strength and significance of intraregional correlations in gene expression have been revealed in the DSC. Whereas no significant differences between L-SO, L-CCI, and naive groups were evident, both R-SO and R-CCI substantially reduced the strength and significance of intraregional correlations in the DSC. Furthermore, differences between the combined group with right-side injury (pooled R-SO and R-CCI groups) and the combined group with left-side injury (pooled L-SO and L-CCI groups) were significant for each of the L-DSC and R-DSC. A pathologic role and mechanism of right-side injury–induced discoordination of EOS genes transcription, however, is still not clear.
In summary, we demonstrated that EOS genes are coexpressed in the spinal cord, and that the intra- and interregional coexpression patterns are specific for CNS area and CNS side. Coregulated EOS genes may represent the components of the distributed transcription network that operates in the spinal cord but not in striatum. In bilaterally symmetric mammalian CNS, 2 hemispheres have mostly similar mechanisms that regulate genome-wide transcription (59, 75, 76). Strikingly, EOS expression levels differ between left and right spinal areas, and inter-regional coexpression patterns are not symmetric (Fig. 6A, B). These observations support the idea of the existence of side-specific spinal transcriptional mechanisms and side-specific neurohormonal regulation of spinal functions.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by grants from the Swedish Science Research Council (VR), the Swedish Council for Working Life and Social Research (FORTE), and the Swedish Research Council Formas (to G.B.); the Swedish Institute (Visby Program; to O.K. and G.B.); the European Union, Swedish Brain Foundation, and Uppsala University Hospital Research Fund (ALF; to N.M.); and U.S. National Institutes of Health National Institute of Neurological Disorders and Stroke Grant NS065866 (to D.L.A.). The authors thank Prof. Olga Korosteleva (Statistics at California State University, Long Beach, Long Beach, CA, USA) for discussion and correction of the manuscript. The authors declare no conflicts of interest.
Glossary
- AI
asymmetry index
- CCI
controlled cortical impact
- ddPCR
droplet digital PCR
- DSC
dorsal spinal cord
- EOS
endogenous opioid system
- Pdyn
prodynorphin
- Penk
proenkephalin
- qRT-PCR
quantitative RT-PCR
- SO
sham operation
- TBI
traumatic brain injury
- TF
transcription factor
- VSC
ventral spinal cord
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
N. Marklund, A. Tonevitsky and G. Bakalkin designed the research; O. Kononenko, V. Galatenko, M. Andersson, D. Sarkisyan, I. Ponomarev, O. Krishtal, N. Marklund and G. Bakalkin analyzed the data; O. Kononenko, V. Galatenko, M. Andersson, I. Bazov, H. Watanabe, X. W. Zhou, A. Iatsyshyna, I. Mityakina, T. Yakovleva, D. Sarkisyan, I. Ponomarev and D. L. Adkins performed the research; O. Kononenko, V. Galatenko, M. Andersson, I. Bazov, T. Yakovleva, D. Sarkisyan, I. Ponomarev and G. Bakalkin wrote the paper; and V. Galatenko, X. W. Zhou, I. Mityakina, D. Sarkisyan and A. Tonevitsky performed statistical treatment.
REFERENCES
- 1.Bargmann C. I. (2012) Beyond the connectome: how neuromodulators shape neural circuits. BioEssays 34, 458–465 [DOI] [PubMed] [Google Scholar]
- 2.Stoop R. (2012) Neuromodulation by oxytocin and vasopressin. Neuron 76, 142–159 [DOI] [PubMed] [Google Scholar]
- 3.Van den Pol A. N. (2012) Neuropeptide transmission in brain circuits. Neuron 76, 98–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bargmann C. I., Marder E. (2013) From the connectome to brain function. Nat. Methods 10, 483–490 [DOI] [PubMed] [Google Scholar]
- 5.Barrios A., Ghosh R., Fang C., Emmons S. W., Barr M. M. (2012) PDF-1 neuropeptide signaling modulates a neural circuit for mate-searching behavior in C. elegans. Nat. Neurosci. 15, 1675–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leinwand S. G., Chalasani S. H. (2014) From genes to circuits and behaviors: neuropeptides expand the coding potential of the nervous system. Worm 3, e27730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwig M., Leng G. (2006) Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 7, 126–136 [DOI] [PubMed] [Google Scholar]
- 8.Marcoli M., Agnati L. F., Benedetti F., Genedani S., Guidolin D., Ferraro L., Maura G., Fuxe K. (2015) On the role of the extracellular space on the holistic behavior of the brain. Rev. Neurosci. 26, 489–506 [DOI] [PubMed] [Google Scholar]
- 9.Herkenham M. (1987) Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience 23, 1–38 [DOI] [PubMed] [Google Scholar]
- 10.Drake C. T., Chavkin C., Milner T. A. (2007) Opioid systems in the dentate gyrus. Prog. Brain Res. 163, 245–263 [DOI] [PubMed] [Google Scholar]
- 11.Ferguson S. M., Eskenazi D., Ishikawa M., Wanat M. J., Phillips P. E., Dong Y., Roth B. L., Neumaier J. F. (2011) Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat. Neurosci. 14, 22–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steffens H., Schomburg E. D. (2011) Spinal motor actions of the μ-opioid receptor agonist DAMGO in the cat. Neurosci. Res. 70, 44–54 [DOI] [PubMed] [Google Scholar]
- 13.Clarke R. W., Harris J. (2004) The organization of motor responses to noxious stimuli. Brain Res. Brain Res. Rev. 46, 163–172 [DOI] [PubMed] [Google Scholar]
- 14.Bardoni R., Tawfik V. L., Wang D., François A., Solorzano C., Shuster S. A., Choudhury P., Betelli C., Cassidy C., Smith K., de Nooij J. C., Mennicken F., O’Donnell D., Kieffer B. L., Woodbury C. J., Basbaum A. I., MacDermott A. B., Scherrer G. (2014) Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron 81, 1312–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan B., Cheng L., Bourane S., Britz O., Padilla C., Garcia-Campmany L., Krashes M., Knowlton W., Velasquez T., Ren X., Ross S. E., Lowell B. B., Wang Y., Goulding M., Ma Q. (2014) Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuzmin A., Sandin J., Terenius L., Ogren S. O. (2000) Dose- and time-dependent bimodal effects of kappa-opioid agonists on locomotor activity in mice. J. Pharmacol. Exp. Ther. 295, 1031–1042 [PubMed] [Google Scholar]
- 17.Hauser K. F., Aldrich J. V., Anderson K. J., Bakalkin G., Christie M. J., Hall E. D., Knapp P. E., Scheff S. W., Singh I. N., Vissel B., Woods A. S., Yakovleva T., Shippenberg T. S. (2005) Pathobiology of dynorphins in trauma and disease. Front. Biosci. 10, 216–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe H., Fitting S., Hussain M. Z., Kononenko O., Iatsyshyna A., Yoshitake T., Kehr J., Alkass K., Druid H., Wadensten H., Andren P. E., Nylander I., Wedell D. H., Krishtal O., Hauser K. F., Nyberg F., Karpyak V. M., Yakovleva T., Bakalkin G. (2015) Asymmetry of the endogenous opioid system in the human anterior cingulate: a putative molecular basis for lateralization of emotions and pain. Cereb. Cortex 25, 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams J. T., Ingram S. L., Henderson G., Chavkin C., von Zastrow M., Schulz S., Koch T., Evans C. J., Christie M. J. (2013) Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev. 65, 223–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chavkin C. (2013) Dynorphin--still an extraordinarily potent opioid peptide. Mol. Pharmacol. 83, 729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole J. T., Yarnell A., Kean W. S., Gold E., Lewis B., Ren M., McMullen D. C., Jacobowitz D. M., Pollard H. B., O’Neill J. T., Grunberg N. E., Dalgard C. L., Frank J. A., Watson W. D. (2011) Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J. Neurotrauma 28, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrow L., Vrtunski P. B., Kim Y., Boller F. (1981) Arousal responses to emotional stimuli and laterality of lesion. Neuropsychologia 19, 65–71 [DOI] [PubMed] [Google Scholar]
- 23.Pearlson G. D., Robinson R. G. (1981) Suction lesions of the frontal cerebral cortex in the rat induce asymmetrical behavioral and catecholaminergic responses. Brain Res. 218, 233–242 [DOI] [PubMed] [Google Scholar]
- 24.Levin B. E., Brown K. L., Pawar G., Dunn-Meynell A. (1995) Widespread and lateralization effects of acute traumatic brain injury on norepinephrine turnover in the rat brain. Brain Res. 674, 307–313 [DOI] [PubMed] [Google Scholar]
- 25.Schaefer S. Y., Haaland K. Y., Sainburg R. L. (2009) Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia 47, 2953–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain Z. M., Fitting S., Watanabe H., Usynin I., Yakovleva T., Knapp P. E., Scheff S. W., Hauser K. F., Bakalkin G. (2012) Lateralized response of dynorphin a peptide levels after traumatic brain injury. J. Neurotrauma 29, 1785–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones T. A., Liput D. J., Maresh E. L., Donlan N., Parikh T. J., Marlowe D., Kozlowski D. A. (2012) Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J. Neurotrauma 29, 1455–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Combs H. L., Jones T. A., Kozlowski D. A., Adkins D. L. (2016) Combinatorial motor training results in functional reorganization of remaining motor cortex after controlled cortical impact in rats. J. Neurotrauma 33, 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jefferson S. C., Clayton E. R., Donlan N. A., Kozlowski D. A., Jones T. A., Adkins D. L. (2016) Cortical stimulation concurrent with skilled motor training improves forelimb function and enhances motor cortical reorganization following controlled cortical impact. Neurorehabil. Neural Repair 30, 155–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDermott G. P., Do D., Litterst C. M., Maar D., Hindson C. M., Steenblock E. R., Legler T. C., Jouvenot Y., Marrs S. H., Bemis A., Shah P., Wong J., Wang S., Sally D., Javier L., Dinio T., Han C., Brackbill T. P., Hodges S. P., Ling Y., Klitgord N., Carman G. J., Berman J. R., Koehler R. T., Hiddessen A. L., Walse P., Bousse L., Tzonev S., Hefner E., Hindson B. J., Cauly T. H. III, Hamby K., Patel V. P., Regan J. F., Wyatt P. W., Karlin-Neumann G. A., Stumbo D. P., Lowe A. J. (2013) Multiplexed target detection using DNA-binding dye chemistry in droplet digital PCR. Anal. Chem. 85, 11619–11627 [DOI] [PubMed] [Google Scholar]
- 31.Hindson C. M., Chevillet J. R., Briggs H. A., Gallichotte E. N., Ruf I. K., Hindson B. J., Vessella R. L., Tewari M. (2013) Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods 10, 1003–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao C. J., Niu L., Ren P. C., Wang W., Zhu C., Li Y. Q., Chai W., Sun X. D. (2012) Hypoxic preconditioning attenuates global cerebral ischemic injury following asphyxial cardiac arrest through regulation of delta opioid receptor system. Neuroscience 202, 352–362 [DOI] [PubMed] [Google Scholar]
- 33.Kraus J., Borner C., Giannini E., Hickfang K., Braun H., Mayer P., Hoehe M. R., Ambrosch A., Konig W., Hollt V. (2001) Regulation of mu-opioid receptor gene transcription by interleukin-4 and influence of an allelic variation within a STAT6 transcription factor binding site. J. Biol. Chem. 276, 43901–43908 [DOI] [PubMed] [Google Scholar]
- 34.Li Q., Goodchild A. K., Pilowsky P. M. (2003) Effect of haemorrhage on the expression of neurotransmitter-related genes in rat ventrolateral medulla: a quantitative real-time RT-PCR study. Brain Res. Mol. Brain Res. 114, 46–54 [DOI] [PubMed] [Google Scholar]
- 35.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim K. I., van de Wiel M. A. (2008) Effects of dependence in high-dimensional multiple testing problems. BMC Bioinformatics 9, 114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman J., Hastie T., Tibshirani R. (2008) Sparse inverse covariance estimation with the graphical lasso. Biostatistics 9, 432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y., Jackson S. A. (2015) Gene network reconstruction by integration of prior biological knowledge. G3 (Bethesda) 5, 1075–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Z. (2014) Using VisANT to analyze betworks. Curr. Protoc. Bioinformatics 45, 8.8.1–8.8.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Z., Mellor J., Wu J., DeLisi C. (2004) VisANT: an online visualization and analysis tool for biological interaction data. BMC Bioinformatics 5, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López-Kleine L., Leal L., López C. (2013) Biostatistical approaches for the reconstruction of gene co-expression networks based on transcriptomic data. Brief. Funct. Genomics 12, 457–467 [DOI] [PubMed] [Google Scholar]
- 42.Gaiteri C., Ding Y., French B., Tseng G. C., Sibille E. (2014) Beyond modules and hubs: the potential of gene coexpression networks for investigating molecular mechanisms of complex brain disorders. Genes Brain Behav. 13, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grange P., Bohland J. W., Okaty B. W., Sugino K., Bokil H., Nelson S. B., Ng L., Hawrylycz M., Mitra P. P. (2014) Cell-type-based model explaining coexpression patterns of genes in the brain. Proc. Natl. Acad. Sci. USA 111, 5397–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jennrich R. I. (1970) An asymptotic X2 test for equality of 2 correlation matrices. J. Am. Stat. Assoc. 65, 904–912 [Google Scholar]
- 45.Ulrich J. D., Kim M. S., Houlihan P. R., Shutov L. P., Mohapatra D. P., Strack S., Usachev Y. M. (2012) Distinct activation properties of the nuclear factor of activated T-cells (NFAT) isoforms NFATc3 and NFATc4 in neurons. J. Biol. Chem. 287, 37594–37609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong S., Song M. R. (2014) STAT3 but not STAT1 is required for astrocyte differentiation. PLoS One 9, e86851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y., Yang L., Mei X., Yu Y. (2014) Selective inhibition of STAT1 reduces spinal cord injury in mice. Neurosci. Lett. 580, 7–11 [DOI] [PubMed] [Google Scholar]
- 48.Thai L., Lee P. H., Ho J., Suh H., Hong J. S. (1992) Regulation of prodynorphin gene expression in the hippocampus by glucocorticoids. Brain Res. Mol. Brain Res. 16, 150–157 [DOI] [PubMed] [Google Scholar]
- 49.Matthews S. G., Challis J. R. (1995) Developmental regulation of preproenkephalin mRNA in the ovine paraventricular nucleus: effects of stress and glucocorticoids. Brain Res. Dev. Brain Res. 86, 259–267 [DOI] [PubMed] [Google Scholar]
- 50.Zaringhalam J., Manaheji H., Mghsoodi N., Farokhi B., Mirzaiee V. (2008) Spinal mu-opioid receptor expression and hyperalgesia with dexamethasone in chronic adjuvant-induced arthritis in rats. Clin. Exp. Pharmacol. Physiol. 35, 1309–1315 [DOI] [PubMed] [Google Scholar]
- 51.Yuen J. W., So I. Y., Kam A. Y., Wong Y. H. (2004) Regulation of STAT3 by mu-opioid receptors in human neuroblastoma SH-SY5Y cells. Neuroreport 15, 1431–1435 [DOI] [PubMed] [Google Scholar]
- 52.Georganta E. M., Agalou A., Georgoussi Z. (2010) Multi-component signaling complexes of the delta-opioid receptor with STAT5B and G proteins. Neuropharmacology 59, 139–148 [DOI] [PubMed] [Google Scholar]
- 53.Park S. W., He Y., Ha S. G., Loh H. H., Wei L. N. (2008) Epigenetic regulation of kappa opioid receptor gene in neuronal differentiation. Neuroscience 151, 1034–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Remage-Healey L. (2014) Frank Beach Award Winner: steroids as neuromodulators of brain circuits and behavior. Horm. Behav. 66, 552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Persson S., Schäfer M. K., Nohr D., Ekström G., Post C., Nyberg F., Weihe E. (1994) Spinal prodynorphin gene expression in collagen-induced arthritis: influence of the glucocorticosteroid budesonide. Neuroscience 63, 313–326 [DOI] [PubMed] [Google Scholar]
- 56.Madsen K. S., Jernigan T. L., Iversen P., Frokjaer V. G., Knudsen G. M., Siebner H. R., Baaré W. F. (2012) Hypothalamic-pituitary-adrenal axis tonus is associated with hippocampal microstructural asymmetry. Neuroimage 63, 95–103 [DOI] [PubMed] [Google Scholar]
- 57.Hausmann M., Hamm J. P., Waldie K. E., Kirk I. J. (2013) Sex hormonal modulation of interhemispheric transfer time. Neuropsychologia 51, 1734–1741 [DOI] [PubMed] [Google Scholar]
- 58.Nguyen T. V., McCracken J., Ducharme S., Botteron K. N., Mahabir M., Johnson W., Israel M., Evans A. C., Karama S.; Brain Development Cooperative Group (2013) Testosterone-related cortical maturation across childhood and adolescence. Cereb. Cortex 23, 1424–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun T., Walsh C. A. (2006) Molecular approaches to brain asymmetry and handedness. Nat. Rev. Neurosci. 7, 655–662 [DOI] [PubMed] [Google Scholar]
- 60.MacNeilage P. F., Rogers L. J., Vallortigara G. (2009) Origins of the left & right brain. Sci. Am. 301, 60–67 [DOI] [PubMed] [Google Scholar]
- 61.Concha M. L., Bianco I. H., Wilson S. W. (2012) Encoding asymmetry within neural circuits. Nat. Rev. Neurosci. 13, 832–843 [DOI] [PubMed] [Google Scholar]
- 62.Duboc V., Dufourcq P., Blader P., Roussigné M. (2015) Asymmetry of the brain: development and implications. Annu. Rev. Genet. 49, 647–672 [DOI] [PubMed] [Google Scholar]
- 63.Marlin B. J., Mitre M., D’amour J. A., Chao M. V., Froemke R. C. (2015) Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DiGiorgio A. (1929) Persistence of postural and motor asymmetries of cerebellar origin in spinal animals: I, II, III. Arch. Fisiol. 27, 518–580 [Google Scholar]
- 65.DiGiorgio A. (1942) Influences of the cerebellum-neocerebellum on the postural tone of the limbs and cerebellar somatotopy in the rhomboencephalic animal. Arch. Fisiol. 42, 25–79 [Google Scholar]
- 66.Chamberlain T. J., Halick P., Gerard R. W. (1963) Fixation of experience in the rat spinal cord. J. Neurophysiol. 26, 662–673 [DOI] [PubMed] [Google Scholar]
- 67.Bakalkin G. Ia., Iarygin K. N., Trushina E. D., Titov M. I., Smirnov V. N. (1980) Preferential development of flexion of the left or right hindlimb as a result of treatment with methionine-enkephalin or leucine-enkephalin, respectively. Dokl. Akad. Nauk SSSR 252, 762–765 [PubMed] [Google Scholar]
- 68.Bakalkin GYa, Kobylyansky A. G. (1989) Opioids induce postural asymmetry in spinal rat: the side of the flexed limb depends upon the type of opioid agonist. Brain Res. 480, 277–289 [DOI] [PubMed] [Google Scholar]
- 69.Bakalkin GYa, Kobylyansky A. G., Nagornaya L. V., Yarygin K. N., Titov M. I. (1986) Met-enkephalin-induced release into the blood of a factor causing postural asymmetry. Peptides 7, 551–556 [DOI] [PubMed] [Google Scholar]
- 70.Chazov E. I., Bakalkin GYa, Yarigin K. N., Trushina E. D., Titov M. I., Smirnov V. N. (1981) Enkephalins induce asymmetrical effects on posture in the rat. Experientia 37, 887–889 [DOI] [PubMed] [Google Scholar]
- 71.Schweickert A., Deissler K., Britsch S., Albrecht M., Ehmann H., Mauch V., Gaio U., Blum M. (2008) Left-asymmetric expression of Galanin in the linear heart tube of the mouse embryo is independent of the nodal co-receptor gene cryptic. Dev. Dyn. 237, 3557–3564 [DOI] [PubMed] [Google Scholar]
- 72.Zink C. F., Kempf L., Hakimi S., Rainey C. A., Stein J. L., Meyer-Lindenberg A. (2011) Vasopressin modulates social recognition-related activity in the left temporoparietal junction in humans. Transl. Psychiatry 1, e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reznikov K., Hauser K. F., Nazarevskaja G., Trunova Y., Derjabin V., Bakalkin G. (1999) Opioids modulate cell division in the germinal zone of the late embryonic neocortex. Eur. J. Neurosci. 11, 2711–2719 [DOI] [PubMed] [Google Scholar]
- 74.Bakalkin GYa, Pivovarov A. S., Kobylyansky A. G., Nesterenko P. N., Yarygin K. N. (1989) Lateralization of opioid receptors in turtle visual cortex. Brain Res. 480, 268–276 [DOI] [PubMed] [Google Scholar]
- 75.Hawrylycz M. J., Lein E. S., Guillozet-Bongaarts A. L., Shen E. H., Ng L., Miller J. A., van de Lagemaat L. N., Smith K. A., Ebbert A., Riley Z. L., Abajian C., Beckmann C. F., Bernard A., Bertagnolli D., Boe A. F., Cartagena P. M., Chakravarty M. M., Chapin M., Chong J., Dalley R. A., Daly B. D., Dang C., Datta S., Dee N., Dolbeare T. A., Faber V., Feng D., Fowler D. R., Goldy J., Gregor B. W., Haradon Z., Haynor D. R., Hohmann J. G., Horvath S., Howard R. E., Jeromin A., Jochim J. M., Kinnunen M., Lau C., Lazarz E. T., Lee C., Lemon T. A., Li L., Li Y., Morris J. A., Overly C. C., Parker P. D., Parry S. E., Reding M., Royall J. J., Schulkin J., Sequeira P. A., Slaughterbeck C. R., Smith S. C., Sodt A. J., Sunkin S. M., Swanson B. E., Vawter M. P., Williams D., Wohnoutka P., Zielke H. R., Geschwind D. H., Hof P. R., Smith S. M., Koch C., Grant S. G., Jones A. R. (2012) An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489, 391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Francks C. (2015) Exploring human brain lateralization with molecular genetics and genomics. Ann. N. Y. Acad. Sci. 1359, 1–13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.