Abstract
The receptor for advanced glycan end products (RAGE) has been identified as a susceptibility gene for chronic obstructive pulmonary disease (COPD) in genome-wide association studies (GWASs). However, less is known about how RAGE is involved in the pathogenesis of COPD. To determine the molecular mechanism by which RAGE influences COPD in experimental COPD models, we investigated the efficacy of the RAGE-specific antagonist FPS-ZM1 administration in in vivo and in vitro COPD models. We injected elastase intratracheally and the RAGE antagonist FPS-ZM1 in mice, and the infiltrated inflammatory cells and cytokines were assessed by ELISA. Cellular expression of RAGE was determined in protein, serum, and bronchoalveolar lavage fluid of mice and lungs and serum of human donors and patients with COPD. Downstream damage-associated molecular pattern (DAMP) pathway activation in vivo and in vitro and in patients with COPD was assessed by immunofluorescence staining, Western blot analysis, and ELISA. The expression of membrane RAGE in initiating the inflammatory response and of soluble RAGE acting as a decoy were associated with up-regulation of the DAMP-related signaling pathway via Nrf2. FPS-ZM1 administration significantly reversed emphysema in the lung of mice. Moreover, FPS-ZM1 treatment significantly reduced lung inflammation in Nrf2+/+, but not in Nrf2−/− mice. Thus, our data indicate for the first time that RAGE inhibition has an essential protective role in COPD. Our observation of RAGE inhibition provided novel insight into its potential as a therapeutic target in emphysema/COPD.—Lee, H., Park, J.-R., Kim, W. J., Sundar, I. K., Rahman, I., Park, S.-M., Yang. S.-R. Blockade of RAGE ameliorates elastase-induced emphysema development and progression via RAGE-DAMP signaling.
Keywords: FPS-ZM1, Nrf2, COPD, emphysema
Chronic obstructive pulmonary disease (COPD) is characterized by the irreversible limitation of airflow caused by small-airway disease (obstructive bronchiolitis) and parenchymal destruction (emphysema). Thus, to identify the practical biomarker of COPD, genome-wide association studies (GWASs) have been applied in patients with the condition. A relatively functional gene has been proposed in 2 cohorts associated with the forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratio;—advanced glycosylation end product-specific receptor (AGER) (1–4). The protein of AGER, the receptor for advanced glycation end products (RAGE), is a multiligand receptor of the immunoglobulin superfamily of cell-surface receptors that function as pattern-recognition receptors (5), leading to intracellular danger signals known as damage-associated molecular patterns (DAMPs), which are molecules released by apoptotic and necrotic cells from the injured tissue (6). DAMPs are released because of the accumulation of RAGE ligands such as high-mobility group box 1 (HMGB1) (7), S100/calgranulins (8), AGERs (9), macrophage antigen-1 (10), amyloid-β-peptide, and heat shock protein 70 (11), leading to many pathologic processes including asthma, lung cancer, and acute respiratory distress syndrome in the lung. The RAGE–ligand interaction induces many signal transductions to mediate diverse cellular responses. The signaling pathways are activated by Raf/MAPK, JNK/p38 MAPKs (12), NADPH oxidase resulting in the activation of transcriptional factors, including NF-κB, activator protein 1 and cAMP response element binding protein (13, 14). Levels of the canonical transmembrane RAGE (mRAGE) isoform and circulating soluble RAGE (sRAGE) isoforms by alternative splicing or proteolysis (15) in alveolar epithelium (16) and serum (1), respectively, have been found to be altered in COPD (17). sRAGE, which is a secreted soluble protein derived from RAGE, has been shown to be protective, signaling via binding to the membrane receptor to prevent ligand-induced homodimerization. Zhang et al. (18) reported that treatment with sRAGE attenuates the increases in neutrophil infiltration, lung permeability, and inflammatory cytokines in LPS-induced lung injury in mice. On the other hand, diverse studies about RAGE and emphysema in in vivo models suggest that RAGE overexpression during embryonic development is 100% lethal, and conditional RAGE overexpression during adulthood results in impairment of alveolar morphogenesis (19), alveolar enlargement, apoptosis, and inflammation (20). Although RAGE is an emerging potential therapeutic target for emphysema, blockade of RAGE and its mechanism has not been studied. We sought to investigate the effect of a high-affinity RAGE-specific blocking agent, FPS-ZM1 in our experimental emphysema model. To our knowledge, this study is the first to characterize a protective role of a RAGE antagonist in the lung wherein inhibition of RAGE ameliorates emphysema symptoms by regulating the DAMP signaling cascade via RAGE-Nrf2, highlighting a potential therapeutic approach for treating COPD.
MATERIALS AND METHODS
Human tissue
The use of human tissue samples was approved by the Ethics Committee of the Kangwon National University Hospital (IRB No. KNUH-2014-10-015). All subjects, including control patients and those with COPD, provided informed consent. Human nonmalignant 3 lung tissue specimens were obtained from a lung cancer resection. The lung tissue of patients undergoing diagnostic surgical lung biopsy for COPD with adenocarcinoma and serum samples of nonsmokers, smokers, and persons with COPD were provided by the Kangwon National University Biobank, Soonchunhyang University Biobank, University Guro Hospital of the National Biobank, and Kyungpook National University Biobank, a member of the National Biobank of Korea (n = 29). The samples from patients were selected based on smoking status and airflow limitation of FEV1/FVC less than 0.7, according to the clinical criteria for COPD. All of the cryopreserved samples were transported quickly to the laboratory on solid carbon dioxide ice and stored at −80°C until testing. The distribution of RAGE in serum and lung tissue specimens collected from patients undergoing lung resection surgery was determined by immunoblot analysis and ELISA.
In vivo porcine pancreatic elastase exposures and RAGE-specific blocker FPS-ZM1 treatment
All animal experiments were performed according to the guidelines of the Kangwon National University. the protocol was approved by and followed the regulations of the Institutional Animal Care and Use Committee (No. KW-150820-1). Male Nrf2-knockout mice with a C57BL/6 background were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and 8-wk-old wild-type male C57BL/6 mice (20–25g) were obtained from DooYeol Biotech (Seoul, Korea). The animals were euthanized with minimal discomfort and handled humanely. They were anesthetized with Zoletil (Virbac Corp., Fort Worth, TX, USA) and intratracheally injected with 5 U/100 g of porcine pancreatic elastase (PPE; 324682; EMD-Millipore, Billerica, MA, USA) in saline or saline alone (control) in 100 μl doses. The 1 mg/kg RAGE-specific blocker FPS-ZM1 was intraperitoneally injected daily after PPE instillation and monitored. Mice were euthanized 14 d after treatment.
Cigarette smoke exposure
Eight-week-old mice were used for cigarette smoke (CS) exposure as described previously (21, 22). For studies involving 6-mo CS exposure, research-grade cigarettes (3R4F; University of Kentucky, Lexington, KY, USA) were used to produce a mixture of sidestream smoke (89%) and mainstream smoke (11%), and the mice were exposed to CS according to the U.S. Federal Trade Commission’s protocol with a model TE-10 smoking machine (Teague Enterprises, Woodland CA, USA). CS concentration was set at a value of ∼100 mg total particulate matter (TPM)/m3 by controlling the flow rate of the diluted medical air to avert possible toxicity to mice by a high concentration of long-term CS exposure, and the level of carbon monoxide in the chamber was 350 ppm (23, 24). Mice received exposures 5 h/d every week for 6 mo and were euthanized 24 h after the last exposure. Control mice were exposed to filtered air in an identical chamber according to the same protocol.
Morphometric analysis of lung sections
On d 14 after PPE administration, mice were anesthetized with Zoletil. Right lungs were fixed with 4% paraformaldehyde for 24 h, then embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin (H&E) to analyze histology. The mean alveolar linear intercept was measured on H&E-stained sections taken at intervals throughout both lungs. Three to 5 images per slide were acquired at ×100 magnification and transferred to a computer screen.
Lung harvest technique, BALF isolation, and cell counts
Left lungs were isolated and inflated by using a tube containing 4% paraformaldehyde overnight, whereas right lungs were extracted and washed several times in ice-cold PBS or normal saline. The lung tissue was homogenized with a tissue homogenizer for 15 s. The lung homogenates were stored at −80°C and used for protein analyses. The fixed right lungs were embedded in paraffin for histologic analyses. BAL was performed with a 22-gauge intravenous catheter inserted into the trachea. The lungs were lavaged 1 time with 1.0 ml PBS and then centrifuged at 4500 g for 4 min (21, 25). Cell pellets were resuspended in 0.2 ml cold PBS, and the total number of cells in the BALF was determine with a hemocytometer. For total and differential cell counts, cytospin preparations were made with a cytocentrifuge on poly-l-lysine–coated slides at 3000 g for 10 min (Sigma-Aldrich, St. Louis, MO, USA). The cells were stained with HEMA3 Stain, a 3-step staining method comparable to the traditional Wright-Giemsa method (Thermo Fisher Scientific, Waltham, MA, USA) (26). In brief, the slide was dipped in HEMA 3 Fixative for 5 min, washed in distilled water, dipped in HEMA 3 Solution I for 5 min, and washed again in distilled water. Finally, the slide was dipped in HEMA 3 Solution II for 5 s and washed in distilled water. All cytospin preparations were evaluated by microscopy (magnification, ×200). The cells were identified by standard morphology and staining characteristics.
Immunohistochemistry and immunofluorescence staining
To measure the level of RAGE in the fixed lung sections, immunohistochemistry was performed by a method described by Bodine et al. (27). The paraffin-embedded sections were cut onto slides at preselected thicknesses of 4–6 μm, dewaxed with xylene, and dehydrated through a series of ethanols of cumulatively increased concentrations. The sections were incubated in 3% H2O2, blocked with 10% goat serum, and incubated with the primary antibody for 1 h at 4°C. The slides were washed with Tris-buffered saline-Tween 20, incubated with an appropriate biotin-conjugated rabbit anti-goat IgG secondary antibody (1:100; Vector Laboratories, Burlingame, CA, USA ), and developed with avidin biotin complex and 3,3′-diaminobenzidine detection reagents (Vector Laboratories), prepared according to the manufacturer’s protocol (28). The RAGE antibodies were used at 1:50 (Santa Cruz Biotechnology, Dallas, TX, USA). The stained slides were counterstained with hematoxylin.
ELISA
The mouse and human lung homogenates were prepared with RIPA lysis buffer supplemented with a protease inhibitor cocktail (GenDepot; P3100, P3200). Tissue homogenates, serum, and BALF were subjected to ELISA for determination of IL-6, TNF-α, sRAGE content, and S100A8 with a Duoset ELISA development kit (DY406-05, DY410-05, DY1179, DY3059, respectively; R&D Systems, Minneapolis, MN, USA). In brief, a 96-well microplate was coated with the diluted rat anti-mouse or goat anti-mouse capture antibody and incubated overnight at room temperature. Each well was aspirated and washed with washing buffer, followed by addition of blocking buffer for 1 h. The lung homogenate sample or standards were added to the plate for 2 h at room temperature, and biotinylated rat anti-mouse or goat anti-mouse detection antibody was subsequently added in the plate for 2 h. Finally, the working dilution of streptavidin–horseradish peroxidase (HRP) conjugated to HRP; the substrate solution, including 1:1 mixture of color reagent A (H2O2) and color reagent B (tetramethylbenzidine) was added; and 2 N H2SO4 stop solution changed the color from blue to yellow to stop the response, and the intensity of the color was measured at 450 nm. Experiments for all treatments were performed in triplicate.
Preparation of CS extract
CS extract (CSE) was prepared by using a modified method described by Lee et al. (29). Research-grade cigarettes (3R4F) were obtained from the Kentucky Tobacco Research Development Center (The Tobacco Research Institute, University of Kentucky, Lexington, KY, USA). The smoke generated from 3R4F research cigarettes contains 11.0 mg TPM, 9.4 mg tar, and 0.73 mg nicotine per cigarette. In brief, Kentucky 3R4F research-reference filtered CSEs were bubbled for 1–2 min through 10 ml serum-free DMEM/F12 supplemented with 1% penicillin-streptomycin with a peristaltic vacuum pump. Next, the solution containing smoke was filtered through a 0.22-μm filter to remove large particles and was regarded as a 10% CSE solution (30). CSE was prepared within 30 min and stored at −80°C for additional experiments. The CSE was diluted and added to cells in submerged culture conditions.
Cell culture and treatment
The murine lung epithelial type II cell line MLE-12 was purchased from American Type Culture Collection (Manassas, VA, USA). MLE-12 cells were cultured in DMEM/F-12 with 2% FBS and 1% penicillin-streptomycin supplemented with 0.005 mg/ml insulin, 0.01 mg/ml transferrin, 30 nM sodium selenite, 10 nM hydrocortisone, 10 nM β-estradiol, and 2 mM l-glutamine (Sigma-Aldrich), and 10 mM HEPES at 37°C and 5% CO2. The cells were seeded at a density of 1 × 105 cells in 6-well plates and treated at 37°C for 24 h with different concentrations of FPS-ZM1 ranging from 500 to 1000 nM. After the plates were washed one time with PBS, 0.1–0.5% CSE was added with FPS-ZM1 of equal concentration for 24 h in the cells. At the end of treatment, the supernatant of cell culture was collected to assess nitrite and ELISA for proinflammatory cytokine TNF-α and IL-6 release. The cells were washed with sterile PBS and were lysed in RIPA buffer according to published procedures (31). All treatments were performed in triplicate.
Immunofluorescence staining
Immunofluorescence staining was performed as describe elsewhere (32). In brief, MLE-12 cells were grown on round coverslips in 24-well plates at 37°C. When the cells reached 60% confluence, they were pretreated with FPS-ZM1 for 24 h, followed by addition of CSE with FPS-ZM1. At 24 h after the final treatment, the cells were fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 10 min. After the reaction was blocked in 10% normal goat serum diluted in PBS (Cell Signaling Technology, Danvers, MA, USA) for 30 min, the cells were stained with anti-RAGE goat polyclonal antibody (1:200) and anti-Nrf2 rabbit polyclonal antibody (1:200) in combination with Alex Fluor 488 rabbit anti-goat (1:2000) and Fluor 594 goat anti-rabbit secondary (1:1000) antibody, respectively. All experimental primary antibodies were purchased from Santa Cruz Biotechnology, and secondary antibodies were purchased from Thermo Fisher Scientific. After a thorough washing, the coverslips were mounted with Fluoroshield Mounting Medium with DAPI (Sigma-Aldrich) to label the nuclei. The immunostained cells were analyzed under an LSM 510 confocal laser-scanning microscope (Zeiss, Jena, Germany).
Immunoblot analysis
Protein samples from human and mouse whole-lung homogenates and whole-cell lysates were prepared with RIPA lysis buffer on ice, and 20 μg of isolated soluble proteins was separated on 8–15% SDS-polyacrylamide gels. The separated proteins were electroblotted onto nitrocellulose transfer membranes (0.45 μm; 162-0115; Bio-Rad, Hercules, CA, USA). The membranes were incubated with 5% skim milk for 1 h and then probed with 1:2000–1:10,000 diluted antibodies: anti-RAGE, anti-IKKα, anti-p-S276-RelA/p65, anti-RelA/p65, anti-Nrf2, anti-lamin A/C, anti-Keap1, and anti-GAPDH (Santa Cruz Biotechnology); anti-IKKβ, anti-pS536-RelA/p65, anti-pJNK1/2, anti-JNK1/2, anti-pERK1/2, anti-ERK1/2, anti-pp38, and anti-38 (Cell Signaling Technology) and anti-S100A8 and anti-RAGE (Abcam, Cambridge, MA, USA), to detect the respective proteins for 24 h at 4°C. After 3 washes for 10 min each, polyclonal anti-rabbit/mouse or goat HRP-conjugated secondary antibodies (NCI1460KR, 31430, 31400; Thermo Fisher Scientific) were linked to HRP-bound protein complexes and developed with a Pierce ECL Western Blot substrate (32209; Thermo Fisher Scientific). Equal loading of the samples was determined by quantitation of proteins, as well as by probing membranes for β-actin or GAPDH. Band densitometry was calculated by ImageJ (National Institutes of Health, Bethesda, MD, USA) analysis and expressed as fold change relative to β-actin or GAPDH.
SOD assay
The superoxide dismutase (SOD) activity was measured with a commercial SOD determination kit (Sigma-Aldrich), according to the manufacturer’s protocol. The SOD activity assay used the production of a water-soluble tetrazolium salt, WST-1 [2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] formazan dye in the presence of superoxide anions (O2⋅−). A quantity of O2⋅− is generated from the conversion of xanthine and oxygen to uric acid and H2O2 by xanthine oxidase activity and is proportional to the absorbance at 440 nm. The results indicate the inhibition activity of SOD. All treatments were performed in triplicate.
GSH activity assay
The assay for quantitative determination of glutathione (GSH) and glutathione disulfide (GSSG) levels was conducted as previously described method by Rahman et al. (33). In brief, the cells were washed in PBS and homogenated in RIPA buffer. The cellular debris was collected by centrifugation, and the supernatant concentration was determined by a BCA Assay Kit (Thermo Fisher Scientific). The equal proteins were incubated with 0.67 mg/ml 5,5'-dithiobis-(2-nitrobenzoic acid) and 10 U/ml glutathione reductase in 0.1 M potassium phosphate buffer with 5 mM EDTA disodium salt (pH 7.5). After 30 s, 0.67 mg/ml β-NADPH was added to the sample. The actual total GSH and GSSG concentrations in the sample were determined by calculated linear regression values from a standard curve every 30 s, for a total time of 2 min at 412-nm absorbance. All experiments were performed in triplicate.
NO production
NO production was detected by the modified Griess method (34). This assay is a simple and well-characterized colorimetric test that involves the formation of a red azo dye on a nitrite generated by stress, which is an NO derivative and accumulates in the culture supernatants. In brief, after treatment of MLE12 cells with the CSE for 24 h, with or without FPS-ZM1, the culture supernatants were collected and mixed with equal volumes of 1× Griess reagent at room temperature in a dark room. The absorbance at 540 nm was measured with a microplate reader within 15 min (BioTek, Inc., Shoreline, WA, USA). All experiments performed in triplicate.
Measurement of intracellular ROS
The level of intracellular reactive oxygen species (ROS) was detected with the cell-permeable fluorescent probe 2′,7′-dichlorofluorescein-diacetate (DCF-DA), which detects oxidation of DCF-DA in the highly fluorescent compound 2′,7′-dichlorofluorescein (DCF) in the presence of ROS. MLE12 cells were loaded with 10 μM DCF-DA (Thermo Fisher Scientific) for 30 min at 37°C, followed by treatment with various concentrations of CSE for 24 h. The cells were detached by 0.25% trypsin and analyzed by fluorescence staining at in FL-1 at an excitation of 495 nm and emission of 520 nm by Accuri-C6 flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA). For DCF-DA fluorescence staining, MLE12 cells were seeded on round coverslips in 24-well plates at 37°C, followed by pretreatment with 500–1000 nM FPS-ZM1 and the addition of 0.1–0.5% CSE with FPS-ZM1. At 24 h after the treatment, the cells were fixed in 4% paraformaldehyde for 10 min. The DCF-DA fluorescence-stained cells were observed under a confocal laser-scanning microscope.
Statistical analysis
All cell line and in vivo experiments were performed at least 3 times for each of the different strains. Each result was tested with a 1-way ANOVA followed by the Bonferroni post hoc test for multigroup comparisons (StatView 5.0; SAS Institute, Cary, NC, USA). All in vitro data are expressed as means ± sem, and all in vivo data are shown as median and single measurements. The ELISA statistical analysis of human samples was conducted with Prism 5 software (GraphPad Software, La Jolla, CA, USA). Data are presented as the means ± se of results in at least 3 independent experiments. Comparison of parameters between 2 groups was made by Student’s t test. Comparison of parameters in more than 2 groups was made by 1-way ANOVA followed by the Bonferroni post hoc test. Minimum statistical significance was accepted at P < 0.05. Mean values appeared to be normally distributed, with each figure documenting an appropriate statistical test.
RESULTS
RAGE is released into the alveolar space and serum of patients with COPD
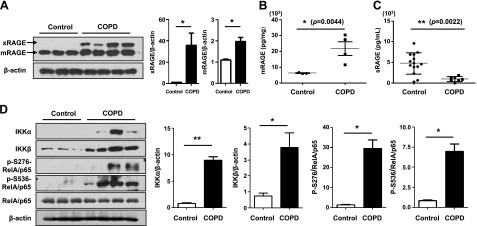
Higher levels of IL-6 and TNF-α were present in COPD serum and lung homogenates compared with control or nonsmoker serum, suggesting systemic and local lung inflammation in COPD. RAGE has several isoforms through alternative splicing, and the dominant isoforms are the full-length membrane-bound RAGE (xRAGE) and mRAGE isoforms and the circulating sRAGE isoform. The levels of xRAGE and mRAGE were significantly increased in peripheral lung tissues of patients with COPD (Fig. 1A). Consistent with the Western blot analysis, mRAGE was highly expressed in COPD lung homogenate compared with expression in control lungs (P = 0.0044; Fig. 1B). We next used ELISA to determine the secretion of sRAGE, and a significant decrease in sRAGE was observed in the serum of smokers and current smokers with COPD vs. controls (P = 0.0022), consistent with the results of Western blot analysis (Fig. 1C). Therefore, these data suggest that the expression of circulating sRAGE and membrane xRAGE and mRAGE is altered in patients with COPD and individuals who are continuous smokers.
Figure 1.
RAGE expression and activation of DAMP signaling in patients with COPD. Western blot of total protein in lung homogenates of patients with COPD and controls (n = 29). A) Membrane-bound RAGE (xRAGE) and mRAGE, 2 isoforms of full-length RAGE in membrane, were detected by immunoblot analysis with an anti-RAGE antibody (N-16), whereas sRAGE was not detected. Protein levels of total RAGE normalized to β-actin revealed a significant increase in expression in COPD-affected lung. The blots were subjected to densitometric analysis and relative quantification. Total circulating and total membrane RAGE levels were measured with an ELISA assay that detects all forms of RAGE. B) mRAGE levels in lung homogenate from controls and patients with COPD (n = 29). C) sRAGE levels in serum samples from control and COPD at baseline (n = 29). D) The IKKα, IKKβ, and phosphorylation of RelA/p65 at 2 sites (Ser276 and Ser536) were determined by Western blot analysis with the appropriate antibodies in human lung homogenate (n = 29). β-Actin and total RelA/p65 were used as the loading control, and the target protein bands were subjected to densitometric analysis. All data are representative of 3 independent experiments. The error bars denote the means ± sd. *P < 0.05, **P < 0.01 vs. control.
RAGE antagonist reverses airspace enlargement and inflammatory response in mice with PPE-induced emphysema
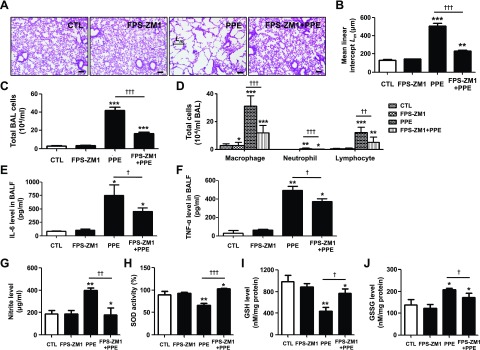
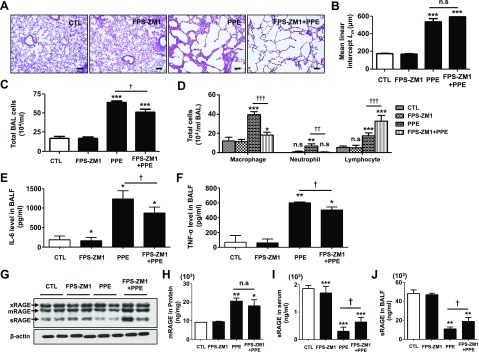
To investigate the protective effect of the RAGE antagonist FPS-ZM1, male C57BL/6J animals were intratracheally exposed to PPE, and 1 mg/kg FPS-ZM1 was intraperitoneally administered daily in the PPE-induced animals. Morphometric quantification demonstrated that FPS-ZM1 treatment effectively inhibited airspace enlargement (Fig. 2A) and epithelium destruction (Supplemental Fig. 1A), and reduced the mean chord length measured at 14 d after PPE instillation (Fig. 2B). BALF neutrophils, macrophages, and lymphocyte counts were significantly higher in PPE-exposed mice. There were significantly lower total cells, including macrophages, neutrophils, and lymphocytes, in PPE treated with FPS-ZM1, compared with no treatment (Fig. 2C, D). BALF levels of IL-6 and TNF-α were higher in the PPE group than in unexposed mice, whereas FPS-ZM1 caused significant reductions in BALF IL-6 and TNF-α levels (Fig. 2E, F).
Figure 2.
Protective effect of FPS-ZM1 against PPE-induced experimental COPD. PPE was applied once through intratracheal injection on d 14, and FPS-ZM1 was applied through intraperitoneal injection daily for 14 d. A) Representative images of H&E-stained lung sections (n = 4 per group) in mice exposed to saline or PPE, with or without administration of FPS-ZM1. Original magnification, ×100. Scale bars, 200 μm. B) Airspace enlargement was quantified by the mean linear intercept (Lm) values of alveoli (n = 4 per group). Values represent the means ± sd. C) Total cell counts in BALF (n = 3 per group). D) Macrophage, neutrophil and lymphocyte counts in BALF (n = 3 per group). E, F) Quantification of BALF (E) IL-6 and TNF-α (F) protein concentrations in mice (n = 3 per group, ELISA). G) Nitrite levels in the BALF (n = 3 per group, Griess assay). H) Total intracellular SOD activity in the lung homogenate (n = 4 per group). I, J) Levels of GSH (I) and GSSG (J) in the lung homogenate (n = 4 per group). Ctl, control group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. controls; †P < 0.05, ††P < 0.01, †††P < 0.001 vs. PPE-induced mice.
The levels of IL-6 and TNF-α were also significantly blunted by prior treatment with FPS-ZM1 in both serum and lung tissue (Supplemental Fig. 1B–E). To determine the inhibitory effect of FPS-ZM1 in response to reactive nitrosative/oxidative stress, the levels of nitrite, SOD, and GSH were assessed in a PPE-induced emphysema model. As shown in Fig. 2, the level of nitrite, the inert end product of endogenous NO metabolism, was increased by PPE in serum (Fig. 2G). Moreover, SOD activity and the levels of GSH were reduced, and levels of the oxidized GSH form, GSSG, were increased, after treatment with PPE. SOD activity and the levels of GSH were reduced by PPE. GSSG was increased after treatment with PPE. However, nitrite, SOD activity, and GSH/GSSG recycling are reversed by treatment with FPS-ZM1 (Fig. 2H–J). Therefore, these data suggest that FPS-ZM1 selectively resists PPE-induced changes in airspace enlargement, accumulation of alveolar macrophages and lymphocytes, influx of excessive inflammatory cytokines in the lung, and pulmonary nitrosative/oxidative stress.
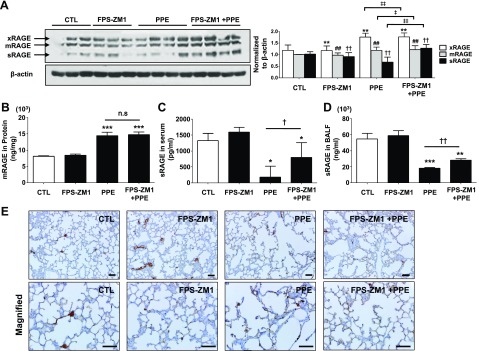
RAGE blockade inhibited the activation of redox-sensitive DAMP signaling in experimental emphysema
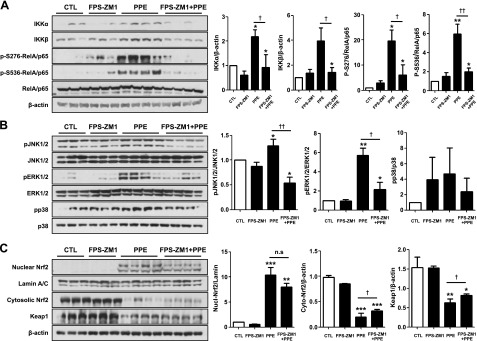
RAGE protein levels were assessed by Western blot analysis from lung tissue homogenates with a RAGE-specific antibody. Significant increases in xRAGE and mRAGE were observed after PPE injury. In contrast, decreased sRAGE was detected in the lower band of ∼45 kDa in PPE-induced mouse lungs. The protein levels of both sRAGE and mRAGE were significantly reversed by FPS-ZM1 treatment (Fig. 3A), according to Western blot analysis. The concentrations of mRAGE and sRAGE were also quantified by using ELISA in lung homogenate, serum, and BALF. Similarly, the levels of increased mRAGE and decreased sRAGE were significantly reversed by FPS-ZM1 treatment (Fig. 3B–D). In immunohistochemistry, FPS-ZM1 protected emphysema from PPE injury associated with increased expression of mRAGE in alveolar epithelium (Fig. 3E). To determine whether the blockade of RAGE is associated with DAMPs or MAPK signaling pathways, we performed Western blot analysis in lungs of mice and patients with COPD. The protein levels of IKKα, IKKβ, and phosphorylated S276- and S536-RelA/p65 were significantly decreased in mouse lung homogenates (Fig. 4A) as well as in patients with COPD (Fig. 1D). The protein levels of phosphorylated JNK1/2 and ERK1/2, but not p38 MAPK, significantly increased after PPE injury, whereas treatment with FPS-ZM1 down-regulated the expression of JNK1/2 and ERK1/2 (Fig. 4B). To investigate whether RAGE blockade is associated with Nrf2/Keap1 interaction, we determined whether FPS-ZM1 protects nuclear translocation of Nrf2 and degradation of Keap1 in mice with PPE-induced emphysema. As shown in Fig. 4C, 14 d after PPE treatment, translocation of Nrf2 from the cytoplasm into the nucleus occurred, whereas 14 d after PPE with daily FPS-ZM1 injection, there was slightly decreased nuclear translocation of Nrf2. The expression of Keap1 was down-regulated by PPE, but not changed as a result of FPS-ZM1 treatment. These results suggest that RAGE blockade suppresses the sustained activation of DAMP signaling, including RelA/p65 NF-κB, JNK1/2, ERK1/2, and p38 MAPK pathways accompanied by the activation of Nrf2 and Keap1, and this inhibition of DAMP signaling is associated with protection in the pathogenesis of COPD.
Figure 3.
Reverse effect of FPS-ZM1 on abnormal change of RAGE isoform level in PPE-induced experimental COPD. A) Western blot analysis of the 3 major isoforms in the lung (n = 4). Corresponding pixel density ratio normalized against β-actin. *P < 0.01 vs. control for xRAGE; ##P < 0.01 vs. control for mRAGE; ††P < 0.01 vs. control for sRAGE; ‡P < 0.05, ‡‡P < 0.01 vs. control for PPE-induced mice. B–D) Levels of mRAGE in lung homogenates (B; n = 4 per group) and circulating sRAGE in serum (C; n = 7 per group) and BALF (D; n = 3 per group) of mice after treatment. Data are means ± sd. E) Effects of FPS-ZM1 on the expression of RAGE in alveolar epithelial cells as determined by immunohistochemistry (n = 4 per group). Top panels: original magnification, ×200; scale bars, 100 μm. Bottom panels: original magnification, ×400; scale bars, 200 μm. Ctl, control group; ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; †P < 0.05, ††P < 0.01 vs. PPE-induced mice.
Figure 4.
Effect of FPS-ZM1 on activation of DAMP signaling in PPE-induced experimental COPD. A) IKKα, IKKβ, and phosphorylation of Rel/p65 at 2 sites (Ser276 and Ser536) were determined by Western blot analysis. B) JNK/ERK/p38 MAPK activation (phosphorylation) was determined by Western blot with the appropriate antibodies. The target protein bands were subjected to densitometric analysis normalized with β-actin. C) The canonical–noncanonical Rel/p65 NF-κB activation and MAPK activation and its correlation to Nrf2 translocation from the cytoplasm into the nucleus was measured by Western blot analysis. Lamin A/C was used for the nuclear protein loading control, and β-actin was used for the cytoplasmic protein loading control. The corresponding density ratio was determined by the average intensity of the bands. Ctl, control group; ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; †P < 0.05, ††P < 0.01 vs. PPE-induced mice.
RAGE blockade inhibited the activation of redox-sensitive DAMP signaling in CSE-induced mouse alveolar type II epithelial cells
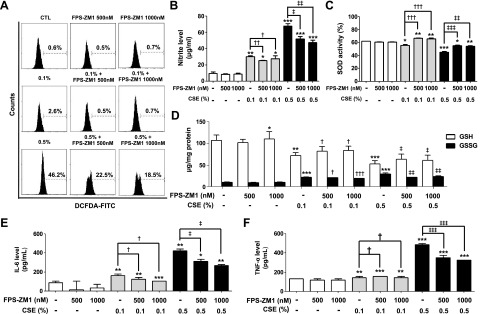
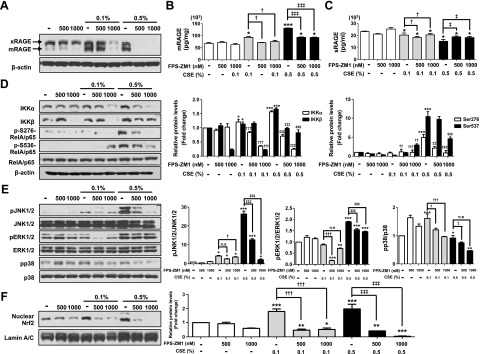
To analyze the effect of FPS-ZM1 in response to CSE-induced stress, MLE-12 cells were exposed to CSE in the presence of FPS-ZM1 (500–1000 nM). Cellular ROS was reduced by FPS-ZM1 treatment, with DCF-DA staining and flow cytometry analysis (0.1% vs. 0.1%+FPS-ZM1 500 nM vs. 0.1%+FPS-ZM1 1000 nM; 2.6% vs. 0.5% vs. 0.7%, 0.5% vs. 0.5%+FPS-ZM1 500 nM vs. 0.5%+1000 nM; 46.2% vs. 22.5% vs. 18.5%) (Fig. 5A and Supplemental Fig. 2A). FPS-ZM1 treatment also relieved nitrite level, SOD activity, and levels of GSH in response to CSE (Fig. 5B–D). Protein expression levels of IL-6 and TNF-α were increased by 0.5% CSE, more so than by than 0.1% CSE, and were significantly reduced in response to FPS-ZM1 in the cells (Fig. 5E, F). To determine whether blockade of RAGE is associated with the DAMP or MAPK signaling pathway, we performed Western blot analysis and ELISA in MLE-12 cell homogenate. The levels of increased mRAGE and decreased sRAGE were significantly reversed by FPS-ZM1 treatment in CSE-exposed MLE-12 (Fig. 6A–C and Supplemental Fig. 2B–D). Moreover, CSE induced the activation of DAMP signaling including the RelA/p65 NF-κB, JNK1/2, ERK1/2, and p38 MAPK pathways accompanied by the nuclear translocation of Nrf2, and this inhibition of DAMP signaling by FPS-ZM1 was associated with protection of the CSE-exposed MLE-12 cells (Fig. 6D–F and Supplemental Fig. 2B–D). The results of the distinct in vitro recovery experiments by FPS-ZM1 in alveolar epithelial cells are in line with those of the in vivo COPD experiments.
Figure 5.
Protective effect of FPS-ZM1 on CSE-induced intracellular ROS/RNS stress and inflammation in MLE-12 cells. The cells were incubated in the presence of FPS-ZM1 (500–1000 nM) for 24 h, followed by incubated in the presence (0.1–0.5%) or absence of CSE for 24 h. A) The dose-dependent effects of CSE on oxidation in cultured MLE-12 cells were evaluated by flow cytometry. The x axis represents the intensity of intracellular DCF fluorescence and the y axis the mean number of cells. B) Nitrite levels were assessed by a modified Griess assay in the culture supernatants. C) The activity of intracellular SOD was detected by a colorimetric method in cell proteins. D) The levels of GSH and GSSG were measured as described in the Supplemental Data. E, F) The protein levels of IL-6 (E) and TNF-α (F) were examined by ELISA. The data showed an increased trend in CSE-exposed cells, whereas the CSE with FPS-ZM1 cotreatment showed protection of intracellular ROS/RNS and proinflammatory cytokine production. Each result is the average of 3 separate experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; †P < 0.05, ††P < 0.01, †††P < 0.001 vs. 0.1% CSE exposure; ‡P < 0.05, ‡‡P < 0.01, ‡‡‡P < 0.001 vs. 0.5% CSE exposure.
Figure 6.
Effect of FPS-ZM1 on CSE-induced activation of DAMP signaling in MLE-12 cells. A–C) After exposure to CSE in the presence or absence of FPS-ZM1 for 24 h, Western blot analysis was perfomed (A), and the expression levels of mRAGE in lung homogenates (B) and of circulating sRAGE (C) were determined by ELISA. D, E) IKKα, IKKβ, and the phosphorylation of Rel/p65 at 2 sites (Ser276 and Ser536) (D) and the phosphorylation of JNK/ERK/p38 MAPK activation (E) were detected with the appropriate antibodies. β-Actin, total RelA/p65, total JNK1/2, total ERK1/2, and total p38 were used for loading controls. F) Correlation to Nrf2 translocation from cytoplasm into the nucleus was measured by Western blot analysis. Lamin A/C was used for nuclear protein loading control, and β-actin was used for cytoplasmic protein loading control. The activation of DAMP signaling was inhibited by FPS-ZM1 treatment. Results are combined data from 3 independent experiments. Ctl, control group; ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control group; †P < 0.05, ††P < 0.01, †††P < 0.001 vs. 0.1% CSE exposure; ‡P < 0.05, ‡‡P < 0.01, ‡‡‡P < 0.001 vs. 0.5% CSE exposure.
RAGE blockade is involved in recovery of inflammation, but not for emphysematous lung change, in response to PPE in Nrf2-deficient mice
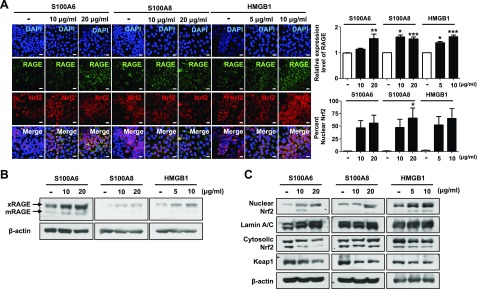
Functions of the Nrf2 pathway have been studied widely in models of emphysema-like phenotype in Nrf2−/− mice. Host defense Nrf2 is a novel transcription factor for cytoprotective genes bearing antioxidant response element (ARE) to maintaining cellular redox balance and homeostasis. To determine how increased levels of RAGE are involved in DAMP and Nrf2 pathways, alveolar epithelial cells were treated wtih various RAGE ligands, including S100A6 (10–20 μg/ml), S100A8 (10–20 μg/ml), and HMGB1 (5–10 μg/ml), for 48 h. As shown in Fig. 7 and Supplemental Fig. 3, multiple RAGE ligands robustly increased the expression of RAGE and induced DAMP activation and Nrf2 nuclear translocation. We next determined the effect of RAGE blockade in Nrf2−/− mice. We detected no difference in airspace enlargement, alveoli destruction, and ROS/reactive nitrogen species (RNS) stress between Nrf2−/− and WT mice in response to PPE-induced injury (Fig. 8A, B and Supplemental Fig. 4E–H). However, we again observed the significant blockade of the expected infiltration of inflammatory cells (macrophages, neutrophils, and lymphocytes) and inflammatory cytokines, such as IL-6 and TNF-α in Nrf2−/− vs. WT mice (Fig. 8C–F and Supplemental Fig. 4A–D). Accordingly, the protein levels of mRAGE and phosphorylation of DAMP pathway were conserved, but the decreased levels of sRAGE were reversed in mice treated with FPS-ZM1 (Fig. 8G–J and Supplemental Fig. 4J–K) and phosphorylation of JNK1/2, ERK1/2, and p38 MAPK were down-regulated in mice treated with FPS-ZM1. These data indicate that FPS-ZM1 contributes to the resolution of accumulation of alveolar macrophages, neutrophils, and lymphocytes, and the influx of excessive inflammatory cytokine in the lung, but not to emphysematous lung changes (airspace enlargement), nitrosative/oxidative stress, and stress and destruction of the scavenger system, in response to PPE.
Figure 7.
The RAGE/ligand axis-induced activation of RAGE-mediated Nrf2 nuclear translocation in MLE-12 cells. MLE-12 cells were treated with S100A6 (10 and 20 μg/ml), S100A8 (10 and 20 μg/ml) and HMGB1 (5 and 10 μg/ml) for 48 h. The protein expression of RAGE and the localization of Nrf2 were analyzed by immunocytochemistry and Western blot. A) Immunocytochemistry of the cells treated with ligands was performed with RAGE and Nrf2 antibodies. Nuclei were stained with DAPI (blue). The number of RAGE+ cells, Nrf2+ cells, and double-positive cells are shown in 3 randomly chosen fields. Magnification, ×600. *P < 0.05, **P < 0.01, ***P < 0.001 (n = 3). B, C) The expression of RAGE protein (B) and Nrf2 (C) nuclear translocation were analyzed by Western blot. Data are means ± sd of 3 independent experiments performed in triplicate.
Figure 8.
The recovery effect of FPS-ZM1 against PPE-induced inflammation, but not emphysematous lung change in experimental COPD of Nrf2−/− mice. PPE was injected once intratracheally and FPS-ZM1 was injected intraperitoneally daily for 14 d. A) Representative images of H&E-stained lung sections (n = 2 per group) in mice. Original magnification ×100; scale bars, 200 μm. B) Morphometric analysis showing mean linear intercept (Lm) values of alveoli (n = 4 per group). Data represent means ± sd. C) Total cell counts in BALF (n = 2 per group). D) Macrophage, neutrophil, and lymphocyte counts in BALF (n = 2 per group). E, F) Quantification of BALF IL-6 (E) and TNF-α (F) protein concentrations in mice by ELISA (n = 2 per group). G) Western blot analysis of the 3 major isoforms in the lung (n = 2). Corresponding pixel density ratio normalized against β-actin. H–J) Levels of mRAGE in lung homogenates (n = 2 per group) (H) and circulating sRAGE in serum (n = 4 per group) (I) and BALF (n = 2 per group) (J) of mice after treatment. Ctl, control; ns, not significant. Data are means ± sd. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; †P < 0.05, ††P < 0.01, †††P < 0.001 vs. PPE-induced mice.
DISCUSSION
GWASs have demonstrated that the AGER gene is functionally associated with susceptibility to COPD (35, 36). Although the gene and its receptor RAGE has been assumed to facilitate a critical role in the pathogenesis of COPD (37–39), it remains to be found how RAGE regulates the pulmonary function and underlying physiologic cellular signaling. RAGE is widely distributed in various tissues and cell types and is highly expressed during development (40). In the lung, RAGE expression is most abundant and increases via accumulation of ligands in pathologic conditions, such as diabetes, cardiovascular diseases, neurodegenerative disorders, and chronic inflammation (41, 42). Like the preceding, in the lung, upregulation of RAGE has been reported with enhancement of HMGB1 in airway epithelia of patients with COPD, particularly current smokers with COPD (43, 44). In addition, CS triggers RAGE and expression of its common ligands in human lung epithelial cells and airways of normal rodent models (2, 37, 45–47). In RAGE-null mouse lung exposed to CS, the secretion of IL-1β, CCL5, MIP-2, and TNF-α, as well as elastolysis (reduction in tissue elastance) and consequent emphysema, were decreased (37, 48). In rat type I-like alveolar cells, RAGE small interfering RNA significantly decreased Ras activity in response to CSE, demonstrating the important role of RAGE signaling for blocking the inflammatory response to CS (49). For direct experimental proof, we confirmed the expression level of RAGE and its ligands (S100A8 and HMGB1) in a CS-induced emphysema mouse model by Western blot analysis. Also, the expression levels of S100A8 and HMGB1 in airway epithelium, macrophages, and alveolar epithelial cells were slightly augmented in immunohistochemistry (data not shown). Chronic CS exposure over 6 mo showed an involvement of decreased sRAGE and increased xRAGE, mRAGE, and ligands in the pulmonary response (Supplemental Fig. 1F-I). Chronic CS exposure induced a less severe form of airspace enlargement associated with a less violent injurious response than did PPE in the lung. In the current study, we conducted an in-depth examination of the pathophysiological role of RAGE and the therapeutic effect of RAGE blockade in emphysema development in a PPE-induced COPD model. Our results demonstrate that membrane xRAGE and mRAGE expression is primarily increased and circulating sRAGE expression is decreased in both PPE-treated mouse lungs and lungs of patients with COPD on concomitant innate immune response, including release of proinflammatory cytokines and macrophage activation.
Increased levels of RAGE exhibited profound activation in redox-sensitive DAMP signaling, including canonical and noncanonical NF-κB, MAPKs, and host defense system Nrf2, highlighting the critical role of RAGE for pathophysiological emphysema models in vivo and in vitro (Fig. 9). Pouwels and colleagues (44) reported that the release of DAMP is increased in induced sputum and serum, both in the stable phase and during exacerbation. Furthermore, LL37, HMGB1, and S100A9 significantly activated RAGE within DAMP during exacerbation, supporting that RAGE constitutes a target in COPD exacerbation. To elucidate the pathway in the pathogenesis of COPD, activation of NF-κB has been implicated in the induction of the sustained inflammation. Moreover, up-regulation of MAPKs is recognized to activate NF-κB, in turn mediating various transcriptional programs. Therefore, we evaluated the role of NF-κB and MAPK signaling pathways to explore the mechanisms by which FPS-ZM1 modulates PPE or CS-induced emphysema. Our data exhibited that IKKα, IKKβ, and RelA/p65 were up-regulated when exposed to PPE or CSE in lungs of mice and treated with type II epithelial cells, as in patients with COPD. In addition, phosphorylation of JNK1/2, ERK1/2, and p38 MAPK were also increased, but inhibited the expression level of these molecules treated with FPS-ZM1 in our experimental models (Fig. 9). It has been reported that RAGE-engaged mechanisms through NF-κB and MAPKs trigger production of inflammatory cytokines, activation of proteases, and oxidative stress in diabetic mice (42). In addition, blockade of RAGE using anti-RAGE serum decreased NF-κB transcriptional activation induced by TNF-α of human monocytic THP-1 cells. In our experimental emphysema models, blockade of RAGE suppressed NF-κB activation through down-regulation of MAPK signaling. This inhibitory signaling pathway resulted in protection against alveolar destruction with anti-inflammatory effects. On the other hand, in Nrf2-knockout mice, the RAGE antagonist FPS-ZM1 failed to protect against airspace enlargement or to regulate NF-κB and MAPK signaling, in terms of conservation of GSH recycling and SOD activity, by suppressing the release of proinflammatory cytokines after PPE injury. These results reflect that not only might Nrf2 play an essential role in mediating intracellular signaling, including NF-κB and MAPK signaling, between RAGE and DAMP that regulates the fate of cells, but also FPS-ZM1 is a potential therapeutic application for inflammatory inhibition during the development of COPD, where sustained inflammation is a big hurdle. In rats with induced Parkinson’s disease, the RAGE–NF-κB/Nrf2 axis was associated with elevated levels of inflammatory, oxidative stress, and apoptotic mediators, resulting in dopaminergic neuronal death and motor impairment (50). In lungs of patients with COPD, the decline in Nrf2-mediated antioxidant transcriptional program correlated positively with decreased Nrf2 protein stability (51). COPD is a disease that accelerates lung aging; the reduction of Nrf2 levels/activity in old age might explain the low efficacy and side effects for medication. Moreover, RAGE is abundantly expressed in both type I and II epithelial cells of the lung. Therefore, RAGE signaling via Nrf2/NF-κB may be active in the lung tissue distal rather than proximal airways, leading to the primary cause: the incremental dilation of alveolar space.
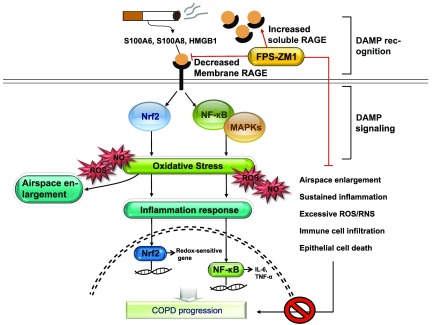
Figure 9.
Proposed mechanism for the effect of FPS-ZM1 in COPD. Exposure to CSE and release of elastase by injured cells during smoking increase ligands, such as S100 calgranulins and HMGB1. The ligands bind to and activate response by RAGE. The engagement of RAGE is linked to the DAMP signaling pathway, notably NF-κB and MAPKs, which overlap considerably with the Nrf2 signaling pathway for RAGE-mediated emphysematous response to stress stimuli in the lung (airspace enlargement, sustained inflammation, excessive ROS/RNS, immune cell infiltration, and epithelial cell death). When mice exposed to PPE or epithelial cells exposed to CSE are treated with FPS-ZM1, RAGE-mediated emphysema and DAMP signaling pathway recover. However, FPS-ZM1 treatment of Nrf2-deficient mice after elastase application fails to repair airspace enlargement, excessive ROS/RNS, and activation of DAMP signaling, including NF-κB and MAPK signaling; FPS-ZM1 reverses only the infiltration of inflammatory cells and cytokines. Eventually, boosted RAGE expression promotes COPD progression. In this respect, FPS-ZM1 may offer a potential therapeutic application to inhibit inflammation during the development of COPD, where this sustained inflammation is a big hurdle. Taken together, there is a need to verify the affirmative action of FPS-ZM1 against excessive infiltration of inflammatory cells and cytokines in COPD.
Inhibition of RAGE signaling is applied in a potent clinical therapy for Alzheimer’s disease (AD). A phase 2 clinical trial of an azole-based first-generation small molecule RAGE inhibitor in patients with AD has shown major safety concerns (52); available anti-RAGE antibodies block only the activation of RAGE ligand, but not the vascular damage by central amyloid β-peptide processing; and high pharmacologic concentrations of sRAGE have shown efficacy in young AD-affected mice, but not in older ones (53). However, FPS-ZM1, a high-affinity novel multimodal and RAGE-specific inhibitor, demonstrated effectively improved cognitive performance and cerebral blood flow response, reducing amyloid β-peptide processing (54). Given that the RAGE–ligand axis is central to pathology in COPD, similar to its effect in AD, strategies that antagonize the RAGE-specific ligand binding to the V domain of RAGE, which underlies COPD pathogenesis, would be a breakthrough over other strategies.
Currently, a range of inhaled, oral, and injectable medicines to reduce the frequency of COPD exacerbation include recommendations for interventions targeted at prevention. Despite such treatment recommendations, exacerbation of COPD still occurs, leading to a high mortality rate. Therefore, it is necessary for more effective strategies to prevent exacerbation. In this perspective, AGE-RAGE disturbances could be a precise and effective strategy with beneficial clinical effects. These results provide the new insights into the pathophysiological role of RAGE. Our findings strongly suggest that blockade of RAGE rescues deleterious effects at the molecular, cellular, and tissue levels through DAMP-Nrf2 signaling. Our study supports the application of a specific RAGE blockade as a novel clinical therapeutic for COPD, and this strong anti-inflammatory effect could apply in various respiratory diseases.
Supplementary Material
ACKNOWLEDGMENTS
The biospecimens and data used for this study were provided by the Biobank of Soonchunhyang, Kyungpook and the Korea University Guro Hospital, a member of the National Biobank of Korea. This work was supported by the National Research Foundation of Korea (NRF; Grant NRF-2014R1A2A2A01003737) funded by the Korean government (to S.-R.Y.) and U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grant 1R01HL085613 (to I.R.). The authors declare no conflicts of interest.
Glossary
- AD
Alzheimer’s disease
- AGER
advanced glycosylation end product-specific receptor
- ARE
antioxidant response element
- BAL
bronchoalveolar lavage
- BALF
BAL fluid
- COPD
chronic obstructive pulmonary disease
- CS
cigarette smoke
- CSE
cigarette smoke extract
- DAMP
damage-associated molecular pattern
- DCF-DA
dichlorofluorescein-diacetate
- FEV1/FVC
forced expiratory volume in 1 second/forced vital capacity
- GSH
glutathione
- GSSG
glutathione disulfide
- GWAS
genome-wide association studies
- H&E
hematoxylin and eosin
- HMGB1
high-mobility group box 1
- HRP
horseradish peroxidase
- mRAGE
transmembrane RAGE
- Nrf2
NF-E2 related factor 2
- PPE
porcine pancreatic elastase
- RAGE
receptor for advanced glycan end products
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- sRAGE
soluble RAGE
- TPM
total particulate matter
- xRAGE
membrane-bound RAGE
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
H. Lee collected the data, performed the experimental analyses, and wrote the manuscript, together with S.-R. Yang and I. Rahman; J.-R. Park, I. K. Sundar, W. J. Kim, and S.-M. Park performed experimental analyses and interpreted the data; and all authors participated in drafting the manuscript, revising it critically for content, and approving its submission.
REFERENCES
- 1.TESRA and ECLIPSE Investigators (2013) Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 188, 948–957 [DOI] [PubMed] [Google Scholar]
- 2.Iwamoto H., Gao J., Pulkkinen V., Toljamo T., Nieminen P., Mazur W. (2014) Soluble receptor for advanced glycation end-products and progression of airway disease. BMC Pulm. Med. 14, 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt W. R., Helfman B. R., McCarty N. A., Hansen J. M. (2016) Advanced glycation end products are elevated in cystic fibrosis-related diabetes and correlate with worse lung function. J. Cyst. Fibros. 15, 681–688 [DOI] [PubMed] [Google Scholar]
- 4.Young R. P., Hay B. A., Hopkins R. J. (2011) Does RAGE protect smokers from COPD? Eur. Respir. J. 38, 743–744, author reply 744 [DOI] [PubMed] [Google Scholar]
- 5.Morbini P., Villa C., Campo I., Zorzetto M., Inghilleri S., Luisetti M. (2006) The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod. Pathol. Inc 19, 1437–1445 [DOI] [PubMed] [Google Scholar]
- 6.Ott C., Jacobs K., Haucke E., Navarrete Santos A., Grune T., Simm A. (2014) Role of advanced glycation end products in cellular signaling. Redox Biol. 2, 411–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pahwa R., Jialal I. (2016) The role of the high-mobility group box1 protein-Toll like receptor pathway in diabetic vascular disease. J. Diabetes Complications 30, 1186–1191 [DOI] [PubMed] [Google Scholar]
- 8.Donato R. (2007) RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr. Mol. Med. 7, 711–724 [DOI] [PubMed] [Google Scholar]
- 9.Xue J., Rai V., Singer D., Chabierski S., Xie J., Reverdatto S., Burz D. S., Schmidt A. M., Hoffmann R., Shekhtman A. (2011) Advanced glycation end product recognition by the receptor for AGEs. Structure 19, 722–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuah Y. K., Basir R., Talib H., Tie T. H., Nordin N. (2013) Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int. J. Inflamm. 2013, 403460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparvero L. J., Asafu-Adjei D., Kang R., Tang D., Amin N., Im J., Rutledge R., Lin B., Amoscato A. A., Zeh H. J., Lotze M. T. (2009) RAGE (receptor for advanced glycation endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 7, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riuzzi F., Sorci G., Sagheddu R., Donato R. (2012) HMGB1-RAGE regulates muscle satellite cell homeostasis through p38-MAPK- and myogenin-dependent repression of Pax7 transcription. J. Cell Sci. 125, 1440–1454 [DOI] [PubMed] [Google Scholar]
- 13.Schmidt A. M., Yan S. D., Yan S. F., Stern D. M. (2001) The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Invest. 108, 949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riehl A., Németh J., Angel P., Hess J. (2009) The receptor RAGE: bridging inflammation and cancer. Cell Commun. Signal. 7, 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harashima A., Yamamoto Y., Cheng C., Tsuneyama K., Myint K. M., Takeuchi A., Yoshimura K., Li H., Watanabe T., Takasawa S., Okamoto H., Yonekura H., Yamamoto H. (2006) Identification of mouse orthologue of endogenous secretory receptor for advanced glycation end-products: structure, function and expression. Biochem. J. 396, 109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stogsdill J. A., Stogsdill M. P., Porter J. L., Hancock J. M., Robinson A. B., Reynolds P. R. (2012) Embryonic overexpression of receptors for advanced glycation end-products by alveolar epithelium induces an imbalance between proliferation and apoptosis. Am. J. Respir. Cell Mol. Biol. 47, 60–66 [DOI] [PubMed] [Google Scholar]
- 17.Sukkar M. B., Postma D. S. (2013) Receptor for advanced glycation end products and soluble receptor for advanced glycation end products: a balancing act in chronic obstructive pulmonary disease? Am. J. Respir. Crit. Care Med. 188, 893–894 [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Tasaka S., Shiraishi Y., Fukunaga K., Yamada W., Seki H., Ogawa Y., Miyamoto K., Nakano Y., Hasegawa N., Miyasho T., Maruyama I., Ishizaka A. (2008) Role of soluble receptor for advanced glycation end products on endotoxin-induced lung injury. Am. J. Respir. Crit. Care Med. 178, 356–362 [DOI] [PubMed] [Google Scholar]
- 19.Fineschi S., De Cunto G., Facchinetti F., Civelli M., Imbimbo B. P., Carnini C., Villetti G., Lunghi B., Stochino S., Gibbons D. L., Hayday A., Lungarella G., Cavarra E. (2013) Receptor for advanced glycation end products contributes to postnatal pulmonary development and adult lung maintenance program in mice. Am. J. Respir. Cell Mol. Biol. 48, 164–171 [DOI] [PubMed] [Google Scholar]
- 20.Stogsdill M. P., Stogsdill J. A., Bodine B. G., Fredrickson A. C., Sefcik T. L., Wood T. T., Kasteler S. D., Reynolds P. R. (2013) Conditional overexpression of receptors for advanced glycation end-products in the adult murine lung causes airspace enlargement and induces inflammation. Am. J. Respir. Cell Mol. Biol. 49, 128–134 [DOI] [PubMed] [Google Scholar]
- 21.Yao H., Arunachalam G., Hwang J. W., Chung S., Sundar I. K., Kinnula V. L., Crapo J. D., Rahman I. (2010) Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc. Natl. Acad. Sci. USA 107, 15571–15576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao H., Chung S., Hwang J. W., Rajendrasozhan S., Sundar I. K., Dean D. A., McBurney M. W., Guarente L., Gu W., Rönty M., Kinnula V. L., Rahman I. (2012) SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J. Clin. Invest. 122, 2032–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao H., Edirisinghe I., Rajendrasozhan S., Yang S. R., Caito S., Adenuga D., Rahman I. (2008) Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L1174–L1186 [DOI] [PubMed] [Google Scholar]
- 24.Sussan T. E., Rangasamy T., Blake D. J., Malhotra D., El-Haddad H., Bedja D., Yates M. S., Kombairaju P., Yamamoto M., Liby K. T., Sporn M. B., Gabrielson K. L., Champion H. C., Tuder R. M., Kensler T. W., Biswal S. (2009) Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc. Natl. Acad. Sci. USA 106, 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueno M., Maeno T., Nishimura S., Ogata F., Masubuchi H., Hara K., Yamaguchi K., Aoki F., Suga T., Nagai R., Kurabayashi M. (2015) Alendronate inhalation ameliorates elastase-induced pulmonary emphysema in mice by induction of apoptosis of alveolar macrophages. Nat. Commun. 6, 6332 [DOI] [PubMed] [Google Scholar]
- 26.Davis B. B., Shen Y. H., Tancredi D. J., Flores V., Davis R. P., Pinkerton K. E. (2012) Leukocytes are recruited through the bronchial circulation to the lung in a spontaneously hypertensive rat model of COPD. PLoS One 7, e33304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodine B. G., Bennion B. G., Leatham E., Jimenez F. R., Wright A. J., Jergensen Z. R., Erickson C. J., Jones C. M., Johnson J. P., Knapp S. M., Reynolds P. R. (2014) Conditionally induced RAGE expression by proximal airway epithelial cells in transgenic mice causes lung inflammation. Respir. Res. 15, 133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motz G. T., Eppert B. L., Wesselkamper S. C., Flury J. L., Borchers M. T. (2010) Chronic cigarette smoke exposure generates pathogenic T cells capable of driving COPD-like disease in Rag2−/− mice. Am. J. Respir. Crit. Care Med. 181, 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H., Park J. R., Kim E. J., Kim W. J., Hong S. H., Park S. M., Yang S. R. (2016) Cigarette smoke-mediated oxidative stress induces apoptosis via the MAPKs/STAT1 pathway in mouse lung fibroblasts. Toxicol. Lett. 240, 140–148 [DOI] [PubMed] [Google Scholar]
- 30.Yang S. R., Yao H., Rajendrasozhan S., Chung S., Edirisinghe I., Valvo S., Fromm G., McCabe M. J. Jr., Sime P. J., Phipps R. P., Li J. D., Bulger M., Rahman I. (2009) RelB is differentially regulated by IkappaB Kinase-alpha in B cells and mouse lung by cigarette smoke. Am. J. Respir. Cell Mol. Biol. 40, 147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park J. R., Lee H., Kim C. H., Hong S. H., Ha K. S., Yang S. R. (2016) Functional characteristics of mesenchymal stem cells derived from the adipose tissue of a patient with achondroplasia. In Vitro Cell. Dev. Biol. Anim. 52, 545–554 [DOI] [PubMed] [Google Scholar]
- 32.Lee H., Park J. R., Yang J., Kim E., Hong S. H., Woo H. M., Ryu S. M., Cho S. J., Park S. M., Yang S. R. (2014) Nicotine inhibits the proliferation by upregulation of nitric oxide and increased HDAC1 in mouse neural stem cells. In Vitro Cell. Dev. Biol. Anim. 50, 731–739 [DOI] [PubMed] [Google Scholar]
- 33.Rahman I., Kode A., Biswas S. K. (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 1, 3159–3165 [DOI] [PubMed] [Google Scholar]
- 34.Mo C., Wang L., Zhang J., Numazawa S., Tang H., Tang X., Han X., Li J., Yang M., Wang Z., Wei D., Xiao H. (2014) The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 20, 574–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim W. J., Lee S. D. (2015) Candidate genes for COPD: current evidence and research. Int. J. Chron. Obstruct. Pulmon. Dis. 10, 2249–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim W. J., Lim M. N., Hong Y., Silverman E. K., Lee J. H., Jung B. H., Ra S. W., Choi H. S., Jung Y. J., Park Y. B., Park M. J., Lee S. W., Lee J. S., Oh Y. M., Lee S. D. (2014) Association of lung function genes with chronic obstructive pulmonary disease. Lung 192, 473–480 [DOI] [PubMed] [Google Scholar]
- 37.Sambamurthy N., Leme A. S., Oury T. D., Shapiro S. D. (2015) The receptor for advanced glycation end products (RAGE) contributes to the progression of emphysema in mice. PLoS One 10, e0118979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sukkar M. B., Ullah M. A., Gan W. J., Wark P. A., Chung K. F., Hughes J. M., Armour C. L., Phipps S. (2012) RAGE: a new frontier in chronic airways disease. Br. J. Pharmacol. 167, 1161–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson A. B., Stogsdill J. A., Lewis J. B., Wood T. T., Reynolds P. R. (2012) RAGE and tobacco smoke: insights into modeling chronic obstructive pulmonary disease. Front. Physiol. 3, 301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlueter C., Hauke S., Flohr A. M., Rogalla P., Bullerdiek J. (2003) Tissue-specific expression patterns of the RAGE receptor and its soluble forms: a result of regulated alternative splicing? Biochim. Biophys. Acta 1630, 1–6 [DOI] [PubMed] [Google Scholar]
- 41.Kierdorf K., Fritz G. (2013) RAGE regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 94, 55–68 [DOI] [PubMed] [Google Scholar]
- 42.Ramasamy R., Yan S. F., Schmidt A. M. (2011) Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci. 1243, 88–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferhani N., Letuve S., Kozhich A., Thibaudeau O., Grandsaigne M., Maret M., Dombret M. C., Sims G. P., Kolbeck R., Coyle A. J., Aubier M., Pretolani M. (2010) Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 181, 917–927 [DOI] [PubMed] [Google Scholar]
- 44.Pouwels S. D., Nawijn M. C., Bathoorn E., Riezebos-Brilman A., van Oosterhout A. J., Kerstjens H. A., Heijink I. H. (2015) Increased serum levels of LL37, HMGB1 and S100A9 during exacerbation in COPD patients. Eur. Respir. J. 45, 1482–1485 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Li S., Wang G., Han D., Xie X., Wu Y., Xu J., Lu J., Li F., Li M. (2014) Changes of HMGB1 and sRAGE during the recovery of COPD exacerbation. J. Thorac. Dis. 6, 734–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zabini D., Crnkovic S., Xu H., Tscherner M., Ghanim B., Klepetko W., Olschewski A., Kwapiszewska G., Marsh L. M. (2015) High-mobility group box-1 induces vascular remodelling processes via c-Jun activation. J. Cell. Mol. Med. 19, 1151–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li M., Guo L., Wang H., Wang T., Shen Y., Liao Z., Wen F., Chen L. (2015) RAGE-ligands axis: a new ‘driving force’ for cigarette smoke-induced airway inflammation in COPD? Respirology 20, 998–999 [DOI] [PubMed] [Google Scholar]
- 48.Wood T. T., Winden D. R., Marlor D. R., Wright A. J., Jones C. M., Chavarria M., Rogers G. D., Reynolds P. R. (2014) Acute secondhand smoke-induced pulmonary inflammation is diminished in RAGE knockout mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L758–L764 [DOI] [PubMed] [Google Scholar]
- 49.Reynolds P. R., Kasteler S. D., Schmitt R. E., Hoidal J. R. (2011) Receptor for advanced glycation end-products signals through Ras during tobacco smoke-induced pulmonary inflammation. Am. J. Respir. Cell Mol. Biol. 45, 411–418 [DOI] [PubMed] [Google Scholar]
- 50.Abdelsalam R. M., Safar M. M. (2015) Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J. Neurochem. 133, 700–707 [DOI] [PubMed] [Google Scholar]
- 51.Biswal S., Thimmulappa R. K., Harvey C. J. (2012) Experimental therapeutics of Nrf2 as a target for prevention of bacterial exacerbations in COPD. Proc. Am. Thorac. Soc. 9, 47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alzheimer’s Disease Cooperative Study (2014) Clinical trial of an inhibitor of RAGE-Aβ interactions in Alzheimer disease. Neurology 82, 1536–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deane R., Du Yan S., Submamaryan R. K., LaRue B., Jovanovic S., Hogg E., Welch D., Manness L., Lin C., Yu J., Zhu H., Ghiso J., Frangione B., Stern A., Schmidt A. M., Armstrong D. L., Arnold B., Liliensiek B., Nawroth P., Hofman F., Kindy M., Stern D., Zlokovic B. (2003) RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 9, 907–913 [DOI] [PubMed] [Google Scholar]
- 54.Deane R., Singh I., Sagare A. P., Bell R. D., Ross N. T., LaRue B., Love R., Perry S., Paquette N., Deane R. J., Thiyagarajan M., Zarcone T., Fritz G., Friedman A. E., Miller B. L., Zlokovic B. V. (2012) A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 122, 1377–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.