Abstract
Induction of resistance of oral anaerobes to the effects of human β-defensin 1 (hβD-1) to hβD-4 was investigated by pretreating cells with either sublethal levels of defensins or environmental factors, followed by a challenge with lethal levels of defensins. Cultures of Porphyromonas gingivalis were (i) pretreated with defensins at 1 ng/ml, (ii) heated to 42°C (heat stress), (iii) exposed to normal atmosphere (oxidative stress), or (iv) exposed to 1 mM hydrogen peroxide (peroxide stress). Samples (10 μl) were distributed among the wells of sterile 384-well plates containing hβD-1 to -4 (100 μg/ml). Plates were incubated at 37°C for 36 h in an anaerobe chamber. Growth inhibition was determined by a system that measures the total nucleic acid of a sample with a DNA binding dye. The MICs of the four defensins for P. gingivalis were 3 to 12 μg/ml. We found that sublethal levels of the defensins and heat and peroxide stress, but not oxidative stress, induced resistance to 100 μg of defensin per ml in P. gingivalis. Resistance induced by sublethal levels of hβD-2 lasted 90 min, and the resistance induced by each defensin was effective against the other three. Multiple strains exposed to hβD-2 all evidenced resistance induction. Defensin resistance is vital to the pathogenic potential of several human pathogens. This is the first report describing the induction of defensin resistance in the oral periodontal pathogen P. gingivalis. Such resistance may have an effect on the ability of oral pathogens to persist in the mouth and to withstand innate human immunity.
Cationic antimicrobial peptides (CAPs) are important components of the innate human immune system (4). They are amphiphilic peptides that preferentially bind to microbial surfaces through electrostatic and hydrophobic interactions. Many CAPs possess antimicrobial activity against gram-positive and/or gram-negative bacteria and/or fungi at micromolar concentrations, levels that can be attained in vivo (8). While antimicrobial activity is considered the primary function of CAPs, recent evidence suggests that these molecules may induce, and possibly control, several key innate immune functions. At nanomolar concentrations, several CAPs have been shown to be chemoattractive to immune cells, including immature dendritic cells important in antigen processing and instruction of cells of the adaptive immune system (36). CAPs are found at the mucous membranes of the human gastroenteric system, including the oral cavity (5). Many are constitutively produced by epithelial cells of the mucous membranes and may perform surveillance functions. However, a number of these molecules are induced by contact with bacterial surface molecules or products of inflammatory immune responses (16).
Various modes of antimicrobial activity have been proposed for CAPs, including (i) critical membrane depolarization, (ii) creation of physical holes in the membranes, (iii) induction of hydralases that degrade the cell wall, and (iv) disturbance of membrane function by changing the relationship between the bilayer leaflets. Recent evidence suggests that CAPs may cause cell death by disruption of critical intracellular processes (8).
The defensins are a group of cysteine-rich β-sheet CAPs with broad-spectrum antimicrobial activity that are believed to kill microbes by inducing physical holes in the membrane (reviewed in reference 29). These small (3- to 5-kDa) peptides are represented in humans by the α- and β-defensins. Human α-defensins consist of four human neutrophil defensins (1 to 4) and two (5 and 6) found in intestinal crypts. Human β-defensins (hβDs) are distinguished from α-defensins by the placement and connectivity of the six cysteine residues characteristic of all defensins. Four hβDs (hβD-1 to -4) have been cloned and characterized, although there is evidence from in silico analysis that many more exist (18). All four have antimicrobial activity against gram-positive and gram-negative bacteria and fungi. hβD-1 is highly effective against gram-negative bacteria (2). hβD-2 has a strong bactericidal effect on gram-negative bacteria and a high antimycotic potency but only a weak bacteriostatic effect on the gram-positive bacterium Staphylococcus aureus (29). The more recently discovered and characterized defensin hβD-3 possesses a broad spectrum of potent antimicrobial activities against gram-negative and gram-positive bacteria (17). hβD-4 inhibits the growth of both gram-positive and gram-negative bacteria and fungi and is especially active against Pseudomonas aeruginosa (9). hβD-1 is expressed constitutively in many epithelial tissues, while the expression of hβD-2, -3, and -4 mRNAs is significantly up-regulated by tumor necrosis factor alpha or induced by bacteria (20). Resistance to CAPs, once thought to be highly unlikely, has been demonstrated to be both adaptive (10) and constitutive (1).
It has been reported that β-defensins are expressed by epithelial cells in the oral cavity and suggested that such expression could be important in the control of the normal oral flora, as well as protection against oral pathogens. However, the nature of the interactions between β-defensins and oral bacteria is poorly understood. We hypothesized that environmental stresses consistent with some oral diseases might be able to induce resistance to defensins similar to that described for other pathogens. Elevated temperatures in the subgingival space have been reported in human periodontal disease (13-15). In addition, P. gingivalis is subject to oxidative stress, not only from occasional exposure to air but also during neutrophil infiltration and oxidative burst or gum bleeding in advanced periodontitis (21). Here we describe these interactions by comparing the antimicrobial activities of β-defensins against Escherichia coli and two important oral bacteria, Fusobacterium nucleatum and Porphyromonas gingivalis. In addition, we present evidence that pretreatment of P. gingivalis with selected environmental stress factors, including sublethal doses of β-defensins, reduces the susceptibility of this organism to subsequent lethal β-defensin concentrations.
MATERIALS AND METHODS
Bacteria. P. gingivalis (ATCC 33277) and F. nucleatum (ATCC 25586) were obtained from the American Type Culture Collection (31), and E. coli D5α was obtained from Invitrogen (Carlsbad, Calif.). The bacteria were maintained by weekly transfer in an anaerobe chamber (Coy Manufacturing, Grass Lake, Mich.) at 37°C on PRAS Brucella agar plates (Anaerobe Systems, Morgan Hill, Calif.) in a 5% hydrogen-10% carbon dioxide-85% nitrogen atmosphere. Broth cultures were grown in BHTS, which is a mixture of 50% brain heart infusion broth, 50% Trypticase soy broth, and 5 g of yeast extract per liter supplemented with 0.01 g of sodium bisulfite per liter, 5 mg of hemin per liter, and 5 μg of vitamin K per liter (34). Bacteria used throughout these experiments were grown to early log phase (A600 = 0.20) because some stress genes are induced at high cell densities without temperature shifts (23).
Bioactive peptides.
hβDs were obtained from Peptides International (Louisville, Ky.).
Estimation of MICs of defensins.
A doubling dilution series of defensins, beginning with 100 μg/ml, was added to the wells of a sterile 384-well microtiter plate (12 replicates per dilution) and dried overnight in a desiccator box. Bacterial suspensions (10 μl, 107/ml) were added to the wells under anaerobic conditions, covered with a sterile plastic film, centrifuged briefly to collect the cells in the bottom of the wells, and incubated in an anaerobic chamber (37°C, 5% hydrogen, 10% carbon dioxide, 85% nitrogen) for 6 to 36 h, depending upon the rate of growth of the bacterial species. The terminal cell numbers were then determined as described below. MICs were set as the lowest concentration of the defensin at which there was no growth above the inoculated level of bacteria (P < 0.05, n = 12). Values expressed in tables represent the log10 growth above the inoculated levels.
Quantitation of bacteria.
A system that measures the total nucleic acid of a sample with a DNA binding dye (SYBR Green I) was adapted to measure the growth of bacteria (22). Ten microliters of a solution (1:100) of SYBR Green I in sterilely filtered water was added to 10 μl of bacterial suspension in the wells of a 384-well growth plate and incubated for 30 min at room temperature, and the fluorescence was measured (excitation, 485 nm; emission, 635 nm) with a GENios plate reader (Tecan, Männedorf, Switzerland). Values are expressed as the log10 of the bacterial equivalents as determined by plotting the log10 of known numbers of each bacterium against the relative fluorescence units to produce a standard dilution curve. Preliminary experiments demonstrated that the method correlated with turbidimetric (A600) estimates with an r value of 0.948 under normal growth conditions. However, it was determined that the kinetics of P. gingivalis growth during a challenge with defensins was highly variable with respect to time and the method of measurement used. Therefore, all measurements of growth were taken after 36 h of incubation, at which time the differences were not significant.
Defensin treatments.
Defensins were dissolved in water, and 10-μl volumes were distributed aseptically among the wells of 384-well plates. Plates were then dried for 24 h in a desiccator cabinet. For inhibition experiments, all defensins were dispensed at 100 μg/ml. Cultures (10 μl, 107/ml) were added to the wells after pretreatment in BHTS. Cultures were incubated at 37°C in an anaerobic chamber for 6 to 36 h, depending upon the bacterial species, and the terminal cell numbers were determined as described above. To determine the duration of the effect of induced defensin resistance, we pretreated cultures of P. gingivalis with 1 ng of defensin per ml for 30 min at 37°C in an anaerobic chamber. The cells (20 μl, 107/ml) were then added to the wells, and 1 μl of defensin (100 μg/ml) was added immediately and 30, 60, 90, and 120 min later. The cells were incubated for an additional 36 h, and the terminal cell numbers were determined as described above.
Pretreatment.
Multiple 10-ml cultures were inoculated with 100 μl of cell suspension from log-phase cultures and grown for 24 to 48 h at 37°C to an optical density (A600) of 0.2 in the anaerobic chamber. Culture samples (1.5 ml) were then either (i) heated to 42°C for 30 min anaerobically, (ii) treated with a defensin (hβD-1, -2, -3, or -4) at a final concentration of 1 ng/ml for 20 min at 37°C anaerobically, (iii) transferred to a 50-ml tube and shaken at 37°C for 30 min in normal atmosphere (28), or (iv) treated anaerobically with 1 mM hydrogen peroxide for 30 min. Control cultures were maintained at 37°C. Ten-microliter samples were distributed in quadruplicate among the wells of sterile 384-well plates containing the dried defensins (final concentration, 100 μg/ml). The plates were then sealed with an adhesive, gas-permeable cover sheet and incubated at 37°C in an anaerobic chamber.
Defensin resistance in multiple strains of P. gingivalis.
Cultures of five laboratory strains and four recent isolates of P. gingivalis were grown to an A600 of 0.20, pretreated with 1 ng of hβD-2 per ml, and tested for resistance to the same defensin by the method described above.
Statistical analysis.
The statistical significance of differences was evaluated with a two-sided t-test routine in the Statistica 6 (StatSoft, Tulsa, Okla.) software package.
RESULTS
MICs of hβD-1, -2, -3, and -4 under anaerobic conditions.
The MIC of each of the defensin molecules was examined with three strains of bacteria to establish concentrations to be used in the resistance induction experiments and to ensure the effectiveness of the preparations. A modification of the standard microdilution technique was used to increase the number of determinations for analytical purposes and to conserve defensins. Each of the defensins was effective against the E. coli cells tested (Table 1). The patterns of growth inhibition were similar for all of the defensins. The range of the MICs of the defensins for E. coli was 3 to 6 μg/ml.
TABLE 1.
MICs of hβD-1 to -4
| Defensin | MIC (μg/ml)a
|
||
|---|---|---|---|
| E. coli | F. nucleatum | P. gingivalis | |
| hβD-1 | 6.25 | >100 | 3.13 |
| hβD-2 | 3.13 | >100 | 6.25 |
| hβD-3 | 3.13 | >100 | 12.5 |
| hβD-4 | 3.13 | >100 | 6.25 |
n = 12 at each point.
Cultures of P. gingivalis were consistently affected by treatment with hβD-1 to -4, as shown in Table 1. The mean bacterial count at each defensin concentration was determined. The apparent MICs for P. gingivalis exposed to the defensins are between 3 and 12 μg/ml. The MIC of hβD-1 was 3.13 μg/ml, that of hβD-2 and hβD-4 was 6.25 μg/ml, and that of hβD-3 was 12.5 μg/ml. We determined the significance of the differences between the means of the log10 values at different defensin dilutions preceding and following the MIC point with a two-tailed t test. For all of the defensins, the log10 values at the MIC were not significantly different from that at the preceding (higher) defensin concentration (P > 0.05) but were significantly different from that at the following (lower) defensin concentration (P < 0.05).
F. nucleatum was consistently resistant to the defensins at all of the concentrations studied (Table 1).
Growth kinetics of P. gingivalis challenged with hβD-1 as measured by different methods.
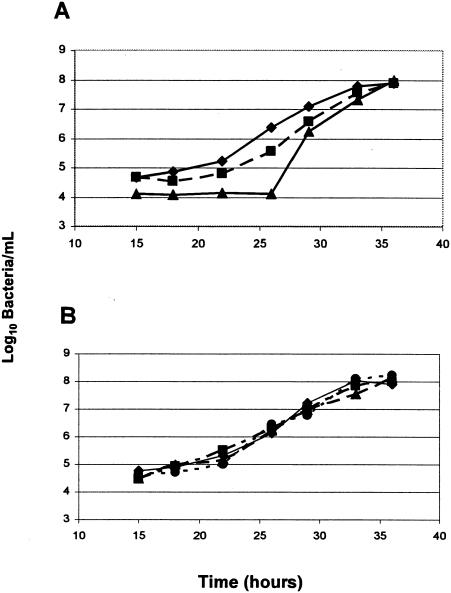
As determined by the three methods used to measure the growth of P. gingivalis, the terminal levels were the same after 36 h of incubation in cultures pretreated with 1 ng of defensin per ml. However, in pretreated cultures challenged with lethal levels of defensins, the period of no apparent growth (lag period) was increased by several hours as measured by the colony counting method compared to either A600 turbidity measurements or the DNA binding assay (Fig. 1.) No comparable change was seen in pretreated cultures not challenged with defensin, which were indistinguishable from nonpretreated cultures.
FIG. 1.
Growth kinetics of P. gingivalis challenged with hβD-1 as measured by different methods. P. gingivalis cells were pretreated with 1 ng of hβD-1 per ml for 30 min, plated in microtiter wells, and allowed to grow anaerobically at 37°C for 36 h either with (A) or without (B) a challenge with 100 μg of hβD-1 per ml. Replicate cultures were sampled at the times indicated, and the number of bacteria was determined by one of three methods: (i) colony counts on PRAS Brucella agar (triangles), (ii) turbidity as determined by A600 (squares), or (iii) the SYBR Green DNA assay described in Materials and Methods (diamonds). The no-treatment, no-challenge control was assayed for turbidity as determined by A600 (panel B, circles). Representative results of three experiments are displayed.
Induction of resistance in P. gingivalis by low levels of defensins.
Experiments testing the induction of resistance in P. gingivalis by low levels of defensins were undertaken to determine if the growth-inhibitory activity of hβDs was affected by pretreatment of P. gingivalis cells with subinhibitory levels of the same hβD or different hβDs. P. gingivalis cells pretreated with sublethal levels of hβDs were significantly more resistant to 100 μg of hβDs per ml than untreated cells were (Table 2). Pretreatment of the cells with each of the four defensins provided protection when the cells were challenged with 100 μg of both homologous and heterologous defensins per ml. There was a statistically significant increase in growth compared to that of cells that received no defensin pretreatment. Each of the defensins was effective against cells that did not receive any pretreatment.
TABLE 2.
Patterns of autoinduced defensin resistance in P. gingivalis ATCC 33277a
| Defensin challenge (100 μg/ml) | Mean log10 no. of bacteria 36 h after pretreatment (1 ng/ml) with:
|
||||
|---|---|---|---|---|---|
| Nothing | hβD-1 | hβD-2 | hβD-3 | hβD-4 | |
| None | 7.80 | 7.62 | 7.50 | 8.16 | 7.96 |
| hβD-1 | 5.36c | 7.87b | 7.16b | 7.30b | 8.04b |
| hβD-2 | 5.41c | 8.09b | 7.18b | 7.47b | 7.94b |
| hβD-3 | 5.35c | 6.65b | 6.94b | 8.16b | 7.72b |
| hβD-4 | 5.29c | 6.63b | 7.21b | 8.11b | 7.68b |
n = 12 at each point.
P ≤ 0.01, t test compared to no-pretreatment control.
P ≤ 0.01, t test compared to no-challenge control.
Induction of resistance in P. gingivalis by heat and oxidative stress.
Experiments testing the induction of resistance in P. gingivalis by heat and oxidative stress were designed to determine if the growth-inhibitory activity of hβDs was affected by pretreatment of P. gingivalis cells with environmental effects consistent with those that might be encountered in the oral cavity. In cells stressed with a +5°C heat shock, a surrogate for the elevated temperatures at inflamed gingival sites (13-15), there was a significant reduction in the growth-inhibitory effect of all four defensins (Table 3). A similar reduction in defensin effectiveness was seen in cells treated with low levels of hydrogen peroxide as a surrogate for the effects of inflammatory cells (Table 3). When we tested the effect of exposure to atmospheric oxygen, there was no reduction in the effectiveness of the defensin (Table 3).
TABLE 3.
Patterns of environmentally induced defensin resistance in P. gingivalis ATCC 33277a
| Defensin challenge (100 μg/ml) | Mean log10 no. of bacteria 36 h after pretreatment with:
|
|||
|---|---|---|---|---|
| Nothing | +5°C heat stress | Atmospheric O2 | Hydrogen peroxide | |
| None | 7.11 | 7.09 | 7.01 | 7.00 |
| hβD-1 | 5.51b | 7.07c | 5.73 | 7.11c |
| hβD-2 | 5.21b | 6.92c | 5.31 | 7.84c |
| hβD-3 | 5.84b | 8.16c | 5.93 | 7.81c |
| hβD-4 | 5.89b | 7.08d | 5.99 | 6.69c |
n = 12 at each point.
P ≤ 0.01, t test compared to no-challenge control.
P ≤ 0.01, t test compared to no-pretreatment control.
P ≤ 0.05, t test compared to no-pretreatment control.
Duration of induced defensin resistance.
To estimate the length of time that induced defensin resistance persisted after pretreatment, we treated cultures with lethal levels of defensins (hβD-1, -2, -3, and -4) for 0, 30, 60, 90, and 120 min after pretreatment with hβD-2 for 30 min. There was a significant reduction in defensin resistance between 90 and 120 min with all of the defensins (Table 4).
TABLE 4.
Duration of induced defensin resistancea
| Defensin challenge (50 μg/ml) | Mean log10 no. of bacteria 36 h after:
|
||||||
|---|---|---|---|---|---|---|---|
| Pretreatment with 1 ng of hβD-2/ml
|
No pretreatment
|
||||||
| 0b | 30 | 60 | 90 | 120 | 0 | 120 | |
| None | 7.73 | 7.89 | 7.92 | 7.51 | 7.32 | 7.64 | 7.97 |
| hβD-1 | 6.94 | NDc | ND | 7.00 | 5.16d | 6.12e | 5.27e |
| hβD-2 | 7.26 | 8.10 | 7.49 | 6.78 | 5.34d | 5.05e | 5.25e |
| hβD-3 | 8.03 | ND | ND | 7.07 | 5.81d | 5.21e | 5.04e |
| hβD-4 | 7.37 | ND | ND | 7.17 | 5.08d | 5.36e | 5.61e |
n = 12 at each point.
Time (minutes) posttreatment when defensin was added.
ND, not done.
P ≤ 0.05, t test compared to previous time point.
P ≤ 0.05, t test compared to no-pretreatment, no-challenge control.
Defensin resistance in multiple strains of P. gingivalis.
In order to determine if the defensin resistance was a characteristic of only a single strain of P. gingivalis, we tested several stains, both laboratory and freshly isolated, for the ability to respond to low levels of defensins with resistance to normally lethal levels. All of the strains tested were initially susceptible to the antimicrobial effects of hβD-2, with MICs between 1 and 10 μg/ml, similar to previous results. When the strains were pretreated with a low level of hβD-2 (1 ng/ml) and then subsequently exposed to higher levels of hβD-2, the apparent MIC was 10 to 100 times higher (Table 5). Therefore, in at least this small sample of P. gingivalis strains, all of the cultured cells were originally sensitive to β-defensin and all could be induced to resist the same defensin after low-level pretreatment.
TABLE 5.
Induction of hβD-2 resistance in P. gingivalis strains
| P. gingivalis strain | MIC of hβD-2 (μg/ml)
|
|
|---|---|---|
| No pretreatment | Pretreatment | |
| ATCC 33277 | 10 | >100 |
| W83 | 1 | >100 |
| 381 | 10 | >100 |
| A7-A28 | 10 | >100 |
| ATCC 49437 | 10 | >100 |
| MLW-47-1(wt) | 1 | >100 |
| MLW-05-5(wt) | 1 | >100 |
| MLW-17-3(wt) | 10 | >100 |
| MLW-42-2(wt) | 1 | >100 |
DISCUSSION
The role of CAPs as potential therapeutic molecules in the oral cavity has been suggested by several authors (6, 26, 35), and although the antimicrobial activities of porcine defensins against F. nucleatum and Prevotella and Porphyromonas species have been described (25), the exact effect of human defensins against these bacteria had not been addressed until recently. In an abstract, it has been reported that a number of oral bacteria, including P. gingivalis, are sensitive to hβD-1 to -3 and that F. nucleatum, as we have also found, seems resistant to those molecules in vitro (19). However, this is the first report describing inducible defensin resistance in P. gingivalis.
In many cases, induction of CAP resistance is vital to the pathogenic potential of bacteria (reviewed in reference 27). For example, if Staphylococcus aureus cells modify their phosphatidylglycerol by adding l-lysine, which causes resistance to CAPs, the ability of human neutrophils to kill the cells is decreased and virulence in mouse sepsis models is increased. In Salmonella enterica serovar Typhimurium, lipid A modification with aminoarabinose facilitated by activation of pmrHFIJKL and other genes confers resistance to the peptide antibiotic polymyxin B, and mutant forms of those genes produce attenuated virulence in mice (11, 12).
Interestingly, exposure to atmospheric levels of oxygen did not induce defensin resistance in P. gingivalis. Like some other anaerobes, P. gingivalis is tolerant of exposure to air but does not grow significantly in its presence. The gene most thought to be responsible for that aerotolerance is sod (24). However, a number of genes have been recently associated with atmospheric oxygen tolerance in the obligate anaerobe Bacteroides fragilis, including that for an aerobic-type ribonucleotide reductase, nrdAB; an aspartate decarboxylase gene, asdA; and genes that code for proteins similar to the P. gingivalis temperature-modulated protein RagA, a cation efflux pump (CzcD), and a chaperone, HtpG (32). These genes are not part of the peroxide response-inducible genes ahpCF, dps, and katB in B. fragilis that are controlled by the redox-sensitive transcriptional activator OxyR (28). Combined with our observation that peroxide pretreatment but not pretreatment with atmospheric oxygen results in defensin resistance, this suggests a method for the dissection of the induction of such resistance by comparison of the gene profiles of these two oxidative stress responses.
The expression of defensin genes in oral tissues and the presence of defensins in crevicular fluid and saliva have been examined. In tissue from subjects with periodontitis, significantly higher levels of hβD-3 expression and a trend toward higher expression of hβD-2 were found in healthy tissue than in diseased tissue (3). Diamond et al. (7) detected hβD-1 and hβD-2 in six crevicular fluid samples and reported relatively higher levels of hβD-2 and lower levels of hβD-1 in samples from an individual with the greatest level of inflammation. Levels of 375 and 530 ng of hβD-1 and hβD-2 per ml, respectively, were reported in the saliva of subjects with oral candidiasis (33); patients had significantly lower levels than controls did. The calculated excretion rate for the two defensins was between 69 and 228 ng/min for the entire salivary space, which, assuming about 20 teeth, could mean about 3 to 10 ng/min in each crevicular space. Since gingival crevicular fluid flow is increased in periodontal disease, this might favor lower concentrations of defensins at diseased sites and induction of defensin resistance over antimicrobial activity. Since we found that the induced defensin resistance phenotype is transient, determination of the levels of these molecules in the subgingival space will be important in evaluating their pathogenic potential in vivo.
With the defensins described here, pretreatment with low levels of defensins or environmental stress produced broad resistance to all four of the molecules we tested. This may mean that induced defensin resistance requires a single mechanism to be effective against all defensins or that a number of bacterial gene systems involved in resistance are coactivated by this exposure. The results obtained with the environmental stressors suggest that the latter may be true. Stress responses that were successful in inducing defensin resistance have been shown to produce activation of a large number of microbial genes, and overlap between the genes induced by different stress factors is common. Preliminary results of reverse transcription-PCR experiments in our laboratory, reported in an abstract (30), also support this notion. However, the exact mechanism of defensin resistance induction in P. gingivalis remains to be determined, as does its occurrence, persistence, and possible significance in vivo.
Acknowledgments
We thank Larry F. Wolff, University of Minnesota School of Dentistry, for the low-passage human isolates of P. gingivalis and Dominica G. Sweier, University of Michigan School of Dentistry, for critical appraisal of the manuscript. We also acknowledge the technical assistance of Taniform Abongwa in the growth and maintenance of the bacterial strains used in these studies and Florence An for running the resistance induction experiments.
This research was supported by NIH grants DE11117 and DE 007256.
REFERENCES
- 1.Baird, R. M., H. Brown, A. W. Smith, and M. L. Watson. 1999. Burkholderia cepacia is resistant to the antimicrobial activity of airway epithelial cells. Immunopharmacology 44:267-272. [DOI] [PubMed] [Google Scholar]
- 2.Bensch, K. W., M. Raida, H. J. Magert, P. Schulz-Knappe, and W. G. Forssmann. 1995. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 368:331-335. [DOI] [PubMed] [Google Scholar]
- 3.Bissell, J., S. Joly, G. K. Johnson, C. C. Organ, D. Dawson, P. B. McCray, Jr., and J. M. Guthmiller. 2004. Expression of beta-defensins in gingival health and in periodontal disease. J. Oral Pathol. Med 33:278-285. [DOI] [PubMed] [Google Scholar]
- 4.Cole, A. M., and T. Ganz. 2000. Human antimicrobial peptides: analysis and application. BioTechniques 29:822-826, 828, 830-831. [DOI] [PubMed] [Google Scholar]
- 5.Dale, B. A., J. R. Kimball, S. Krisanaprakornkit, F. Roberts, M. Robinovitch, R. O'Neal, E. V. Valore, T. Ganz, G. M. Anderson, and A. Weinberg. 2001. Localized antimicrobial peptide expression in human gingiva. J. Periodontal Res. 36:285-294. [DOI] [PubMed] [Google Scholar]
- 6.Dale, B. A., and S. Krisanaprakornkit. 2001. Defensin antimicrobial peptides in the oral cavity. J. Oral Pathol. Med. 30:321-327. [DOI] [PubMed] [Google Scholar]
- 7.Diamond, D. L., J. R. Kimball, S. Krisanaprakornkit, T. Ganz, and B. A. Dale. 2001. Detection of beta-defensins secreted by human oral epithelial cells. J. Immunol. Methods 256:65-76. [DOI] [PubMed] [Google Scholar]
- 8.Ganz, T., and R. I. Lehrer. 1998. Antimicrobial peptides of vertebrates. Curr. Opin. Immunol. 10:41-44. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, J. R., A. Krause, S. Schulz, F. J. Rodriguez-Jimenez, E. Kluver, K. Adermann, U. Forssmann, A. Frimpong-Boateng, R. Bals, and W. G. Forssmann. 2001. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 15:1819-1821. [PubMed] [Google Scholar]
- 10.Groisman, E. A., C. Parra-Lopez, M. Salcedo, C. J. Lipps, and F. Heffron. 1992. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11939-11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 12.Gunn, J. S., S. S. Ryan, J. C. Van Velkinburgh, R. K. Ernst, and S. I. Miller. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haffajee, A. D., S. S. Socransky, and J. M. Goodson. 1992. Subgingival temperature. I. Relation to baseline clinical parameters. J. Clin. Periodontol. 19:401-408. [DOI] [PubMed] [Google Scholar]
- 14.Haffajee, A. D., S. S. Socransky, and J. M. Goodson. 1992. Subgingival temperature. II. Relation to future periodontal attachment loss. J. Clin. Periodontol. 19:409-416. [DOI] [PubMed] [Google Scholar]
- 15.Haffajee, A. D., S. S. Socransky, C. Smith, S. Dibart, and J. M. Goodson. 1992. Subgingival temperature. III. Relation to microbial counts. J. Clin. Periodontol. 19:417-422. [DOI] [PubMed] [Google Scholar]
- 16.Hancock, R. E., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 18.Jia, H. P., B. C. Schutte, A. Schudy, R. Linzmeier, J. M. Guthmiller, G. K. Johnson, B. F. Tack, J. P. Mitros, A. Rosenthal, T. Ganz, and P. B. McCray, Jr. 2001. Discovery of new human beta-defensins using a genomics-based approach. Gene 263:211-218. [DOI] [PubMed] [Google Scholar]
- 19.Kim, S. O., Z. Feng, and A. Weinberg. 2003. Characteristics of human β-defensin 1, 2, and 3 killing of oral bacterial species. In 32nd Annual Meeting of the American Association for Dental Research, abstr. 0716.
- 20.Krisanaprakornkit, S., J. R. Kimball, A. Weinberg, R. P. Darveau, B. W. Bainbridge, and B. A. Dale. 2000. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect. Immun. 68:2907-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, W. K., and P. M. Dickie. 2001. Monitoring phytoplankton, bacterioplankton, and virioplankton in a coastal inlet (Bedford Basin) by flow cytometry. Cytometry 44:236-246. [DOI] [PubMed] [Google Scholar]
- 23.Lopatin, D. E., A. Combs, D. G. Sweier, J. C. Fenno, and S. Dhamija. 2000. Characterization of heat-inducible expression and cloning of HtpG (Hsp90 homologue) of Porphyromonas gingivalis. Infect. Immun. 68:1980-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch, M. C., and K. H. Kuramitsu. 1999. Role of superoxide dismutase activity in the physiology of Porphyromonas gingivalis. Infect. Immun. 67:3367-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyasaki, K. T., R. Iofel, A. Oren, T. Huynh, and R. I. Lehrer. 1998. Killing of Fusobacterium nucleatum, Porphyromonas gingivalis and Prevotella intermedia by protegrins. J. Periodontal Res. 33:91-98. [DOI] [PubMed] [Google Scholar]
- 26.Miyasaki, K. T., and R. I. Lehrer. 1998. Beta-sheet antibiotic peptides as potential dental therapeutics. Int. J. Antimicrob. Agents 9:269-280. [DOI] [PubMed] [Google Scholar]
- 27.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 28.Rocha, E. R., G. Owens, Jr., and C. J. Smith. 2000. The redox-sensitive transcriptional activator OxyR regulates the peroxide response regulon in the obligate anaerobe Bacteroides fragilis. J. Bacteriol. 182:5059-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroder, J. M., and J. Harder. 1999. Human beta-defensin-2. Int. J. Biochem. Cell Biol. 31:645-651. [DOI] [PubMed] [Google Scholar]
- 30.Shelburne, C. E., R. M. Gleason, D. G. Sweier, W. A. Coulter, and D. E. Lopatin. 2002. Gene activation in Porphyromonus gingivalis by human beta-defensins. In 80th General Session of the International Association for Dental Research, abstr. 4008.
- 31.Shelburne, C. E., G. P. Sandberg, C. A. Binsfeld, L. F. Wolff, and R. A. Curry. 1993. Monoclonal antibodies to lipopolysaccharide of four oral bacteria associated with periodontal disease. J. Periodontal Res. 28:1-9. [DOI] [PubMed] [Google Scholar]
- 32.Smalley, D., E. R. Rocha, and C. J. Smith. 2002. Aerobic-type ribonucleotide reductase in the anaerobe Bacteroides fragilis. J. Bacteriol. 184:895-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanida, T., T. Okamoto, A. Okamoto, H. Wang, T. Hamada, E. Ueta, and T. Osaki. 2003. Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis. J. Oral Pathol. Med. 32:586-594. [DOI] [PubMed] [Google Scholar]
- 34.Tanner, A., M. F. Maiden, P. J. Macuch, L. L. Murray, and R. L. Kent, Jr. 1998. Microbiota of health, gingivitis, and initial periodontitis. J. Clin. Periodontol. 25:85-98. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg, A., S. Krisanaprakornkit, and B. A. Dale. 1998. Epithelial antimicrobial peptides: review and significance for oral applications. Crit, Rev. Oral Biol. Med. 9:399-414. [DOI] [PubMed] [Google Scholar]
- 36.Yang, D., Q. Chen, O. Chertov, and J. J. Oppenheim. 2000. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 68:9-14. [PubMed] [Google Scholar]