Abstract
Intestinal inflammation is associated with low levels of mucosal ATP, highlighting the importance of mitochondrial function associated with ATP production in the pathophysiology of the disease. In the inflamed colon of humans and mice, we found decreased levels of mitochondrial complex cytochrome c oxidase I/IV and lower ATP levels. Thus, we generated colonic ρ0 cells with reduced mitochondrial function linked to ATP production by selective depletion of mitochondrial DNA. In these cells, RNA sequencing revealed a substantial number of differentially expressed transcripts, among which 240 belonged to inflammatory pathways activated in human inflamed colon and TNF-α-treated cells (false discovery rate < 0.05). TNF-α treatment of colonic ρ0 cells augmented IL-8 expression by 9-fold (P < 0.01) via NF-κB compared to TNF-α-treated control. Moreover, reduced mitochondrial function facilitated TNF-α-mediated NF-κB luciferase promoter activity as a result of lowered inhibitory IκBα (nuclear factor of κ light polypeptide gene enhancer in B-cell inhibitor, α), leading to elevated NF-κB. In cells with reduced mitochondrial function, TNF-α facilitated AMPKα2 activation by 8-fold (P < 0.01), which was involved in NF-κB-dependent IL-8 expression. Last, in human and mouse colon, anti-TNF-α treatment restored reduced mitochondria-dependent inflammation. We propose that selective targeting of this novel mechanism provides new treatment opportunities for intestinal inflammation.—Heller, S., Penrose, H. M., Cable, C., Biswas, D., Nakhoul, H., Baddoo, M., Flemington, E., Crawford, S. E., Savkovic, S. D. Reduced mitochondrial activity in colonocytes facilitates AMPKα2-dependent inflammation.
Keywords: intestine, RNA-seq, inflammatory bowel disease, ρ0 cells
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, has mainly been viewed as an immune syndrome (1). In addition, IBD-affected intestinal tissue displays low levels of mucosal ATP (2), highlighting the importance of mitochondrial function associated with ATP production in intestinal inflammation. Polymorphisms in mitochondrial genes leading to diminished ATP production exacerbate intestinal inflammation, while a mutation in another mitochondrial gene leading to increased ATP levels was found to be protective (3, 4). However, the role of reduced mitochondrial function associated with ATP production and the mechanisms involved in driving intestinal inflammation are not well understood or explored.
The inflamed intestine is associated with increased levels of inflammatory mediators, infiltration of inflammatory cells, reactive oxygen species (ROS), and mucosal injury (1, 5–7). Increased expression of proinflammatory TNF-α facilitates disease progression by stimulating production of other cytokines (1, 8). Additionally, IBD pathophysiology is driven by extensive mucosal injury due to infiltrated inflammatory cells, leading to elevated ROS (6, 9, 10), which could trigger inflammasome-mediated cytokine production (11, 12). Because ROS are mainly produced by mitochondria (5) and TNF-α signaling is linked to ROS production, the role of mitochondrial dysfunction in intestinal inflammation has been studied for the most part in this respect (13). In addition, TNF-α stimulates mitophagy, a process involved in the removal of damaged mitochondria, which could also be contributing to intestinal inflammation in part through generation of ROS and activation of inflammasome pathways (14, 15). Moreover, sufficient mitochondrial ATP production has been suggested to be critical for epithelial proliferation in healing of mucosal injury and maintaining intestinal barrier function (4, 16–18). Because intestinal inflammation is driven by both increased inflammatory mediators and impaired healing of mucosal injuries, it is clear that alterations in diverse mitochondrial function could play a critical role in this pathophysiology.
Mitochondria are critical for cellular homeostasis because of their production of ATP through oxidative phosphorylation (oxphos). Products of cellular oxidation enter the mitochondrial citric acid cycle, generating redox equivalents that are used to build a proton gradient across the inner membrane, consequently leading to ATP production (19, 20). Because multiple respiratory chain complex (I–V) proteins encoded by nuclear and mitochondrial DNA are required for oxphos function, targeting individual genes encoding oxphos protein to study ATP production has many limitations. A more effective means to do this uses a more global approach that utilizes cells devoid of mitochondrial DNA, so-called ρ0 cells (21, 22). These ρ0 cells are unable to generate mitochondrial ATP, but they are viable because they still perform the citric acid cycle after supplementation with pyruvate (21, 23). Also, these cells are unable to produce ROS and have a lower susceptibility to apoptosis (24–27). Therefore, ρ0 cells represent a suitable model to study the effects of oxphos on nuclear gene expression, cell function, and pathophysiology associated with mitochondrial disorders.
Here we show in inflamed human and mouse colon tissue decreased levels of mitochondrial cytochrome c oxidase (COX) complex and ATP levels. In colon cells with reduced mitochondrial activity (ρ0 cells), next-generation RNA sequencing (RNA-seq) demonstrated activated inflammatory pathways similar to those observed in human IBD colon and colon cells stimulated with TNF-α, with IL-8 being highly expressed. We found in colon cells that TNF-α-mediated IL-8 expression is facilitated by reduced mitochondrial activity through AMPKα2. In human and mouse inflamed colon, anti-TNF-α treatment restored this mitochondrial axis, thus highlighting this novel mechanism of intestinal inflammation as a potentially new therapeutic strategy in targeting mitochondria for IBD treatment.
MATERIALS AND METHODS
Cells
Human colonic cells HCT116 and HT29 (American Type Culture Collection, Manassas, VA, USA; passages 7–10) were propagated in McCoy’s 5A medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA). Semiconfluent colonic monolayers were serum starved overnight before experimental procedures.
Generation of colonic ρ0 cells
Human colonic HCT116 cells were propagated in low doses of ethidium bromide (2.5 µM) for 8 to 10 passages in adequate medium supplemented with FBS (15%), pyruvate (100 µM, Thermo Fisher Scientific), and uridine (50 µg/ml; Sigma-Aldrich) (21, 28). This low ethidium bromide concentration inhibits mitochondrial, but not nuclear, DNA polymerase (21, 22), as confirmed by PCR using primers amplifying mitochondrial DNA (mtDNA-FOR: 5′-CCAAACCCCAAAAACAAAGAA-3′, mtDNA-REV: 5′-TTTGAGCTGCATTGCTGCGTG-3′) and nuclear DNA (18S-FOR: 5′-TCGGAACTGAGGCCATGATTA-3′, 18S-REV: 5′-GCGGGTCATGGGAATAACG-3′) (29).
Treatments and inhibitors
Experimental cells were treated with human recombinant TNF-α (10 ng/ml; R&D Systems, Minneapolis, MN, USA) over a designated time period. For blockade of mitochondrial respiratory chain complex III activity, the inhibitor antimycin A (AntiA, 500 nM; Sigma-Aldrich) was used overnight. AMPK activation was blocked with 1 h pretreatment with compound C (CompC; 40 µM; Cayman Chemicals, Ann Arbor, MI, USA).
Immunofluorescence staining
Colon cells grown on glass coverslips were fixed with 3.7% formaldehyde and immunofluorescently stained for translocase of outer membrane (TOM20; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and phosphorylated AMPK (pAMPK; Cell Signaling Technology, Danvers, MA, USA) as previously described (30, 31). Stained coverslips were washed and mounted with Prolong Gold antifade reagent containing DAPI (Thermo Fisher Scientific). Finally, slides were observed using fluorescent microscopy (Olympus DP80; Olympus, Tokyo, Japan) with camera software for CellSens Dimensions [Shinjuku, Tokyo, Japan]). For analysis of the mitochondrial network, experimental cells stained with the mitochondrial marker TOM20 were imaged using confocal microscopy (Olympus FluoView FV1000 confocal laser scanning microscope; Morphology and Imaging Core, Louisiana State University Health Sciences Center, New Orleans, LA, USA). An xy plane from the 3-dimensional images was subjected to tubeness analysis (σ = 0) followed by the 2-dimensional skeletonize plugin (no elimination of loops or end points; ImageJ, Image Processing and Analysis in Java; National Institutes of Health, Bethesda, MD, USA). The skeletonize imprints of mitochondria were analyzed for total branch length, and the percentage of branches shorter or longer than 5 µm per cell was calculated.
Extraction of protein and immunoblotting
Total protein was extracted from experimental cells and colon mucosa of mice as previously described (30, 31). Protein extraction from nuclear fractions was performed using the Cell Fractionation kit (Abcam, Cambridge, MA, USA) following the manufacturer’s instructions. Proteins were separated by SDS-PAGE and immunoblotted with specific antibodies: COXI was from Thermo Fisher; COXIV, total AMPK and pAMPK, AMPKα2, p65, phospho-(Ser536)-p65, IκBα (nuclear factor of κ light polypeptide gene enhancer in B-cell inhibitor, α), and β-actin were from Cell Signaling Technology. Proteins were visualized with IRDye-conjugated secondary antibodies (Li-Cor Biosciences, Lincoln, NE, USA) using the Odyssey infrared imaging system (Li-Cor Biosciences).
RNA isolation and next-generation RNA sequencing
Total RNA from colonic HCT116 and HCT116 ρ0 cells with or without TNF-α treatment (6 h) were isolated using the miRNeasy kit (Qiagen, Germantown, MD, USA), and the quality was determined by Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The samples, with RNA integrity numbers ranging from 7 to 8, were used for library preparation before RNA-seq (University of Wisconsin Biotechnology Center DNA Sequencing Facility; http://www.biotech.wisc.edu/services/dnaseq). High-quality mRNAs were enriched by poly-A selection from total RNA samples (1 μg input per sample), and library preparation was accomplished using the Illumina Truseq Stranded mRNA preparation kit (Illumina, San Diego, CA, USA). After poly-A purification, mRNA underwent fragmentation and priming for cDNA synthesis, followed by first- and second-strand cDNA synthesis and 3′ adenylation. After indexing adapters were ligated to the ends of cDNA, PCR was used to enrich DNA fragments with adapter molecules. This was followed by an Agilent DNA 1000 chip (Agilent Technologies) assay in order to confirm the cDNA library’s size and profile. Samples underwent single-end 100-bp strand-specific sequencing using an Illumina HiSeq 2500 instrument.
Differential expression analysis and ingenuity pathway analysis
Data analysis was carried out in the Tulane Cancer Center Next Generation Sequence Analysis Core using core computational resources (http://www.tulane.edu/som/cancer/research/core-facilities/cancer-crusaders). RNA-seq reads were mapped to an index containing the human reference haploid genome sequence (Genome Reference Consortium human genome build 37, GRCm37). Quantification of transcript expression from RNA-seq data was accomplished by RSEM 1.2.25 software (32). To calculate differentially expressed transcripts at the whole-gene level across different conditions, EBseq (33) was used, and lists of differentially expressed transcripts were obtained, with a false discovery rate (FDR) controlled at 0.05. These data, including design of study, have been submitted through the National Center for Biotechnology Information (NCBI) Sequence Read Archive and are available through study accession number SRP093357. Qiagen’s ingenuity pathway analysis (IPA; http://www.qiagen.com/ingenuity) was used to generate all network, function, and heat-map analyses. Transcripts input into IPA were expressed at a minimum threshold of >|1.5|-fold change relative to control HCT116 colonic cells with FDR < 0.05. For analysis of gene expression in human IBD samples, we used GSE4183 and GSE16879 data sets using NCBI GEO2R (34). Only those transcripts differentially expressed using the Benjamini and Hochberg FDR testing adjustment were used for analysis (35).
Quantitative PCR
Total RNA from experimental cells extracted with Qiazol Lysis Reagent (Qiagen) was reverse transcribed using Oligo-dT12-18 primers of the SuperScript First-Strand Synthesis System (Thermo Fisher Scientific) according to the standard protocol. The C1000 Thermal Cycler system (Bio-Rad, Hercules, CA, USA) and iQ SYBR Green DNA double-strand binding dye (iQ SYBR Green Supermix; Bio-Rad) were used for quantification of cDNA using primers specifically amplifying IL-8 (CXCL8-FOR: 5′-GTGCAGTTTTGCCAAGGAGT-3′, CXCL8-REV: 5′-CTCTGCACCCAGTTTTCCTT-3′), AMPKα1 (PRKAA1-FOR: 5′-TGTGATGGGATCTTCTATACCCC-3′, PRKAA1-REV: 5′-CCATTCATGTTCCCTGATATCTTTG-3′), AMPKα2 (PRKAA2-FOR: 5′-CTGTAAGCATGGACGGGTTG-3′, PRKAA2-REV: 5′-TTGGCATTCATGTGTGCATCC-3′), and HPRT1 (HPRT1-FOR: 5′-GACCAGTCAACAGGGGACAT-3′, HPRT1-REV: 5′-AACACTTCGTGGGGTCCTTTTC-3′) (36). The Ct method was used to determine relative levels of IL-8, AMPKα1, and AMPKα2 mRNA.
Electroporation
Semiconfluent monolayers were trypsinized, washed in serum-free medium, and resuspended to a final concentration of 2 × 107 cells/ml. The cell suspension was mixed with 200 nM small interfering RNA (siRNA) (Silencer Select PRKAA2 siRNA; Thermo Fisher Scientific) or 4 µg plasmid (pNFKB-MetLuc2-reporter vector; Takara Bio USA, Mountain View, CA, USA), transferred to a precooled Gene Pulser Cuvette (Bio-Rad; 0.4 cm electrode gap), and electroporated with a pulse of 200 V, 350 µF or 260 V, 950 µF (Gene Pulser Xcell Electroporation System; Bio-Rad). After electroporation, cells were transferred to 1 ml of medium containing 20% FBS, plated at desired densities, and collected 48 h after transfection.
NF-κB promoter luciferase activity
Cells transfected with pNFKB-MetLuc2-reporter vector (Takara Bio USA) were serum starved overnight before TNF-α treatment. Cell supernatant was collected and analyzed for secreted luciferase according to the manufacturer’s protocol (Ready-to-Glow Secreted Luciferase Assay; Takara Bio USA).
ELISA
ELISA was used to quantify intracellular IL-8 levels in cell lysates (37) according to the manufacturer’s protocol. In brief, supernatants of experimental cells lysed (with 100 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1 mM EGTA; 1 mM EDTA; 1% Triton X-100; 0.5% sodium deoxycholate; and 10 µl/ml protease inhibitor) were used with the human IL-8 ELISA kit (R&D Systems). The IL-8 values were normalized to cellular protein.
ATP synthase activity
ATP synthase–specific activity was determined in cells after freeze–thawing according to the manufacturer’s instructions (Microplate Assay Kit; Abcam). Relative specific ATP synthase activity was calculated as a ratio of ATP synthase activity and total quantity of the enzyme. When comparing ρ0 to control cells, the difference between enzyme activity with and without 1 µM oligomycin (Cayman Chemicals) reflects ATP synthase activity (38). Finally, the specific oligomycin-sensitive ATP synthase activity was displayed as the ratio of oligomycin-sensitive activity and total quantity.
ATP quantification
ATP levels were determined by luciferase-driven bioluminescence (ApoSensor Assay Kit; BioVision, Milpitas, CA, USA) according to the manufacturer’s instructions and normalized to total protein level. For in vitro experiments, cell lysates (1 × 104 cells in 60 µl nucleotide-releasing buffer) were transferred to a luminometer plate, followed by measuring luminescence with the CentroXS3 LB 960 luminometer (Berthold Technologies, Bad Wildbad, Germany). For tissue ATP level assessment, scraped colon mucosa of experimental mice was resuspended in nucleotide-releasing buffer (10 mg mucosa in 300 µl), and ATP levels were subsequently measured using the same luminescence protocol.
Animals
C57BL/6J strain mice, housed in the Biologic Resources Laboratory at Tulane University, were used in these studies. Intestinal inflammation was induced with 2.5% dextran sulfate sodium (DSS) in drinking water (MP Biomedicals, Solon, OH, USA) for 5 d. For suppression of inflammation, DSS-treated animals were injected with mouse anti-TNF-α antibody (20 pg/100 µl) (MP6-XT22; Abcam) daily for 5 d. All guidelines and experimental procedures were approved by the animal ethics committee, and procedures were performed according to approved animal care protocols.
Histologic analysis
Paraffin-embedded mouse colon tissue sections were immunohistostained after antigen retrieval with anti-COXIV (1:1000, Cell Signaling Technology) or anti-p65 (1:200, Cell Signaling Technology) after incubation with horseradish peroxidase–conjugated secondary antibody. After incubation with 3,3′-diaminobenzidine (DAB) chromogen and hematoxylin counterstain, tissue sections were dehydrated in graded alcohol and xylene, then coverslipped using Permount (Thermo Fisher Scientific). Stained tissue was imaged with the slide scanner Aperio CS2 and prepared by ImageScope software (Leica Biosystems, Buffalo Grove, IL, USA). Frozen colon tissue sections fixed with 3.7% formaldehyde were blocked with 2.5% bovine serum albumin in PBS for 1 h before immunofluorescence staining for COXI (Thermo Fisher Scientific), pAMPK (Santa Cruz Biotechnology), or macrophage inflammatory protein 2 (MIP-2; R&D Systems). After mounting with Prolong Gold antifade reagent (Thermo Fisher Scientific), sections were imaged using an Olympus DP80 fluorescence microscope. Measurements of staining intensity were achieved by ImageJ.
Statistical analysis
ANOVA and Student–Newman–Keuls posttest or unpaired Student’s t test were used for statistical analysis by GraphPad Instat 3 software (La Jolla, CA, USA). All data are shown as means ± se for a sequence of experiments, and P < 0.05 was considered to be statistically significant.
RESULTS
Colon inflammation is associated with attenuated mitochondrial activity and low ATP levels
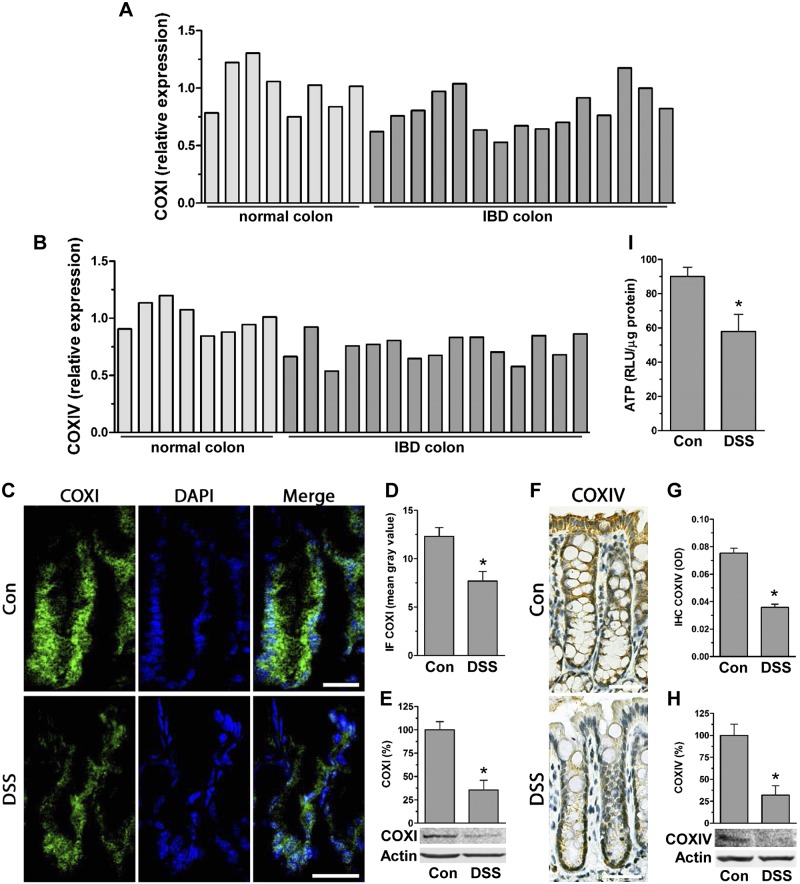
Because IBD is linked to low ATP levels in affected tissue (2), we assessed expression of the COX complex, known to be critical for ATP production (19), in human IBD patients as well as in mouse inflamed colon. Comparing microarray gene expression (GSE4183) from human IBD-affected colon against control, we found lower transcript levels of mitochondria-encoded COXI by 20 ± 8% and considerably lower nuclear-encoded COXIV by 26 ± 5% (Fig. 1A, B). In mouse inflamed colon tissue from DSS-treated animals, immunofluorescent staining revealed decreased levels of COXI and COXIV compared to control (Fig. 1C, D, F, G); these decreases were confirmed by immunoblot analysis to be 64 ± 9% for COXI and 68 ± 13% for COXIV (Fig. 1E, H). The decreases in COXI/IV levels in inflamed colon suggested attenuated mitochondrial ATP production, which was confirmed by lower mucosal ATP levels in inflamed colon of DSS-treated mice (Fig. 1I).
Figure 1.
Intestinal inflammation is associated with decreased levels of mitochondrial complexes COXI and IV. A, B) Microarray relative expression of COXI (A) and COXIV (B) in human IBD colon (GSE4183) (normal colon, n = 8; IBD colon, n = 15). P < 0.05, IBD compared to normal colon, Student’s t test. C, D) Frozen colon tissue from DSS-treated mice was immunofluorescently stained for COXI (Alexa Fluor 488; n = 4 mice). Scale bars, 40 μm. Graph shows quantification of fluorescence displayed as mean gray value (ImageJ; n = 10 crypts.) *P < 0.05 compared to control (con), Student’s t test. E) Total protein of scraped mucosa from con and DSS-treated mice was immunoblotted for COXI (densitometric analysis of n = 3). *P < 0.05, compared to con, Student’s t test. F, G) Paraffin-embedded colon tissue of mice treated with DSS was immunohistostained for COXIV (n = 4 mice). Scale bars, 40 μm. Graph represents quantification of DAB staining expressed as optical density (OD) (ImageJ; n = 20 crypts). *P < 0.05 compared to con, Student’s t test. H) Total protein extracted from scraped mucosa of DSS-treated mice was immunoblotted for COXIV (densitometric analysis of n = 3). *P < 0.05, compared to con, Students t test. I) ATP levels [relative luciferase unit (RLU)/µg protein] were quantified by luciferase driven bioluminescence in scraped mucosa of DSS-treated mice (n = 6). *P < 0.05, compared to con, Student’s t test.
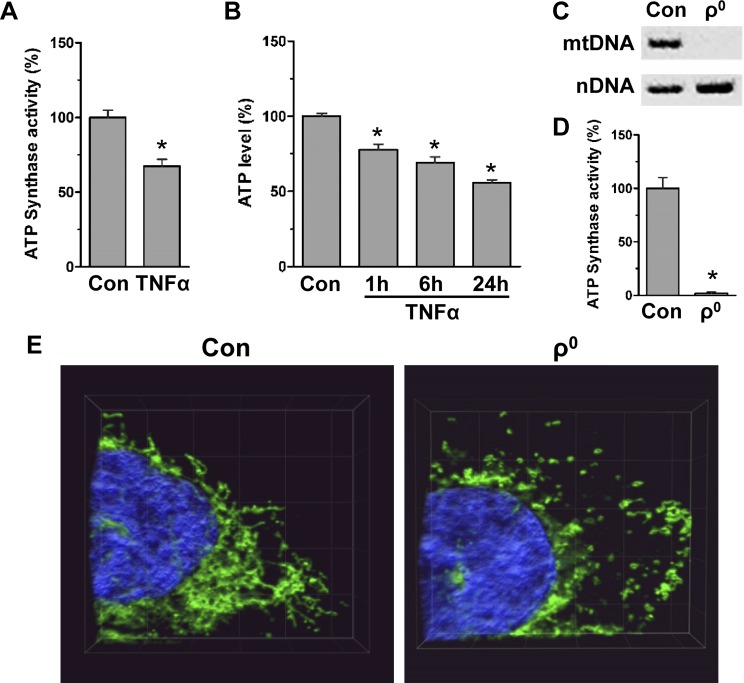
Because TNF-α is critical for driving intestinal inflammation (1), we assessed in colon cells whether TNF-α alone lowers ATP levels. We found that TNF-α attenuated ATP synthase activity by 33 ± 1% (Fig. 2A); it also progressively diminished ATP levels during 24 h of treatment (Fig. 2B). We then generated human colonic HCT116 cells with reduced mitochondrial activity (i.e., ρ0 cells, by selectively targeting mitochondrial but not nuclear DNA polymerase) (21, 22). Colonic ρ0 cell phenotype was confirmed by PCR, showing the loss of mitochondrial DNA, but not nuclear DNA, as well as low ATP synthase activity (98 ± 1%) (Fig. 2C, D). Also, because ρ0 cells have been characterized by fragmentation and disruption of the mitochondrial network (39), we immunofluorescently stained colonic ρ0 cells for TOM20, a transmembrane protein of the outer mitochondrial membrane (40), to analyze the length of mitochondrial branches. Confocal microscopy and tubeness analysis by the 2-dimensional skeletonize plug-in (ImageJ) showed decreased mitochondrial branch length in colonic ρ0 cells [control 15 ± 1% and ρ0 5 ± 1% (>5 µm), n = 40 cells] (Fig. 2E). These human colonic ρ0 cells were further utilized to determine the possible mechanisms by which reduced mitochondrial activity might promote intestinal inflammation.
Figure 2.
In colon cells, TNF-α lowers mitochondrial ATP production, and depletion of mtDNA (ρ0 cells) leads to shortened mitochondrial network. A) Specific ATP synthase activity in HT29 cells treated with TNF-α (6 h; ratio of ATP synthase activity and amount of enzyme; n = 3). *P < 0.05, compared to control (con), Student’s t test. B) ATP levels in colonic HCT116 con treated with TNF-α (1–24 h) normalized to total protein (n = 4). *P < 0.05, compared to con, ANOVA. C) Total DNA from HCT116 con and ρ0 cells was amplified by PCR with primers specific for mitochondrial DNA (mtDNA, D-Loop) and nuclear DNA (nDNA, 18S gene), followed by separation on agarose gel. D) In HCT116 con and ρ0 cells, specific oligomycin-sensitive ATP synthase activity was assessed [oligomycin-sensitive ATP synthase activity normalized to amount of enzyme (n = 3)]. *P < 0.05, compared to con, Student’s t test. E) Confocal 3-D images of HCT116 con and ρ0 cell immunofluorescently stained for mitochondrial protein TOM20 (Alexa Fluor 488; representative image of 3 independent experiments, 35 slices, 0.16 µm per slice, 5-µm grid).
In colon cells, reduced mitochondrial activity facilitates activation of inflammatory pathways
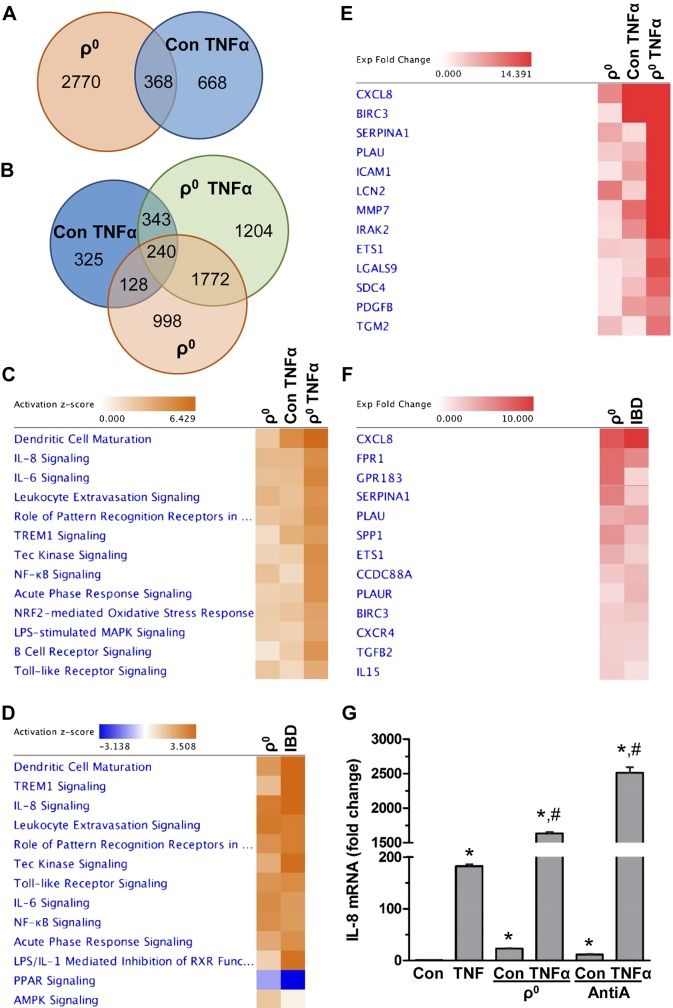
Next we examined in colon cells the effect of reduced mitochondrial function (ρ0 cells) on the TNF-α-mediated gene expression profile using RNA sequencing. As expected, we found a substantial number of differentially expressed transcripts in colonic TNF-α-treated and ρ0 cells relative to control (FDR < 0.05, EBseq) (Fig. 3A), including 240 differentially expressed transcripts that were shared among these groups (Fig. 3B). This finding suggests that reduced mitochondrial activity in colonic ρ0 cells leads to an expression profile similar to induction by inflammatory TNF-α. Pathway analysis of these transcripts showed activation of multiple pathways associated predominantly with inflammation (FDR < 0.05, IPA) including IL-8, IL-6, NF-κB, TREM1, Tec kinase, and acute phase response, as well as pathways associated with leukocytes, B cells, and dendritic cells (Fig. 3C). Detailed pathways with their differentially expressed transcripts are provided in Supplemental Unit 1. Additionally, we found a considerable similarity in transcriptional signatures and pathways between colonic ρ0 cells and human IBD tissues (GSE4183) (Fig. 3D) (FDR < 0.05, IPA). One of the top differentially expressed transcripts shared among colonic ρ0 cells, TNF-treated cells, and human IBD tissue was IL-8 (Fig. 3E, F), a cytokine critical in driving intestinal inflammation through the recruitment of inflammatory neutrophils (41, 42). Quantitative PCR (qPCR) showed that in colon cells, TNF-α-mediated increases in IL-8 expression were additionally facilitated when mitochondrial activity was reduced, as observed in ρ0 cells (by 9-fold), or after pharmacologic inhibition of the mitochondrial complex III with AntiA (by 14-fold) (Fig. 3G). These findings reveal that in colon cells, reduced mitochondrial activity leads to activation of inflammatory pathways and that IL-8 is one of the top differentially expressed transcripts.
Figure 3.
Reduced mitochondrial activity in colonic ρ0 cells promotes inflammatory pathways with IL-8 as top expressed transcript. A, B) Venn diagrams represent number of transcripts commonly or uniquely expressed in control (con) and colonic ρ0 cells without (A) or with (B) TNF-α (6 h) treatment (FDR < 0.05, EBseq). C) Top canonical pathways activated in colonic ρ0 cells and TNF-α-treated (con and ρ0) cells relative to con cells (FDR < 0.05, IPA). D) Top canonical pathways altered in ρ0 cells and human IBD colon (GSE4183) (FDR < 0.05, IPA). E) Top transcripts upregulated in inflammatory response signaling in colonic ρ0 cells and TNF-α-treated (con and ρ0) cells relative to control cells (FDR < 0.05, IPA). F) Top transcripts upregulated in inflammatory response signaling in ρ0 cells and human IBD colon (GSE4183) (FDR < 0.05, IPA). G) Total RNA was extracted from HCT116 con, ρ0 cells, or cells incubated with AntiA and basal and TNF-induced (2 h) IL-8 mRNA levels were quantified using qPCR (n = 3). *P < 0.05, compared to con; #P < 0.05 compared to TNF-α alone, ANOVA.
Moreover, in colonic ρ0 cells we found activation of pathways associated with signaling triggered by bacteria (LPS, TLR, and pattern recognition receptors in response to bacteria and viruses) (Fig. 3C), as well as dysregulation of barrier function (Supplemental Unit 1). Collectively, these findings suggest that reduced mitochondrial function stimulates activation of multiple pathways critical for intestinal epithelial function.
In colon cells, TNF-α-stimulated IL-8 expression facilitated by reduced mitochondrial activity depends on AMPKα2 activation
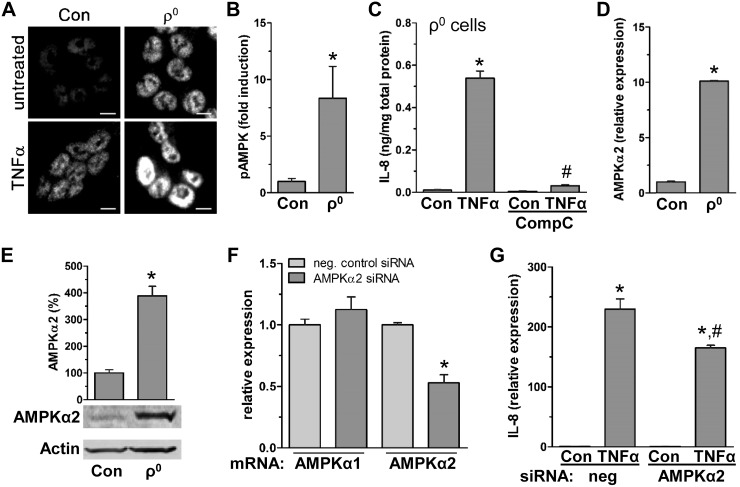
Next we assessed in colon cells the potential mechanism of how reduced mitochondrial activity facilitates TNF-α-induced IL-8 expression. Pathway analysis comparing human colonic ρ0 cells and human IBD colon tissues showed activation of the AMPK pathway (Fig. 3D), which is critical for regulation of cellular energy (43). In colonic ρ0 cells, we found 19 differentially expressed transcripts associated with activation of the AMPK pathway (Supplemental Units 2 and 3). In TNF-α-treated colon cells, immunofluorescent staining revealed increased AMPK phosphorylation, which was additionally augmented 8-fold (pAMPK/AMPK ratio) with reduction of mitochondrial function in ρ0 cells (Fig. 4A, B). Pharmacologic inhibition of AMPK with CompC blocked TNF-α induced IL-8 expression (Fig. 4C), supporting dependency of IL-8 production on TNF-α-stimulated AMPK activity. Specifically, we found AMPK-dependent IL-8 expression was mediated by AMPKα2, a subunit known for enhanced sensitivity to low ATP levels relative to other subunits (43). In colonic ρ0 cells, increases in both AMPKα1 (2-fold, data not shown) and AMPKα2 were observed, but increases in AMPKα2 transcripts and protein (8- and 4-fold, respectively) were more profound (Fig. 4D, E). Additionally, siRNA silencing of AMPKα2 (reduction of 47 ± 6%) attenuated TNF-α induced IL-8 production by 28 ± 4% (Fig. 4F, G). These findings indicate that in colon cells, reduced mitochondrial activity facilitates TNF-stimulated IL-8 expression in part via AMPKα2 activation.
Figure 4.
TNF-α induced IL-8 is facilitated by reduced mitochondrial function via increased AMPK activity. A) HCT116 control (con) and ρ0 cells treated with TNF-α (15 min) were immunofluorescently stained for pAMPK (Alexa Fluor 594; representative image of 3 independent experiments). Scale bars, 5 µm. B) TNF-α-induced fold increase in ratio of pAMPK to total AMPK after immunoblotting of total protein from HCT116 con and ρ0 cells treated with TNF-α (15 min) (densitometric analysis; n = 3). *P < 0.05, compared to con, Student’s t test. C) In ρ0 cells, intracellular IL-8 was measured in presence of CompC after 4 h TNF-α treatment by ELISA (n = 3). *P < 0.05, compared to con; #P < 0.05compared to TNF-α alone, ANOVA. D, E) In HCT116 con and ρ0 cells, AMPKα2 subunit was quantified by qPCR (D) and immunoblot (E) (n = 3). *P < 0.05 compared to con, Student’s t test. F, G) As a control for specific silencing of AMPKα2, AMPKα subunits were quantified by qPCR (F), and IL-8 was quantified by qPCR in HCT116 con cells transfected with negative con (neg) and AMPKα2 siRNA in presence of TNF-α (2 h; n = 3) (G). *P < 0.05, compared to con; #P < 0.05 compared to TNF-α alone, Student’s t test, ANOVA.
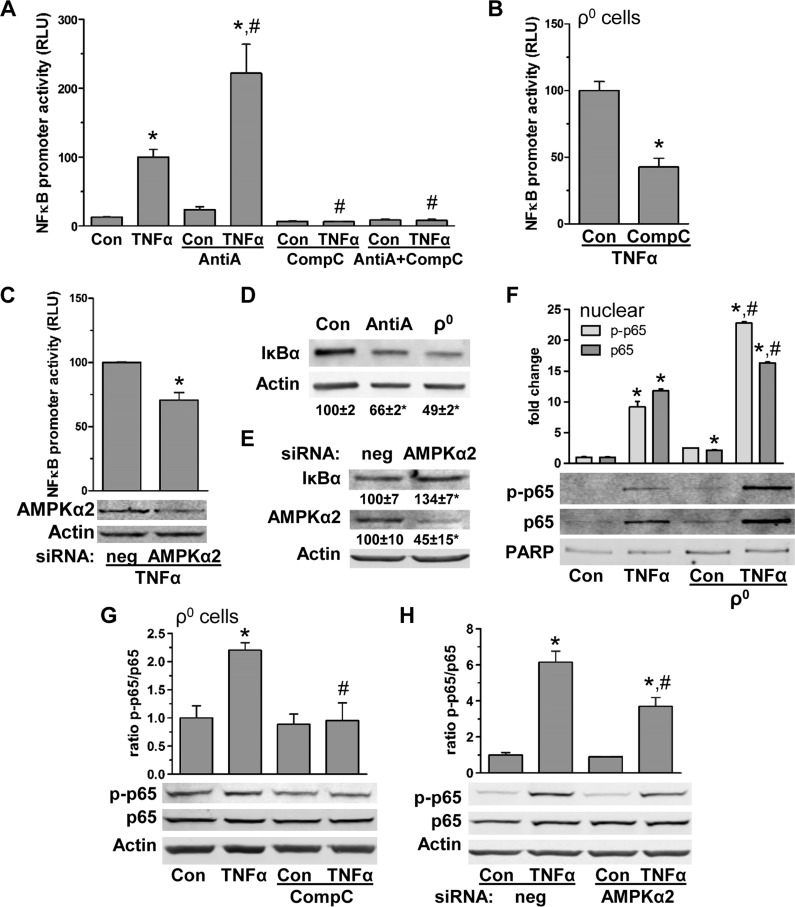
In colon cells, TNF-α promotes NF-κB transcription activity via reduced mitochondrial activity-mediated AMPKα2 activation
As IL-8 transcription is mainly controlled by NF-κB (44), we assessed if reduced mitochondrial activity facilitates IL-8 production in part via AMPKα2-dependent NF-κB activation. First, we found that in colon cells, TNF-α-induced NF-κB-dependent luciferase activity was enhanced with reduction in mitochondrial function (AntiA treatment) (Fig. 5A). However, TNF-α-facilitated NF-κB luciferase activity with reduced mitochondrial function was inhibited with AMPK blockade (CompC) (Fig. 5A). Similar results were found in control and ρ0 cells (Fig. 5B, C), supporting the notion that IL-8 expression depends in part on reduced mitochondrial activity, leading to AMPK-dependent enhancement of NF-κB activity. We next found that NF-κB inhibitory protein IκBα levels were decreased in colon cells with AntiA treatment by 34 ± 1% and in ρ0 cells by 51 ± 2% (Fig. 5D). Moreover, in control colon cells, AMPKα2 silencing led to increased IκBα levels (Fig. 5E), supporting dependency of IκBα on reduced mitochondrial function mediated by AMPKα2 activation. Additionally, we found that nuclear levels of basal and TNF-α-induced NF-κB subunit p65, total and phosphorylated, were elevated by 2- and 2.5-fold, respectively, after reduction of mitochondrial function (Fig. 5F). Blockade of AMPKα2 (CompC or siRNA) reversed this effect on p65 in control and ρ0 cells (Fig. 5G, H). These data demonstrate that in colon cells, reduced mitochondrial function facilitates IL-8 by promoting NF-κB activity via low levels of cytosolic IκBα, leading to increases in nuclear p65 mediated by AMPKα2 activation.
Figure 5.
In colon cells, TNF-α-stimulated NF-κB depends on reduced mitochondrial function leading to AMPK activation. A) Luciferase activity (relative luciferase unit, RLU) driven by NF-κB promoter in HCT116 control (con) cells transfected with pNFKB-MetLuc2-reporter vector with and without TNF-α (4 h) treatment in presence of AntiA and CompC (n = 3). *P < 0.05, compared to con; #P < 0.05 compared to TNF-α alone, ANOVA. B) Colonic ρ0 cells were transfected with pNFKB-MetLuc2-reporter vector and treated with TNF-α (4 h) with and without CompC; relative luciferase activity (RLU) (n = 3). *P < 0.05, compared to con, Student’s t test. C) HCT116 con cells were transfected with pNFKB-MetLuc2-reporter vector and siRNA for AMPKα2; TNF-induced (4 h) relative luciferase activity (RLU) (n = 3). Total protein of negative con (neg) and AMPKα2 siRNA transfected cells was immunoblotted for AMPKα2. *P < 0.05 compared to con, Student’s t test. D) In HCT116 con cells in presence of AntiA and ρ0 cells, IκBα protein levels were assessed by immunoblotting (densitometric analysis of n = 3). *P < 0.05; *P < 0.05 compared to con, ANOVA. E) In HCT116 con cells transfected with negative con (neg) and AMPKα2 siRNA, total protein was analyzed for IκBα and AMPKα2 (densitometric analysis of n = 3). *P < 0.05, compared to con, Student’s t test. F) Nuclear protein fraction of HCT116 con and ρ0 cells stimulated with TNF-α (15 min) were immunoblotted for phosphorylated p65 (p-p65), total p65, and poly(ADP-ribose)polymerase (PARP) as loading con (densitometric analysis of n = 3). *P < 0.05, compared to con, #P < 0.05 compared to TNF-α alone, ANOVA. G) Total protein of HCT116 ρ0 cells treated with TNF-α (15 min) in presence of CompC were immunoblotted for phosphorylated p65 (p-p65) and total p65 (densitometric analysis of n = 3). *P < 0.05, compared to con; #P < 0.05 compared to TNF-α alone, ANOVA. H) HCT116 con cells transfected with negative control (neg) and AMPKα2 siRNA, were treated with TNF-α (15 min) and total protein was immunoblotted for phosphorylated p65 (p-p65) and total p65 and (densitometric analysis of n = 3). *P < 0.05, compared to con; #P < 0.05 compared to TNF-α alone, ANOVA.
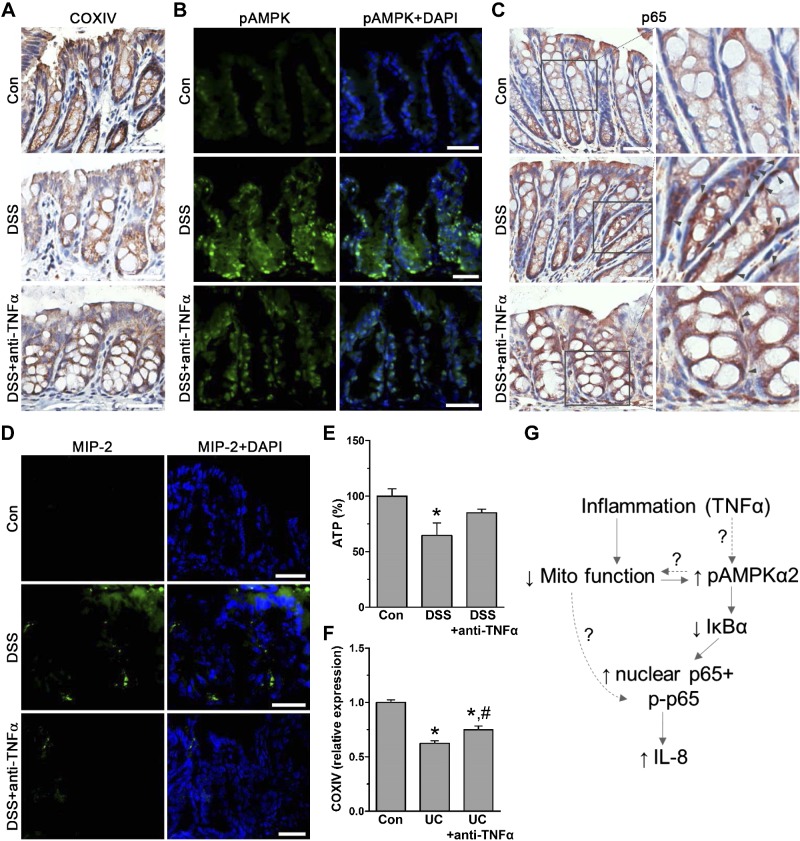
Last, we assessed in inflamed mouse and human colon if the pathways triggered by reduced mitochondrial function could be reversed by anti-TNF-α treatment. We found in inflamed colon of DSS-treated mice reduced COXIV, increased AMPK phosphorylation, nuclear p65 presence, and elevated levels of the IL-8 ortholog MIP-2 compared to normal tissue (Fig. 6A–D). After anti-TNF-α treatment, mitochondrial COXIV and ATP levels, as well as decreases in AMPK phosphorylation, nuclear p65, and MIP-2, were recovered (Fig. 6A–E). Ultimately, when we compared COXIV microarray expression (GSE16879) in colon of human IBD (ulcerative colitis) patients against control, we found that anti-TNF therapy attenuated COXIV decreases in IBD-affected colon (Fig. 6F). These data support the physiologic relevance of this novel mechanism involving reduced mitochondrial function associated with ATP production–dependent intestinal inflammation (Fig. 6G).
Figure 6.
In human and mouse colon, anti-TNF-α treatment restores reduced mitochondrial dependent inflammation. A) Immunohistostaining for COXIV in paraffin-embedded colon tissue from control (con) and DSS-treated mice with or without anti-TNF-α (n = 4 mice). Scale bars, 40 μm. B) Immunofluorescence staining of frozen colon tissue for pAMPK of con and DSS-treated mice with or without anti-TNF-α (Alexa Fluor 488; n = 4 mice). Scale bars, 20 μm. C) Immunohistostaining for NF-κB subunit p65 of paraffin-embedded colon tissue from con and DSS-treated mice with or without anti-TNF-α. Magnified area shows nuclear p65 localization (gray arrows; n = 4 mice). Scale bars, 40 μm. D) Immunofluorescently stained frozen colon tissue of DSS and anti-TNF-α-treated mice for IL-8 ortholog MIP-2 (Alexa Fluor 488; n = 4 mice). Scale bars, 20 μm. E) ATP levels in scraped mucosa of con, DSS, and anti-TNF-α-treated mice (n = 6). *P < 0.05, compared to con, ANOVA. F) Microarray expression of COXIV relative to con (n = 6) in human ulcerative colitis (UC) biopsy samples before (n = 24) and 6 wk after anti-TNF-α treatment (n = 24; GSE16879). *P < 0.05, compared to con; #P < 0.05 compared to UC alone, ANOVA. G) Proposed regulatory mechanism of colon inflammation dependent on reduced mitochondrial function leading to AMPKα2 activity.
DISCUSSION
IBD pathophysiology, while multifaceted, is associated with altered mitochondrial function as a contributing factor in maintaining the disease (3, 16, 17, 45, 46). However, it is unclear how reduced mitochondrial function associated with low ATP production could drive intestinal inflammation. Here we demonstrated in human and mouse inflamed colon tissue decreased levels of mitochondrial COXI/IV complex and lower ATP levels. In colon cells with reduced mitochondrial activity (ρ0 cells), RNA-seq and pathway analysis revealed activation of inflammatory pathways similar to those seen in human IBD tissues and colon cells stimulated with TNF-α, with IL-8 being one of the most abundantly expressed transcripts. We found that TNF-α-mediated IL-8 expression is dependent on reduced mitochondrial activity via AMPKα2-dependent NF-κB transcription activation. Anti-TNF-α treatment restored this mitochondrial axis in human and mouse inflamed colon. These findings highlight a novel mechanism of intestinal inflammation mediated by reduced mitochondrial activity.
We found that in colon cells, intestinal inflammation is facilitated by reduced mitochondrial energy production. Impairment of the oxphos system, while not well studied, has been implicated to underlie IBD pathophysiology. IBD is associated with polymorphisms in the mitochondrial gene UCP2-866 G/A (rs659366) (3), which has been linked with stronger immunoresponse to infections in mouse models deficient for this gene (47, 48). On the other hand, a polymorphism in the mt-tRNAArg gene that enhances mitochondrial oxphos complex activity, leading to elevated ATP levels, protects against intestinal inflammation (4). Yu et al. (3) speculated that dysregulation in mitochondria leading to ROS production or low ATP synthesis may contribute to the pathophysiology driving intestinal inflammation. However, colonic ρ0 cells are unable to produce ROS (24), but they still exhibit activation of inflammatory pathways, supporting the role of diminished oxphos in driving intestinal inflammation. This is supported by findings that show that decreased levels of PGC1α, a master regulator of mitochondrial biogenesis, promote intestinal inflammation and lower mtDNA, similar to colonic ρ0 cells (49). Cunningham et al. (49) suggested that reduced mitochondrial activity could facilitate intestinal inflammation via breakdown of the intestinal barrier, subsequently causing bacterial translocation. Because increased ATP levels provide energy to support epithelial cell proliferation, which is required for healing of inflammation-mediated injury (4, 18), it is plausible that this is one of the roles of increased mitochondrial oxphos in protecting the intestine. Nevertheless, our findings reveal that reduced mitochondrial activity directly activates not only inflammatory pathways, but also those triggered by bacterial products, highlighting a direct role for mitochondria in sensitizing the intestine to commensal bacteria and exacerbating inflammation. Moreover, because reduced mitochondrial activity leads to activation of inflammatory pathways due to attenuated oxphos activity, it is important to take into account the possibility of compensatory glycolysis (50) as a contributing factor.
These findings demonstrate that intestinal inflammation is mediated in part by reduced mitochondrial activity, leading to activation of AMPKα2 and consequently facilitating NF-κB-dependent IL-8 expression. In human IBD colon tissues, we observed concurrent reduction in mitochondrial oxphos protein and activation of AMPK pathways. The activity of AMPK and oxphos are interconnected, as low mitochondrial activity (e.g., elevated AMP/ATP ratio) leads to AMPK activation, which could in turn stimulate oxphos ATP production (43). Thus, it is reasonable that in colon cells, reduced mitochondrial activity leads to AMPK activation; yet, if mitochondria are unable to produce ATP in return, AMPK overactivation could potentially facilitate increased NF-κB activity. In lung epithelia, activation of AMPK leads to increases in cytokine levels, while in keratinocytes it has the opposite effect (51–53). We speculate that AMPK could have a tissue-specific role and that its proinflammatory role could be exerted in cells with mitochondrial dysfunction. Moreover, activated AMPK mediates the negative effects of cytokines on intestinal barrier function (54), supporting its diverse roles in driving intestinal inflammation. Several studies showed that AMPK activation with pharmacologic compounds such as 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), 6-gingerol, and metformin attenuated intestinal inflammation in mice, in part by protecting barrier function (55–57). However, these compounds have various effects on other cellular pathways (58) and are used for extended periods of time in animal models, suggesting that they might exert AMPK-independent effects. Also, it is plausible that different AMPK subunits might have unique effects on signaling pathways, suggesting that the AMPKα2 subunit mediates NF-κB activation as a mechanism that in colon cells is utilized by reduced mitochondrial activity to promote cytokine production.
Mitochondria have returned to the forefront of research because of their roles in apoptosis and in the metabolic reprogramming of cancer cells (59, 60). Defects in genes encoding mitochondrial oxphos protein lead to mitochondrial disorders, including mitochondrial myopathy, diabetes mellitus and deafness, Leigh syndrome, neuropathy, and myoneurogenic gastrointestinal encephalopathy (61). Emerging findings reveal that even a functional polymorphism in a mitochondrial gene leading to low oxphos activity is associated with inflammatory diseases including multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus, Wegener granulomatosis, Churg-Strauss syndrome, Crohn’s disease, and ulcerative colitis (3). These associations indicate that active mitochondria are protective against chronic inflammatory diseases. Here we demonstrated that in the intestine, reduced mitochondrial activity causes activation of pathways associated with inflammation through an AMPK-mediated mechanism. These findings provide insights into the novel mechanisms involved in driving intestinal inflammation; they also identify mitochondrial oxphos as a potential and novel therapeutic target for IBD treatment.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a U.S. National Institutes of Health, National Cancer Institute RO1 award (CA160809). The authors thank the University of Wisconsin Biotechnology Center DNA Sequencing Facility for providing next-generation sequencing facilities and services.
Glossary
- AntiA
antimycin A
- CompC
compound C
- COX
cytochrome c oxidase
- DAB
3,3′-diaminobenzidine
- DSS
dextran sulfate sodium
- FBS
fetal bovine serum
- FDR
false discovery rate
- IBD
inflammatory bowel disease
- IPA
ingenuity pathway analysis
- IκBα
nuclear factor of κ light polypeptide gene enhancer in B-cell inhibitor, α
- MIP-2
macrophage inflammatory protein 2
- mtDNA
mitochondrial DNA
- NCBI
National Center for Biotechnology Information
- oxphos
oxidative phosphorylation
- pAMPK
phosphorylated AMPK
- qPCR
quantitative PCR
- RNA-seq
RNA sequencing
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- TOM20
translocase of outer membrane
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Heller and S. D. Savkovic conceived and designed the research; S. Heller, H. Penrose, and C. Cable performed experiments; S. Heller, H. Penrose, H. Nakhoul, M. Baddoo, and S. D. Savkovic analyzed the data; S. Heller and H. M. Penrose prepared the figures; S. Heller, D. Biswas, S. E. Crawford, E. Flemington, and S. D. Savkovic interpreted the results; and S. D. Savkovic wrote the article.
REFERENCES
- 1.Geremia A., Biancheri P., Allan P., Corazza G. R., Di Sabatino A. (2014) Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 13, 3–10 [DOI] [PubMed] [Google Scholar]
- 2. Roediger W. E. (1980) The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet 2(8197), 712–715 [DOI] [PubMed] [Google Scholar]
- 3.Yu X., Wieczorek S., Franke A., Yin H., Pierer M., Sina C., Karlsen T. H., Boberg K. M., Bergquist A., Kunz M., Witte T., Gross W. L., Epplen J. T., Alarcón-Riquelme M. E., Schreiber S., Ibrahim S. M. (2009) Association of UCP2-866 G/A polymorphism with chronic inflammatory diseases. Genes Immun. 10, 601–605 [DOI] [PubMed] [Google Scholar]
- 4.Bar F., Bochmann W., Widok A., von Medem K., Pagel R., Hirose M., Yu X., Kalies K., Konig P., Bohm R., Herdegen T., Reinicke A. T., Buning J., Lehnert H., Fellermann K., Ibrahim S., Sina C. (2013) Mitochondrial gene polymorphisms that protect mice from colitis. Gastroenterology 145, 1055–1063e3 [DOI] [PubMed] [Google Scholar]
- 5.Kowaltowski A. J., de Souza-Pinto N. C., Castilho R. F., Vercesi A. E. (2009) Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 47, 333–343 [DOI] [PubMed] [Google Scholar]
- 6.Beltrán B., Nos P., Dasí F., Iborra M., Bastida G., Martínez M., O’Connor J. E., Sáez G., Moret I., Ponce J. (2010) Mitochondrial dysfunction, persistent oxidative damage, and catalase inhibition in immune cells of naïve and treated Crohn’s disease. Inflamm. Bowel Dis. 16, 76–86 [DOI] [PubMed] [Google Scholar]
- 7.Kmieć Z. (1998) Cytokines in inflammatory bowel disease. Arch. Immunol. Ther. Exp. (Warsz.) 46, 143–155 [PubMed] [Google Scholar]
- 8.Li J., Yin Q., Wu H. (2013) Structural basis of signal transduction in the TNF receptor superfamily. Adv. Immunol. 119, 135–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buffinton G. D., Doe W. F. (1995) Altered ascorbic acid status in the mucosa from inflammatory bowel disease patients. Free Radic. Res. 22, 131–143 [DOI] [PubMed] [Google Scholar]
- 10.Oz H. S., Chen T. S., McClain C. J., de Villiers W. J. (2005) Antioxidants as novel therapy in a murine model of colitis. J. Nutr. Biochem. 16, 297–304 [DOI] [PubMed] [Google Scholar]
- 11.Kepp O., Galluzzi L., Kroemer G. (2011) Mitochondrial control of the NLRP3 inflammasome. Nat. Immunol. 12, 199–200 [DOI] [PubMed] [Google Scholar]
- 12.Dashdorj A., Jyothi K. R., Lim S., Jo A., Nguyen M. N., Ha J., Yoon K. S., Kim H. J., Park J. H., Murphy M. P., Kim S. S. (2013) Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 11, 178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garg A. K., Aggarwal B. B. (2002) Reactive oxygen intermediates in TNF signaling. Mol. Immunol. 39, 509–517 [DOI] [PubMed] [Google Scholar]
- 14.Kim I., Rodriguez-Enriquez S., Lemasters J. J. (2007) Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 462, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardet A., Xavier R. J. (2012) Common alleles that influence autophagy and the risk for inflammatory bowel disease. Curr. Opin. Immunol. 24, 522–529 [DOI] [PubMed] [Google Scholar]
- 16.Hsieh S. Y., Shih T. C., Yeh C. Y., Lin C. J., Chou Y. Y., Lee Y. S. (2006) Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics 6, 5322–5331 [DOI] [PubMed] [Google Scholar]
- 17.Shkoda A., Werner T., Daniel H., Gunckel M., Rogler G., Haller D. (2007) Differential protein expression profile in the intestinal epithelium from patients with inflammatory bowel disease. J. Proteome Res. 6, 1114–1125 [DOI] [PubMed] [Google Scholar]
- 18.Podolsky D. K. (1997) Healing the epithelium: solving the problem from two sides. J. Gastroenterol. 32, 122–126 [DOI] [PubMed] [Google Scholar]
- 19.Hatefi Y. (1985) The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 54, 1015–1069 [DOI] [PubMed] [Google Scholar]
- 20.Boekema E. J., Braun H. P. (2007) Supramolecular structure of the mitochondrial oxidative phosphorylation system. J. Biol. Chem. 282, 1–4 [DOI] [PubMed] [Google Scholar]
- 21.King M. P., Attardi G. (1989) Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246, 500–503 [DOI] [PubMed] [Google Scholar]
- 22.Meyer R. R., Simpson M. V. (1969) DNA biosynthesis in mitochondria. Differential inhibition of mitochondrial and nuclear DNA polymerases by the mutagenic dyes ethidium bromide and acriflavin. Biochem. Biophys. Res. Commun. 34, 238–244 [DOI] [PubMed] [Google Scholar]
- 23.Arnould T., Mercy L., Houbion A., Vankoningsloo S., Renard P., Pascal T., Ninane N., Demazy C., Raes M. (2003) mtCLIC is up-regulated and maintains a mitochondrial membrane potential in mtDNA-depleted L929 cells. FASEB J. 17, 2145–2147 [DOI] [PubMed] [Google Scholar]
- 24.Bell E. L., Klimova T. A., Eisenbart J., Moraes C. T., Murphy M. P., Budinger G. R., Chandel N. S. (2007) The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. 177, 1029–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szczepanowska J., Malinska D., Wieckowski M. R., Duszynski J. (2012) Effect of mtDNA point mutations on cellular bioenergetics. Biochim. Biophys. Acta 1817, 1740–1746 [DOI] [PubMed] [Google Scholar]
- 26.Sidoti-de Fraisse C., Rincheval V., Risler Y., Mignotte B., Vayssière J. L. (1998) TNF-alpha activates at least two apoptotic signaling cascades. Oncogene 17, 1639–1651 [DOI] [PubMed] [Google Scholar]
- 27.Chevallet M., Lescuyer P., Diemer H., van Dorsselaer A., Leize-Wagner E., Rabilloud T. (2006) Alterations of the mitochondrial proteome caused by the absence of mitochondrial DNA: a proteomic view. Electrophoresis 27, 1574–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li K., Neufer P. D., Williams R. S. (1995) Nuclear responses to depletion of mitochondrial DNA in human cells. Am. J. Physiol. 269, C1265–C1270 [DOI] [PubMed] [Google Scholar]
- 29.Heller S., Schubert S., Krehan M., Schäfer I., Seibel M., Latorre D., Villani G., Seibel P. (2013) Efficient repopulation of genetically derived rho zero cells with exogenous mitochondria. PLoS One 8, e73207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snoeks L., Weber C. R., Turner J. R., Bhattacharyya M., Wasland K., Savkovic S. D. (2008) Tumor suppressor Foxo3a is involved in the regulation of lipopolysaccharide-induced interleukin-8 in intestinal HT-29 cells. Infect. Immun. 76, 4677–4685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snoeks L., Weber C. R., Wasland K., Turner J. R., Vainder C., Qi W., Savkovic S. D. (2009) Tumor suppressor FOXO3 participates in the regulation of intestinal inflammation. Lab. Invest. 89, 1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B., Dewey C. N. (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leng N., Dawson J. A., Thomson J. A., Ruotti V., Rissman A. I., Smits B. M., Haag J. D., Gould M. N., Stewart R. M., Kendziorski C. (2013) EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 29, 1035–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett T., Wilhite S. E., Ledoux P., Evangelista C., Kim I. F., Tomashevsky M., Marshall K. A., Phillippy K. H., Sherman P. M., Holko M., Yefanov A., Lee H., Zhang N., Robertson C. L., Serova N., Davis S., Soboleva A. (2013) NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 41, D991–D995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoav B., Hochberg J. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodological) 57, 289–300 [Google Scholar]
- 36.Zhang X., Ding L., Sandford A. J. (2005) Selection of reference genes for gene expression studies in human neutrophils by real-time PCR. BMC Mol. Biol. 6, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heller S., Cable C., Penrose H., Makboul R., Biswas D., Cabe M., Crawford S. E., Savkovic S. D. (2016) Intestinal inflammation requires FOXO3 and prostaglandin E2 dependent lipogenesis and elevated lipid droplets. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G844–G854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García J. J., Ogilvie I., Robinson B. H., Capaldi R. A. (2000) Structure, functioning, and assembly of the ATP synthase in cells from patients with the T8993G mitochondrial DNA mutation. Comparison with the enzyme in Rho(0) cells completely lacking mtDNA. J. Biol. Chem. 275, 11075–11081 [DOI] [PubMed] [Google Scholar]
- 39.Kukat A., Kukat C., Brocher J., Schäfer I., Krohne G., Trounce I. A., Villani G., Seibel P. (2008) Generation of rho0 cells utilizing a mitochondrially targeted restriction endonuclease and comparative analyses. Nucleic Acids Res. 36, e44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lister R., Hulett J. M., Lithgow T., Whelan J. (2005) Protein import into mitochondria: origins and functions today [review] Mol. Membr. Biol. 22, 87–100 [DOI] [PubMed] [Google Scholar]
- 41.Harada A., Sekido N., Akahoshi T., Wada T., Mukaida N., Matsushima K. (1994) Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 56, 559–564 [PubMed] [Google Scholar]
- 42.Kucharzik T., Hudson J. T. III, Lügering A., Abbas J. A., Bettini M., Lake J. G., Evans M. E., Ziegler T. R., Merlin D., Madara J. L., Williams I. R. (2005) Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut 54, 1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardie D. G., Ross F. A., Hawley S. A. (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mukaida N., Hishinuma A., Zachariae C. O., Oppenheim J. J., Matsushima K. (1991) Regulation of human interleukin 8 gene expression and binding of several other members of the intercrine family to receptors for interleukin-8. Adv. Exp. Med. Biol. 305, 31–38 [DOI] [PubMed] [Google Scholar]
- 45.Shekhawat P. S., Srinivas S. R., Matern D., Bennett M. J., Boriack R., George V., Xu H., Prasad P. D., Roon P., Ganapathy V. (2007) Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2(−/−)) mice. Mol. Genet. Metab. 92, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H., Kuai X. Y., Yu P., Lin L., Shi R. (2012) Protective role of uncoupling protein-2 against dextran sodium sulfate–induced colitis. J. Gastroenterol. Hepatol. 27, 603–608 [DOI] [PubMed] [Google Scholar]
- 47.Arsenijevic D., Onuma H., Pecqueur C., Raimbault S., Manning B. S., Miroux B., Couplan E., Alves-Guerra M. C., Goubern M., Surwit R., Bouillaud F., Richard D., Collins S., Ricquier D. (2000) Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 26, 435–439 [DOI] [PubMed] [Google Scholar]
- 48.Vogler S., Pahnke J., Rousset S., Ricquier D., Moch H., Miroux B., Ibrahim S. M. (2006) Uncoupling protein 2 has protective function during experimental autoimmune encephalomyelitis. Am. J. Pathol. 168, 1570–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cunningham K. E., Vincent G., Sodhi C. P., Novak E. A., Ranganathan S., Egan C. E., Stolz D. B., Rogers M. B., Firek B., Morowitz M. J., Gittes G. K., Zuckerbraun B. S., Hackam D. J., Mollen K. P. (2016) Peroxisome proliferator-activated receptor-γ coactivator 1-α (PGC1α) protects against experimental murine colitis. J. Biol. Chem. 291, 10184–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samavati L., Lee I., Mathes I., Lottspeich F., Hüttemann M. (2008) Tumor necrosis factor alpha inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J. Biol. Chem. 283, 21134–21144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang G. J., Wang H. Y., Wang J. Y., Lee C. C., Tseng H. W., Wu Y. L., Shyue S. K., Lee T. S., Kou Y. R. (2011) Novel role of AMP-activated protein kinase signaling in cigarette smoke induction of IL-8 in human lung epithelial cells and lung inflammation in mice. Free Radic. Biol. Med. 50, 1492–1502 [DOI] [PubMed] [Google Scholar]
- 52.Park D. W., Jiang S., Liu Y., Siegal G. P., Inoki K., Abraham E., Zmijewski J. W. (2014) GSK3β-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 307, L735–L745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barroso E., Eyre E., Palomer X., Vázquez-Carrera M. (2011) The peroxisome proliferator–activated receptor β/δ (PPARβ/δ) agonist GW501516 prevents TNF-α-induced NF-κB activation in human HaCaT cells by reducing p65 acetylation through AMPK and SIRT1. Biochem. Pharmacol. 81, 534–543 [DOI] [PubMed] [Google Scholar]
- 54.Scharl M., Paul G., Barrett K. E., McCole D. F. (2009) AMP-activated protein kinase mediates the interferon-gamma-induced decrease in intestinal epithelial barrier function. J. Biol. Chem. 284, 27952–27963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai A., Yong M., Ma A. G., Ma Y., Weiss C. R., Guan Q., Bernstein C. N., Peng Z. (2010) Novel anti-inflammatory action of 5-aminoimidazole-4-carboxamide ribonucleoside with protective effect in dextran sulfate sodium-induced acute and chronic colitis. J. Pharmacol. Exp. Ther. 333, 717–725 [DOI] [PubMed] [Google Scholar]
- 56.Chang K. W., Kuo C. Y. (2015) 6-Gingerol modulates proinflammatory responses in dextran sodium sulfate (DSS)-treated Caco-2 cells and experimental colitis in mice through adenosine monophosphate-activated protein kinase (AMPK) activation. Food Funct. 6, 3334–3341 [DOI] [PubMed] [Google Scholar]
- 57.Koh S. J., Kim J. M., Kim I. K., Ko S. H., Kim J. S. (2014) Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J. Gastroenterol. Hepatol. 29, 502–510 [DOI] [PubMed] [Google Scholar]
- 58.Rena G., Pearson E. R., Sakamoto K. (2013) Molecular mechanism of action of metformin: old or new insights? Diabetologia 56, 1898–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pagliarini D. J., Rutter J. (2013) Hallmarks of a new era in mitochondrial biochemistry. Genes Dev. 27, 2615–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim A. (2015) Mitochondria in cancer energy metabolism: culprits or bystanders? Toxicol. Res. 31, 323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smeitink J. A., Zeviani M., Turnbull D. M., Jacobs H. T. (2006) Mitochondrial medicine: a metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab. 3, 9–13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.