Abstract
High-risk neuroblastoma is characterized by undifferentiated neuroblasts and low schwannian stroma content. The tumor stroma contributes to the suppression of tumor growth by releasing soluble factors that promote neuroblast differentiation. Here we identify heparin-binding epidermal growth factor–like growth factor (HBEGF) as a potent prodifferentiating factor in neuroblastoma. HBEGF mRNA expression is decreased in human neuroblastoma tumors compared with benign tumors, with loss correlating with decreased survival. HBEGF protein is expressed only in stromal compartments of human neuroblastoma specimens, with tissue from high-stage disease containing very little stroma or HBEGF expression. In 3 human neuroblastoma cell lines (SK-N-AS, SK-N-BE2, and SH-SY5Y), soluble HBEGF is sufficient to promote neuroblast differentiation and decrease proliferation. Heparan sulfate proteoglycans and heparin derivatives further enhance HBEGF-induced differentiation by forming a complex with the epidermal growth factor receptor, leading to activation of the ERK1/2 and STAT3 pathways and up-regulation of the inhibitor of DNA binding transcription factor. These data support a role for loss of HBEGF in the neuroblastoma tumor microenvironment in neuroblastoma pathogenesis.—Gaviglio, A. L., Knelson, E. H., Blobe, G. C. Heparin-binding epidermal growth factor-like growth factor promotes neuroblastoma differentiation.
Keywords: HBEGF, heparan sulfate, cancer, signaling, stroma
Neuroblastoma (NB), the most common cancer in infancy, arises from neural crest–derived sympathoadrenal precursor cells (1). The 5-yr survival rate for patients with low-stage disease is approximately 90% with some tumors regressing spontaneously, whereas less than 50% of patients with high-stage disease survive due to disease relapse and the persistence of residual tumor cells after cytotoxic therapy (1, 2). High-stage tumors are often poorly differentiated and stroma poor, and many have MYCN amplification (1). Differentiation therapy with retinoic acid is part of the standard of care in NB and significantly prolongs survival when administered after myeloablative chemotherapy and bone marrow transplant (3, 4). However, many patients with NB either do not respond to retinoic acid or develop resistance to its differentiating effects, highlighting the importance of understanding mechanisms that regulate neuroblast differentiation.
The schwannian stroma is known to secrete soluble factors that promote neuroblast differentiation (5, 6). We recently demonstrated that heparan sulfate proteoglycans (HSPGs) and their soluble ectodomains released from the stroma can promote basic fibroblast growth factor (FGF)2-induced neuroblast differentiation to suppress tumor growth via enhanced ERK1/2 signaling and downstream up-regulation of the transcription factor inhibitor of DNA binding 1 (ID1) (7, 8). FGF2 is a heparan sulfate–binding ligand that binds to glycosaminoglycan modifications on HSPGs to mediate downstream signaling and biology. Interestingly, treatment with 2-O, 3-O desulfated heparin (ODSH), a selectively desulfated heparin that is structurally similar to HSPGs, enhanced FGF2-induced differentiation, leading to suppressed tumor growth and metastasis in xenograft models of NB (8).
The epidermal growth factor (EGF) family of ligands consists of 11 members that share structural and functional characteristics. Three of these members, heparin-binding EGF-like growth factor (HBEGF) (9, 10), amphiregulin (AREG) (11), and neuregulin (NRG)-1 (12, 13), bind to their respective ERBB family receptors, as well as heparan sulfate moieties, to trigger distinct biologic consequences. Previous data from our lab demonstrate that HBEGF expression is decreased in patients with NB with aggressive, stroma-poor tumors (8, 14). Furthermore, HBEGF has been shown to promote neurite outgrowth of pheochromocytoma cells, a process that depends on epidermal growth factor receptor (EGFR) activation and ERK1/2 signaling (15). Here we investigate the role of HBEGF in NB.
MATERIALS AND METHODS
Cell lines and reagents
SK-N-AS (ATCC CRL-2137), SK-N-SH-SY5Y (5Y; CRL-2266), and SK-N-BE (2) (BE2; CRL-2271) cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and were verified by short tandem repeat analysis. 5Y and BE2 were grown in a 1:1 mixture of Eagle’s minimum essential medium and Ham’s F12 with 10% fetal bovine serum and 2 mM l-glutamine. SK-N-SH-SHEP (SHEP; gift of M. A. Armstrong, Duke University, Durham, NC, USA) and SK-N-AS were grown in Dulbecco’s modified Eagle’s minimum essential medium with 10% fetal bovine serum and 1% nonessential amino acids. 293FT and COS7 were sourced and grown as described previously (16). All cells were grown at 37°C in 5% CO2. All human cell lines have tested negative for mycoplasma contamination. The MEK1/2 inhibitor U0126 (9903) and recombinant FGF2 (8910) were purchased from Cell Signaling (Danvers, MA, USA). The MEK1/2 inhibitor CI-1040 (S1020) and the STAT3 inhibitor Ruxolitinib (no. S1378) were purchased from Selleck Chemical (Houston, TX, USA). The EGFR inhibitor erlotinib (10483) was purchased from Cayman Chemical (Ann Arbor, MI, USA). The EGFR inhibitors lapatinib and gefitinib were acquired from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, at the National Cancer Institute (Bethesda, MD, USA). Recombinant soluble human HBEGF (259-HE), type III TGF-β receptor (TβRIII) (242-R3), glypican (GPC)1 (4519-GP), GPC3 (2119-GP), syndecan (SDC)3 (3539-SD), and CD44 (3660-CD) were purchased from R&D Systems (Minneapolis, MN, USA). All-trans retinoic acid (ATRA; R2625) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Coculture experiments used restrictive 0.4-μM transwells in 12-well dishes (Corning, Inc., Corning, NY, USA)
DNA constructs, short hairpin RNA/short interfering RNA
The TβRIII adenoviral short hairpin RNA (shRNA) construct used in this study has been described previously and was used at a multiplicity of infection of 50 particles per cell (7). The EGFR-GFP overexpression plasmid was a gift from Alexander Sorkin (University of Pittsburgh, Pittsburgh, PA, USA) (Addgene plasmid 32751). The EF.STAT3DN.Ubc.GFP plasmid was a gift from Linzhao Cheng (Johns Hopkins University, Baltimore, MD, USA) (Addgene plasmid 24984). Transient DNA transfections were performed using lipofectamine (Thermo Fisher Scientific, Waltham, MA, USA) or X-tremeGene 9 (Sigma-Aldrich) according to the manufacturer’s instructions.
HBEGF shRNA (62203, 62204) knockdown constructs (Sigma Mission TRC1) were purchased from the Duke University RNAi Core Facility. The sequence for shRNA#1 was: CCGGGAGGAGGTTATGATGTGGAAACTCGAGTTTCCACATCATAACCTCCTCTTTTTG, and the sequence for shRNA#2 was: CCGGCCCATGTCTTCGGAAATACAACTCGAGTTGTATTTCCGAAGACATGGGTTTTTG. Pooled ID1 small interfering RNA (siRNA) (sc-29356) and control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and used according to the manufacturer’s instructions.
Microarray dataset analysis
Our microarray meta-dataset was generated as previously described (7). Data for the ERBB receptors are from the 3 U133 plus datasets only because the Human Exon 1.0 ST Arrays lacked an EGFR probe. Expression analysis in stroma-rich and stroma-poor tumors was performed using the publicly available Albino dataset (GSE7529) (14). Survival analysis was conducted using the NB prognosis (17) dataset from the Oncogenomics website (http://home.ccr.cancer.gov/oncology/oncogenomics/). The SEQC (18–21) dataset (GSE49710) was used for survival, gene expression, and linear correlation analysis. Data were visualized using the R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl). The microarray probes are listed in Table 1. All Affymetrix (Santa Clara, CA, USA) probes listed are from the HG-U133 Plus 2.0 platform. The GSE49710 probes listed are from the Agilent-020382 Human Custom Microarray platform (Agilent Technolgies, Santa Clara, CA, USA).
TABLE 1.
Affymetrix probe list
| Probe ID | |||
|---|---|---|---|
| Gene | GSE49710 | Affymetrix | Oncogenomics |
| HBEGF | A_24_P140608 | 38037_at | NB prognosis (35828) |
| EGF | 206254_at | ||
| AREG | 1557285_at | ||
| BTC | 207326_at | ||
| EREG | 205767_at | ||
| TGF-α | 205015_s_at | ||
| NRG1 | 208230_s_at | ||
| NRG2 | 206879_s_at | ||
| NRG4 | 242426_at | ||
| MYCN | A_24_P94402 | ||
| SOXIO | A_23_PI43694 | 209843_s_at | |
| TGFBR3 | A_23_P200780 | 20473 l_at | |
| EGFR | A_23_P215790 | 201983_s_at | |
| HER2 | 216836_s_at | ||
| HER3 | 202454_s_at | ||
| HER4 | 214053_at | ||
| ID1 | A_23_P252306 | 208937_s_at | |
| CDKN1A | A_23_P59210 | 202284_s_at | |
| MKI67 | A_24_P346855 | 212022_s_at | |
Western blotting
Western blotting was performed as previously described using standard techniques (7). Each experiment was conducted a minimum of 3 independent times. The following antibodies for differentiation and signaling markers were purchased from Cell Signaling: neurofilament 160 kD (NF160) (2838), β3-tubulin (5568), neuron-specific enolase (9536), growth-associated protein 43 (5307 and 8945), phospho-ERK1/2 (p-ERK1/2; 9101), ERK1/2 (4695), p21 (2947), phospho-AKT (p-AKT; 4058), AKT (4691), phospho-STAT3 (p-STAT3; 9145), STAT3 (9139), and EGFR (4267). The β-actin (A5441) antibody was purchased from Sigma-Aldrich. The ID1 (sc488) Western antibody was purchased from Santa Cruz Biotechnology. The HBEGF (ab92620) Western antibody was purchased from Abcam (Cambridge, MA, USA).
Real-time quantitative PCR
RNA was isolated from cells using the Quick RNA Miniprep Kit (11-328; Zymo Research Group, Irvine, CA, USA) per the manufacturer’s instructions. RNA (1 μg) was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Each PCR reaction contained 2 μl cDNA in addition to iQ SYBR Green Supermix (Bio-Rad), and cDNA was amplified with the following PCR program: 50 cycles consisting of 30 s of denaturation at 94°C, 30 s of annealing at 55°C, and 30 s of extension at 72°C with the following specific PCR primers (22): HB-EGF-F 5′-GGACCCATGTCTTCGGAAAT-3′ and HB-EGF-R 5′-CCCATGACACCTCTCTCCAT-3′.
Neurite outgrowth analysis
Neurites were measured from phase-contrast images taken with a Nikon Eclipse TE2000-U inverted microscope (Nikon Instruments, Inc., Melville, NY, USA) at ×10 magnification using the ImageJ (National Institutes of Health, Bethesda, MD, USA) plugin NeuronJ (23). Three images were taken of each condition, and all visible neurites were measured (∼100–150 neurites per field).
HBEGF immunofluorescence
NB tissue was obtained from the Children’s Oncology Group Biorepository with approval from the Neuroblastoma Biology Subcommittee. Tissue immunofluorescence was performed as previously described (24, 25) using an HBEGF antibody (HPA053243) from Sigma-Aldrich and the S100 Schwann cell marker antibody (4066) from Abcam.
Proximity ligation assays
The interaction between EGFR and TβRIII was detected in situ using DuoLink In Situ secondary antibodies and detection reagents (no. 92002, 92006, and 92008) purchased from Sigma-Aldrich according to the manufacturer’s instructions. Secondary antibodies in the absence of primary antibodies were used as a negative control. Ten random images per condition were taken with the CoolSnap HQ2 camera (Photometrics, Tucson, AZ, USA) attached to a Nikon Eclipse TE2000-U inverted microscope (Nikon Instruments, Inc.) at ×40 magnification (∼75–100 cells total). Positive signals were quantified using an ImageJ Batch Processing Macro with the following settings: rolling ball 50, threshold 255, analyze particles 5–1000, show outlines. Data were represented as signals per cell.
Proliferation assays
Tritiated thymidine incorporation was used to measure cell proliferation, as previously described (7). Proliferation indices (normalized to control = 1.0) were calculated and averaged for a minimum of 3 individual experiments. Cells were plated in a 96-well plate at a concentration of 5 × 103 cells/well (SK-N-AS, 5Y) or 1 × 103 cells/well (BE2). Each condition was plated and treated in triplicate or quadruplicate prior to a 4-h [3H] thymidine pulse (1 μCi) (Amersham Biosciences/GE Healthcare, Pittsburgh, PA, USA). After incubation with [3H] thymidine, cells were washed with PBS and 5% trichloracetic acid prior to overnight lysis with 0.1 M NaOH. Incorporation of [3H] thymidine was determined by scintillation counting.
In vivo xenograft
SK-N-AS cells were transduced with a nontargeting control (NTC) shRNA or an shRNA targeting HBEGF (shHBEGF#1) and selected with puromycin. SK-N-AS NTC and shHBEGF#1 cell lines were injected subcutaneously into the left flank of 10-wk-old athymic female nude mice. One million cells per mouse were resuspended in 0.2 ml serum-free DMEM with 50% growth factor reduced matrigel (no. 354230; BD Biosciences). Tumor dimensions were measured twice a week, and tumor volume was determined using the formula (A2 × B)/2, where A is the shorter diameter and B is the longer diameter. Animals were killed when tumors reached 2000 mm3. Animal use and care followed the institutional and national guidelines and was approved by the Duke Division for Laboratory Resources.
Statistical analysis
All experiments were repeated a minimum of 3 times. Clinical data were analyzed using nonparametric metrics (Kruskal-Wallis global test with Mann-Whitney post hoc tests) and presented as median with upper and lower quartiles. Kaplan-Meier survival curves were analyzed with log-rank statistics. All in vitro experiments were analyzed using parametric statistics (ANOVA global test followed by Bonferroni-corrected 2-tailed Student’s t tests) and presented as means ± sem. When data were normalized to control, 1-sample Student’s t tests were used with an expected value of 1 or 100%. Significance was set at P < 0.05 for all experiments. Slope and P value for the line of best fit, as well as R2 value for the association, were reported for all linear regression analyses. All statistical analyses were conducted with GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
Study approval
All patient samples were deidentified, and the project was exempted by the Duke University Health System Institutional Review Board (Protocol 00034541).
RESULTS
HBEGF expression is decreased in neuroblastoma with loss correlating with decreased survival
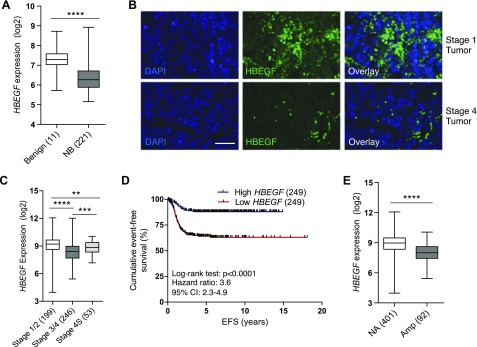
We determined mRNA expression of EGF family ligands using our microarray meta-dataset (7). HBEGF expression was significantly decreased in NB tumors compared with benign neuroblastic tumors, whereas EGF, AREG, β-cellulin (BTC), epiregulin (EREG), TGF-α, NRG1, NRG2, and NRG4 remained unchanged (Fig. 1A and Supplemental Fig. 1A). We then compared HBEGF protein expression by performing immunofluorescence microscopy on clinical specimens. In patients with stage I/II disease, HBEGF was readily detectable, whereas patients with stage IV disease displayed minimal HBEGF staining (Fig. 1B and Supplemental Fig. 2B). Using a publicly available dataset (18–21), we determined that HBEGF expression decreases with advanced-stage disease (Fig. 1C). Interestingly, patients with stage 4S disease, which spontaneously regresses over time, have HBEGF levels comparable to patients with low-stage disease. Patients with high HBEGF expression had a significant survival advantage over those with low HBEGF expression using 2 unique microarray datasets (17–21) (Fig. 1D and Supplemental Fig. 1B). Further, because MYCN amplification is associated with more aggressive tumors and worse survival, we compared HBEGF expression in MYCN-amplified vs. nonamplified patients (18–21). Patients with NB who have MYCN-amplified disease had significantly decreased HBEGF compared with patients with nonamplified disease (Fig. 1E). These data support a role for HBEGF in NB pathogenesis.
Figure 1.
HBEGF is decreased in patients with NB and correlates with survival. A) HBEGF expression in the microarray meta-dataset in benign neuroblastic tumors (ganglioneuroma/ganglioneuroblastoma) or NB tumors. ****P < 0.0001 (Mann-Whitney U test). B) Immunofluorescence in neuroblastoma specimens for HBEGF (green). DAPI nuclear stain in blue. Original magnification, ×40. Scale bar, 50 μM. C) HBEGF expression in patients with NB by stage. P < 0.0001, Kruskal-Wallis test. **P < 0.01, ***P < 0.001, ****P < 0.0001 Mann-Whitney U test for intergroup comparisons. D) Event-free survival (EFS) in patients with NB with low (bottom 50%; red) and high (top 50%; blue) HBEGF expression in the GSE49710 dataset. E) HBEGF expression in the GSE49710 dataset in nonamplified (NA) or MYCN-amplified (Amp) NB tumors. ****P < 0.0001 (Mann-Whitney U test). Box plots are presented as median (horizontal bars) and interquartile range (boxes).
HBEGF promotes neuroblast differentiation
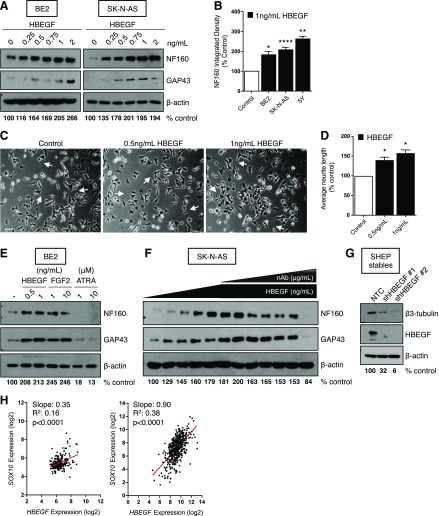
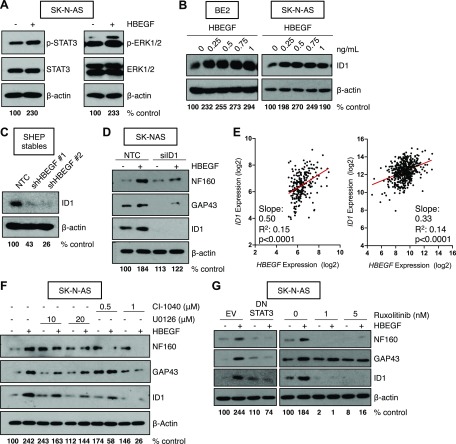
Because HBEGF was highly expressed in more differentiated, low-stage tumor specimens (Fig. 1B), we examined whether HBEGF could enhance neuroblast differentiation in widely used models of neuroblast differentiation: neurite outgrowth in BE2 cells (26) and expression of neuron-specific proteins, including NF160 and neuron growth–associated protein 43 in BE2, SK-N-AS, and 5Y cells (8, 27–31), β3-tubulin in SHEP (8, 32), and neuron-specific enolase in 5Y (8, 27, 33). Neuroblast differentiation markers were enhanced in a dose-dependent manner in BE2, SK-N-AS, and 5Y NB cells (Fig. 2A, B and Supplemental Fig. 1C). Likewise, HBEGF treatment led to a dose-dependent increase in neurite outgrowth (Fig. 2C, D). Importantly, HBEGF was able to promote neuroblast differentiation in the ATRA-insensitive BE2 cell line (Fig. 2E). The prodifferentiating effects of HBEGF were similar to FGF2, a ligand we have previously demonstrated to be important for TβRIII-induced suppression of NB tumor growth (Fig. 2E), suggesting these ligands are both important for the regulation of neuroblast differentiation (7, 8). The effects of HBEGF were specific; an HBEGF-neutralizing antibody inhibited HBEGF-induced differentiation in a dose-dependent manner (Fig. 2F). Further, in SHEP cells, which are often used as a model of schwannian stroma and display a differentiated phenotype (34), stable knockdown of HBEGF with 2 independent shRNAs led to a decrease in neuroblast differentiation as determined by β3-tubulin levels (Fig. 2D). In our microarray meta-dataset and the GSE49710 dataset (7, 18–21), HBEGF expression positively correlated with the neural crest differentiation marker SOX10 (35, 36) (Fig. 2E), suggesting that HBEGF may regulate neuroblast differentiation in vivo.
Figure 2.
HBEGF promotes neuroblast differentiation in NB cells. A) Western blot for differentiation markers after 72 h of HBEGF treatment in BE2 and SK-N-AS. Densitometry for NF160 normalized to β-actin is shown as the percentage of control. B) Quantification of NF160 densitometry normalized to β-actin from 3 independent Western blots (5Y, BE2) or 8 independent Western blots (SK-N-AS) after 72-h treatment with 1 ng/ml HBEGF and presented as means ± sem. P < 0.001 (1-way ANOVA). *P < 0.05, **P < 0.01, ****P < 0.0001, 1-sample Student’s t test. C) Representative phase-contrast images of BE2 cells after 72 h of treatment with HBEGF. Arrows identify long neurites. Original magnification, ×10. Scale bar, 100 μM. D) Quantification of neurite length using NeuronJ after 72 h of treatment with HBEGF from 3 independent experiments. P < 0.01 (1-way ANOVA). *P < 0.05, 1-sample Student’s t test. E) Western blot for differentiation markers after 72 h of HBEGF (0.5 or 1 ng/ml), FGF2 (1 or 10 ng/ml), or ATRA (1 or 10 μM). Densitometry for NF160 normalized to β-actin is shown as the percentage of control. F) Western blot for differentiation markers after 72 h HBEGF (0, 0.25, 0.5, 0.75, 1, 2 ng/ml) and a neutralizing HBEGF antibody (nAb; 0.0075, 0.015, 0.03, 0.05, 0.1, or 0.5 μg/ml). Densitometry for NF160 normalized to β-actin is shown as the percentage of control. G) Western blot for β3-tubulin and HBEGF in SHEP stably expressing an NTC shRNA or shRNA to HBEGF (shHBEGF #1, #2). Densitometry for β3-tubulin normalized to β-actin is shown as the percentage of control. H) Linear regression analyses using the microarray meta-dataset (left) or the GSE49710 dataset (right).
Soluble HBEGF derived from schwannian stroma enhances neuroblast differentiation
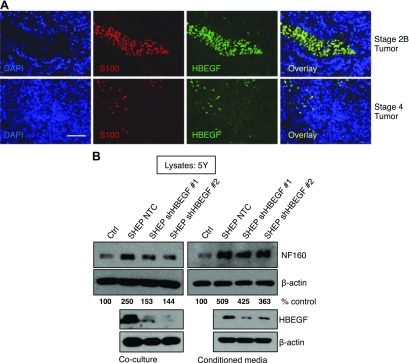
We identified that HBEGF expression is increased in low-stage tumors that are often surrounded by an abundant schwannian stroma (Fig. 1A–C); therefore, we investigated whether the schwannian stroma was a source of HBEGF. We determined mRNA expression of EGF family ligands in a publicly available dataset that delineates tumors as stroma poor or stroma rich (GSE7529) (14). In this dataset, the authors used tumor microdissection to identify a ranked list of genes whose expression was higher in the stromal cells compared with neuroblastic cells. Interestingly, HBEGF was in the top 40 genes that were more highly expressed in the stromal compartment of the isolated tumors. Our analysis also determined that HBEGF was increased in stroma-rich tumors as previously described (8, 14), whereas other EGF-family ligands, including EGF, AREG, BTC, EREG, TGF-α, NRG1, and NRG2, were unchanged (Supplemental Fig. 2A). Immunofluorescence on patient specimens revealed that HBEGF colocalized with stromal cells labeled with the Schwann cell marker S100 (6, 37), with low-stage tumors displaying an increased number of stromal cells and thus more HBEGF expression compared with high-stage tumors (Fig. 3A and Supplemental Fig. 2B).
Figure 3.
Schwannian stroma-derived HBEGF promotes neuroblast differentiation. A) Immunofluorescence in NB specimens using HBEGF (green) and S100 schwannian stroma (red) antibodies. DAPI nuclear stain in blue. Original magnification, ×40. Scale bar, 50 μM. B) Western blot for differentiation markers in 5Y after 72 h of coculture or treatment with conditioned medium from SHEP stably expressing an NTC shRNA construct or shRNA to HBEGF (shHBEGF #1, #2). Densitometry for NF160 normalized to β-actin is shown as the percentage of control.
Because HBEGF is highly expressed in the stroma and can promote neuroblast differentiation, we used direct coculture assays to determine whether stromal-derived HBEGF could promote neuroblast differentiation of tumor cells. SHEP cells were used to model the schwannian stroma (34) and were plated suspended above 5Y NB cells. SHEP cells stably expressing a NTC shRNA promoted differentiation of 5Y, whereas stable HBEGF knockdown with two independent shRNAs abrogated this effect (Fig. 3B). Similar results were obtained when conditioned medium from SHEP cells was used. Conditioned medium from SHEP cells with stable HBEGF knockdown failed to promote differentiation in 5Y to the same extent as SHEP NTC cells (Fig. 3B). These data suggest that HBEGF is stromally derived and that its release from schwannian stroma cells contributes to neuroblast differentiation in the NB tumor microenvironment.
HSPGs and EGFR interact to promote HBEGF-induced neuroblast differentiation
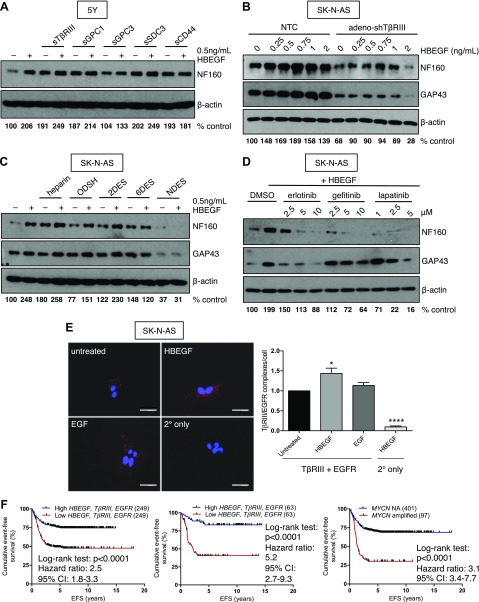
HBEGF requires binding to HSPGs as well as its receptor, EGFR, to initiate signaling (9, 38–40). We have previously demonstrated a critical role for HSPGs in promoting NB differentiation (7, 8). Therefore, we investigated whether HBEGF could potentiate HSPG-induced differentiation. Treatment with HBEGF enhanced differentiation compared with cells treated with soluble TβRIII, GPC1, GPC3, and SDC3 alone (Fig. 4A and Supplemental Fig. 3A). In contrast, the HSPG CD44 had no effect on HBEGF-mediated differentiation (Fig. 4A). In a reciprocal manner, silencing of TβRIII expression blunted the differentiation response to HBEGF (Fig. 4B). However, these cells were still able to undergo neuronal differentiation with HBEGF treatment (Fig. 4B), suggesting that other HSPGs may be compensating for the loss of TβRIII.
Figure 4.
HSPGs and EGFR interact to promote HBEGF-mediated neuroblast differentiation. A) Western blots for differentiation markers after 72-h treatment with 10 ng/ml soluble (s)TβRIII or sCD44 or 100 ng/ml sGPC1, sGPC3, or sSDC3 in the absence or presence of 0.5 ng/ml HBEGF in 5Y cells. B) Densitometry for NF160 normalized to β-actin is shown as the percentage of control, a dosecourse of HBEGF in SK-N-AS after 96-h TβRIII knockdown. C) Densitometry for NF160 normalized to β-actin is shown as the percentage of control, 0.5 μg/ml heparin, ODSH, 2-O desulfated heparin (2DES), 6-O desulfated heparin (6DES), or N desulfated heparin (NDES) in the absence or presence of 0.5 ng/ml HBEGF in SK-N-AS. D) Densitometry for NF160 normalized to β-actin is shown as the percentage of control, EGFR inhibitors for 72 h followed by 48 h of 1 ng/ml HBEGF treatment. Densitometry for NF160 normalized to β-actin is shown as the percentage of control. E) In situ proximity ligation assay in SK-N-AS after 5 min of treatment with 1 ng/ml HBEGF or 1 ng/ml EGF. Original magnification, ×40. Scale bars, 50 μM. Normalized TβRIII/EGFR complexes per cell (75–100 cells/condition) from 6 independent experiments. P < 0.001 (1-way ANOVA). *P < 0.05, ****P < 0.0001, 1-sample Student’s t test. F) Analysis of event-free survival in the GSE49710 dataset stratified by the top and bottom 50% for HBEGF, then TGFBR3, then EGFR expression (left) and analysis of event-free survival stratified by the top and bottom 12.5% for HBEGF, then TGFBR3, then EGFR expression (middle) compared with stratification by MYCN amplification status (right). NA, nonamplified.
The anticoagulant heparin is structurally similar to the heparan sulfate modifications on HSPGs. Heparin and heparan sulfate consist of repeating disaccharide units of glucuronic acid linked to glucosamine. These moieties are variably sulfated at the 3-O, 6-O, and N sites on glucosamine as well as the 2-O site on glucuronic acid (41). Distinct sulfation patterns determine the binding affinity for different ligands, including HBEGF, although the critical sulfation sites required for HBEGF to interact with heparan sulfate are unknown (41, 42). We have previously demonstrated that heparin and its variably sulfated derivatives can mimic the differentiating effects of HSPGs on NB cells with varying degrees of efficacy (8). Therefore, we examined whether HBEGF could enhance neuroblast differentiation induced by heparan sulfate mimetics. Treatment with HBEGF in addition to low doses of heparin, ODSH, or a 2-O desulfated heparin enhanced neuroblast differentiation, whereas a heparin derivative lacking N-sulfation had no effect (Fig. 4C). Treatment with 6-O desulfated heparin had inconsistent effects on HBEGF-induced differentiation in our experiments. These data suggest that the N-sulfation site is critical for HBEGF-induced neuroblast differentiation and supports the use of heparan sulfate mimetics for NB differentiation treatment.
HBEGF is known to actively bind its receptor, EGFR, in addition to HSPGs to mediate its downstream effects (39). In our microarray meta-dataset (7), we identified a decrease in EGFR expression in patients with NB compared with those with benign tumors (Supplemental Fig. 3B). High EGFR expression was also correlated to decreased stage of disease and improved prognosis (Supplemental Fig. 3C, D). To investigate whether EGFR is involved in HBEGF-induced differentiation, we used 3 clinically available EGFR inhibitors. Treatment with erlotinib, gefitinib, and lapatinib prevented HBEGF-induced differentiation in a dose-dependent manner (Fig. 4D), suggesting a role for EGFR in promoting neuroblast differentiation.
Because our data indicate that both EGFR and HSPGs are required for HBEGF-induced neuroblast differentiation, we investigated whether HSPGs act as an HBEGF coreceptor in NB cells. Consistent with a coreceptor role, exogenous expression of TβRIII coimmunoprecipitated with exogenous EGFR in COS-7 cells (Supplemental Fig. 3E) and soluble HBEGF increased this interaction (Supplemental Fig. 3E). To determine whether endogenous TβRIII and EGFR form a complex, we performed proximity ligation assays in SK-N-AS NB cells. We observed an interaction among these endogenous proteins that was enhanced when cells were treated with HBEGF ligand but not with the closely related EGF ligand (Fig. 4E). Using a publicly available microarray dataset (18–21), sequential stratification identified that patients with high HBEGF, TβRIII, and EGFR expression had excellent event-free survival. This 3-gene signature was comparable to MYCN oncogene amplification as a prognostic biomarker (Fig. 4F). Interestingly, HBEGF, TβRIII, and EGFR expression positively correlate in 2 unique microarray datasets (GSE49710) (7, 8, 18–21) (Supplemental Fig. 4F), suggesting these proteins may be coregulated in patients with NB. These data demonstrate that HBEGF, HSPGs, and EGFR interact to potentiate neuroblast differentiation in the tumor microenvironment.
HBEGF promotes differentiation via ERK/STAT3 signaling and ID1 up-regulation
To investigate which signaling pathways are critical for HBEGF-induced differentiation, we treated NB cells with recombinant HBEGF over 72 h to induce differentiation. As expected, HBEGF treatment enhanced phosphorylation of ERK, AKT, and STAT3 (Fig. 5A and Supplemental Fig. 4A). The ID1 transcription factor has been shown to act downstream of ERK in neuroblast differentiation, and its expression was recently revealed to be regulated by STAT3 signaling (7, 8, 43, 44). Consistent with these data, we identified that HBEGF promoted ID1 expression at concentrations that were also found to promote differentiation in NB cells (Fig. 5B and Supplemental Fig. 5B). Conversely, SHEP NB cells that are basally differentiated expressed high levels of ID1, which was decreased with stable shRNA-mediated HBEGF knockdown (Fig. 5C). Knockdown of ID1 with siRNA in NB cells also attenuated the differentiating effects of HBEGF (Fig. 5D). In 2 microarray datasets, HBEGF and ID1 positively correlated in patient samples, supporting the clinical relevance of our in vitro studies (7, 8, 18–21) (Fig. 5E).
Figure 5.
HBEGF induces neuroblast differentiation via ERK and STAT3 signaling and up-regulation of ID1. A) Western blot in SK-N-AS for phosphorylated and total STAT3 or ERK1/2 after 72 h treatment with 1 ng/ml HBEGF. Densitometry for phosphorylated STAT3 or phosphorylated ERK1/2 normalized to β-actin is shown as the percentage of control. B) Western blot for ID1 in BE2 and SK-N-AS after 72 h treatment with a dosecourse of HBEGF. Densitometry for ID1 normalized to β-actin is shown as the percentage of control. C) Western blot for ID1 in SHEP stably expressing an NTC shRNA or shRNA to HBEGF (shHBEGF #1, #2). Densitometry for ID1 normalized to β-actin is shown as the percentage of control. D) Western blot in SK-N-AS after 96 h ID1 knockdown and 72 h HBEGF treatment. Densitometry for NF160 normalized to β-actin is shown as the percentage of control. E) Linear regression analyses using the microarray meta-dataset (left) or the GSE49710 dataset (right). F) Western blot for differentiation markers and ID1 after 24 h cotreatment with 1 ng/ml HBEGF and the indicated doses of U0126 or CI-1040. Densitometry for NF160 normalized to β-actin is shown as the percentage of control. G) Western blot for differentiation markers after 72 h expression of an empty-vector control (EV) or a dominant negative STAT3 (DN STAT3) construct and 48 h treatment with 1 ng/ml HBEGF or 48 h treatment with ruxolitinib and 24 h treatment with 1 ng/ml HBEGF. Densitometry for NF160 normalized to β-actin is shown as the percentage of control.
Because ERK signaling has been implicated in neuronal differentiation in response to α-lipoic acid and retinoic acid (45, 46) and is the critical pathway for FGF2-induced differentiation (7, 8), we sought to determine the contribution of the ERK pathway to HBEGF-induced differentiation. Pharmacologic inhibition of MEK/ERK with U0126 and CI-1040 abrogated HBEGF-induced differentiation and ID1 up-regulation (Fig. 5F; Supplemental Fig. 5C). Previous data have implicated STAT3 activation in the differentiating process downstream of TGF-α, an EGFR ligand, and ERα, a nuclear hormone receptor, in NB cells (47). Similarly, inhibition of STAT3 signaling with a dominant-negative STAT3 and pharmacologic inhibitor treatment also reduced differentiation and ID1 up-regulation in the presence of HBEGF (Fig. 5G). These data support a role for the STAT3/ID1 axis in HBEGF-induced differentiation and further implicate the ERK/ID1 signaling pathway in neuroblast differentiation.
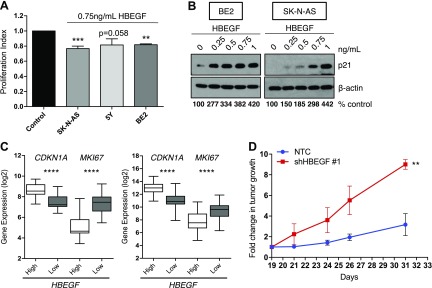
Soluble HBEGF promotes differentiation to suppress neuroblast proliferation and tumor growth
Because neuroblast differentiation is often associated with cell-cycle arrest and decreased tumor growth, we examined whether HBEGF treatment could reduce proliferation. Indeed, treatment with HBEGF led to a decrease in proliferation in 3 NB cell lines (Fig. 6A). Because increased p21 expression has been implicated in neuronal differentiation downstream of ERK (7, 8, 45, 46), we determined whether HBEGF expression altered p21 levels. Indeed, HBEGF led to enhanced expression of the cell cycle inhibitor p21 (Fig. 6B and Supplemental Fig. 4C). In patient microarray data, CDKN1A (cyclin-dependent kinase inhibitor 1A) (p21) positively correlated with HBEGF expression, whereas there was a negative correlation with marker of proliferation Ki-67 (MKI67) (Fig. 6C). In a subcutaneous mouse model of NB, HBEGF knockdown led to an increase in tumor growth, suggesting HBEGF is important for the suppression of NB tumorigenesis (Fig. 6D and Supplemental Fig. 4E).
Figure 6.
HBEGF suppresses neuroblast proliferation. A) Proliferation index from 3 (5Y) or 4 (SK-N-AS, BE2) replicates (means ± sem) of thymidine incorporation after HBEGF treatment for 24 h (SK-N-AS), 48 h (5Y), or 72 h (BE2), normalized to untreated control. P < 0.0001 (1-way ANOVA). **P < 0.01, ***P < 0.001 (1-sample Student’s t test). B) Western blot for p21 after 72 h of HBEGF treatment in BE2 and SK-N-AS. Densitometry for p21 normalized to β-actin is shown as the percentage of control. C) Linear regression analyses using the microarray meta-dataset (left) or the GSE49710 dataset (right). D) SK-N-AS NTC and SK-N-AS shHBEGF#1 subcutaneous xenograft. Tumors were measured at d 19 using calipers, and this measurement was used to calculate the fold change in tumor growth after 21, 24, 26, and 31 d. **P < 0.01, Kruskal-Wallis to compare curves.
Our data demonstrate that HBEGF forms a complex with EGFR and TβRIII to promote neuroblast differentiation, leading to a decrease in NB cell proliferation.
DISCUSSION
Here, we have used clinical data in addition to in vitro and in vivo experiments to identify a novel member of the prodifferentiating stroma secretome. Because the stroma has been implicated in promoting neuroblast differentiation (5, 6) and differentiation is a validated treatment strategy in NB, it is imperative to understand the differentiating factors secreted by the stroma in an effort to reveal new therapeutic strategies for patients with NB.
Our data demonstrate that HBEGF-induced neuronal differentiation requires HSPGs and EGFR to promote neuroblast differentiation. This is consistent with previous reports demonstrating a role for HSPGs (7, 8) and EGFR (15, 48, 49) in promoting neurite outgrowth and enhancement of differentiation markers. Previous research has also determined that HBEGF interacts with SDC3 (50) and CD44 (51) and that GPC1 (52, 53) and SDC1 (54) can mediate HBEGF activity. As demonstrated here, HBEGF is able to potentiate GPC1-, GPC3-, SDC3-, and TβRIII-induced differentiation, suggesting a possible interaction between the heparin-binding domain of HBEGF and the heparan sulfate modifications attached to these receptors. Previous work has also demonstrated an interaction between CD44 and HBEGF (51); however, CD44 did not promote HBEGF-induced differentiation in our studies. This observation may be due to the requirement for an alternatively spliced exon V3 that lends CD44 accessible to heparan sulfate modifications (51, 55, 56). Treatment with variably sulfated forms of heparin also reveals that N-sulfation is required to mediate HBEGF binding to heparan sulfate chains, whereas the 6-O site appears to be dispensable in some experiments. This is in contrast to reports that 6-O sulfation is required for HBEGF signaling in ovarian cancer cells (57). The sulfation requirements are likely cell context specific and may be determined by the relative amounts of functional heparan sulfate and competing heparin-binding ligands, such as FGF2, that are present in the tumor microenvironment (58).
Previous studies have identified HBEGF and ERBB receptors as potential targets for NB treatment due to their effects on proliferation and angiogenesis (59–62). Our data demonstrate that HBEGF and EGFR expression is often suppressed in NB tumors, decreases with advanced stage of disease, and is correlated with increased survival. HBEGF is also highly expressed in the stromal compartment of tumors, which also correlates with an improved prognosis (Figs. 1D and 3A). These data suggest that HBEGF and EGFR are not likely driving a proproliferative or proangiogenic phenotype in the majority of NBs. Further, we demonstrate that these proteins are involved in promoting neuronal differentiation and decreasing cell proliferation. Although inhibition of HBEGF and ERBB receptors has been shown to induce apoptosis (60, 62), it is possible that the doses required to promote apoptosis in a clinical setting may be too high for children with NB, leading to an increased toxicity profile. Further, the antitumor effect may not be sustained due to a propensity for tumor cells to adapt and become resistant, particularly in response to apoptotic stimuli. Our data caution against the therapeutic use of nonspecific tyrosine kinase inhibitors and neutralizing antibodies that are aimed at further reducing HBEGF and EGFR in the tumor microenvironment, which may lead to inhibition of differentiation and tumor resistance.
We have identified a 3-gene gene signature that may identify patients who will benefit from differentiation therapy aimed at enhancing expression of prodifferentiating proteins, including TβRIII and HBEGF (Fig. 4F). We have previously determined that TβRIII expression is epigenetically suppressed by direct N-Myc binding to SP1 sites on the TβRIII promoter (7). Our data demonstrate that HBEGF expression is decreased in MYCN-amplified NB (Fig. 1E) and that its expression positively correlates with TBRIII expression (Supplemental Fig. 3F), suggesting a similar mechanism may contribute to the decreased HBEGF expression seen in patients with NB. Further, HBEGF also contains SP1 binding sites that may contribute to its regulation (63, 64). Restoring expression of this important differentiation pathway using epigenetically targeted therapies may be necessary to re-establish a functional differentiation program.
Our data demonstrate a critical role for the STAT3 pathway in neuroblast differentiation (Fig. 5G). Prior research suggests the use of STAT3 inhibitors in patients with NB, noting their effectiveness in decreasing tumor growth in mouse models (65); however, the dosages used may not be clinically achievable in children, as seen previously with the aurora kinase A inhibitor MLN8237 (66, 67). As demonstrated here, the importance of STAT3 signaling in promoting neuroblast differentiation urges consideration when designing clinical trials aimed at inhibiting this critical differentiating pathway.
In summary, we have identified a novel and clinically relevant differentiating complex consisting of HBEGF, HSPGs, and EGFR. Our work cautions against targeting this important prodifferentiation pathway and instead provides rationale for the use of HBEGF and heparin derivatives for NB differentiation therapy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by U.S. National Institutes of Health, National Cancer Institute Grant F31CA192558 (to A.L.G.), and by a grant from the Alex’s Lemonade Stand Foundation Reach and Innovation Grants (to G.C.B.). The authors thank Michael Armstrong (Duke University), the Children’s Oncology Group Neuroblastoma Biology Subcommittee, Wendy London, and Evan Plunkett (both from the Children's Oncology Group) for assistance in obtaining patient tissue samples; Jennifer Huang for technical help with the animal experiment; David MacAlpine, Donald McDonnell, Ann Marie Pendergast, and Daniel Wechsler for crucial mentoring throughout this project; Elaine Justice for technical assistance; and Cheryl Alles (all from Duke University) for clerical assistance. The authors declare no conflicts of interest.
Glossary
- AREG
amphiregulin
- ATRA
all-trans retinoic acid
- BTC
β-cellulin
- EGFR
epidermal growth factor receptor
- EREG
epiregulin
- FGF
fibroblast growth factor
- GPC
glypican
- HBEGF
heparin-binding epidermal growth factor-like growth factor
- HSPG
heparan sulfate proteoglycan
- ID1
inhibitor of DNA binding 1
- NB
neuroblastoma
- NF160
neurofilament 160 kD
- NRG
neuregulin
- NTC
nontargeting control
- ODSH
2-O, 3-O desulfated heparin
- SDC
syndecan
- shRNA
short hairpin RNA
- siRNA
short interfering RNA
- TβRIII
type III TGF-β receptor
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. L. Gaviglio, E. H. Knelson, and G. C. Blobe designed the research, analyzed the data, and wrote the paper; A. L. Gaviglio performed the experiments; E. H. Knelson optimized model systems necessary for this project; and A. L. Gaviglio and E. H. Knelson contributed to the analysis of microarray data.
REFERENCES
- 1.Maris J. M., Hogarty M. D., Bagatell R., Cohn S. L. (2007) Neuroblastoma. Lancet 369, 2106–2120 [DOI] [PubMed] [Google Scholar]
- 2.Maris J. M. (2010) Recent advances in neuroblastoma. N. Engl. J. Med. 362, 2202–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullassery D., Dominici C., Jesudason E. C., McDowell H. P., Losty P. D. (2009) Neuroblastoma: contemporary management. Arch. Dis. Child. Educ. Pract. Ed. 94, 177–185 [DOI] [PubMed] [Google Scholar]
- 4.Matthay K. K., Villablanca J. G., Seeger R. C., Stram D. O., Harris R. E., Ramsay N. K., Swift P., Shimada H., Black C. T., Brodeur G. M., Gerbing R. B., Reynolds C. P.; Children’s Cancer Group (1999) Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N. Engl. J. Med. 341, 1165–1173 [DOI] [PubMed] [Google Scholar]
- 5.Kwiatkowski J. L., Rutkowski J. L., Yamashiro D. J., Tennekoon G. I., Brodeur G. M. (1998) Schwann cell-conditioned medium promotes neuroblastoma survival and differentiation. Cancer Res. 58, 4602–4606 [PubMed] [Google Scholar]
- 6.Liu S., Tian Y., Chlenski A., Yang Q., Salwen H. R., Cohn S. L. (2005) ‘Cross-talk’ between schwannian stroma and neuroblasts promotes neuroblastoma tumor differentiation and inhibits angiogenesis. Cancer Lett. 228, 125–131 [DOI] [PubMed] [Google Scholar]
- 7.Knelson E. H., Gaviglio A. L., Tewari A. K., Armstrong M. B., Mythreye K., Blobe G. C. (2013) Type III TGF-β receptor promotes FGF2-mediated neuronal differentiation in neuroblastoma. J. Clin. Invest. 123, 4786–4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knelson E. H., Gaviglio A. L., Nee J. C., Starr M. D., Nixon A. B., Marcus S. G., Blobe G. C. (2014) Stromal heparan sulfate differentiates neuroblasts to suppress neuroblastoma growth. J. Clin. Invest. 124, 3016–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aviezer D., Yayon A. (1994) Heparin-dependent binding and autophosphorylation of epidermal growth factor (EGF) receptor by heparin-binding EGF-like growth factor but not by EGF. Proc. Natl. Acad. Sci. USA 91, 12173–12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higashiyama S., Lau K., Besner G. E., Abraham J. A., Klagsbrun M. (1992) Structure of heparin-binding EGF-like growth factor: multiple forms, primary structure, and glycosylation of the mature protein. J. Biol. Chem. 267, 6205–6212 [PubMed] [Google Scholar]
- 11.Cook P. W., Mattox P. A., Keeble W. W., Pittelkow M. R., Plowman G. D., Shoyab M., Adelman J. P., Shipley G. D. (1991) A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol. Cell. Biol. 11, 2547–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeb J. A., Fischbach G. D. (1995) ARIA can be released from extracellular matrix through cleavage of a heparin-binding domain. J. Cell Biol. 130, 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meier T., Masciulli F., Moore C., Schoumacher F., Eppenberger U., Denzer A. J., Jones G., Brenner H. R. (1998) Agrin can mediate acetylcholine receptor gene expression in muscle by aggregation of muscle-derived neuregulins. J. Cell Biol. 141, 715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albino D., Scaruffi P., Moretti S., Coco S., Truini M., Di Cristofano C., Cavazzana A., Stigliani S., Bonassi S., Tonini G. P. (2008) Identification of low intratumoral gene expression heterogeneity in neuroblastic tumors by genome-wide expression analysis and game theory. Cancer 113, 1412–1422 [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y., Besner G. E. (2010) Heparin-binding epidermal growth factor-like growth factor is a potent neurotrophic factor for PC12 cells. Neurosignals 18, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanks B. A., Holtzhausen A., Evans K. S., Jamieson R., Gimpel P., Campbell O. M., Hector-Greene M., Sun L., Tewari A., George A., Starr M., Nixon A., Augustine C., Beasley G., Tyler D. S., Osada T., Morse M. A., Ling L., Lyerly H. K., Blobe G. C. (2013) Type III TGF-β receptor downregulation generates an immunotolerant tumor microenvironment. J. Clin. Invest. 123, 3925–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei J. S., Greer B. T., Westermann F., Steinberg S. M., Son C. G., Chen Q. R., Whiteford C. C., Bilke S., Krasnoselsky A. L., Cenacchi N., Catchpoole D., Berthold F., Schwab M., Khan J. (2004) Prediction of clinical outcome using gene expression profiling and artificial neural networks for patients with neuroblastoma. Cancer Res. 64, 6883–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SEQC/MAQC-III Consortium (2014) A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium. Nat Biotechnol. 32, 903–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munro S. A., Lund S. P., Pine P. S., Binder H., Clevert D. A., Conesa A., Dopazo J., Fasold M., Hochreiter S., Hong H., Jafari N., Kreil D. P., Łabaj P. P., Li S., Liao Y., Lin S. M., Meehan J., Mason C. E., Santoyo-Lopez J., Setterquist R. A., Shi L., Shi W., Smyth G. K., Stralis-Pavese N., Su Z., Tong W., Wang C., Wang J., Xu J., Ye Z., Yang Y., Yu Y., Salit M. (2014) Assessing technical performance in differential gene expression experiments with external spike-in RNA control ratio mixtures. Nat. Commun. 5, 5125 [DOI] [PubMed] [Google Scholar]
- 20.Su Z., Fang H., Hong H., Shi L., Zhang W., Zhang W., Zhang Y., Dong Z., Lancashire L. J., Bessarabova M., Yang X., Ning B., Gong B., Meehan J., Xu J., Ge W., Perkins R., Fischer M., Tong W. (2014) An investigation of biomarkers derived from legacy microarray data for their utility in the RNA-seq era. Genome Biol. 15, 523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Gong B., Bushel P. R., Thierry-Mieg J., Thierry-Mieg D., Xu J., Fang H., Hong H., Shen J., Su Z., Meehan J., Li X., Yang L., Li H., Łabaj P. P., Kreil D. P., Megherbi D., Gaj S., Caiment F., van Delft J., Kleinjans J., Scherer A., Devanarayan V., Wang J., Yang Y., Qian H. R., Lancashire L. J., Bessarabova M., Nikolsky Y., Furlanello C., Chierici M., Albanese D., Jurman G., Riccadonna S., Filosi M., Visintainer R., Zhang K. K., Li J., Hsieh J. H., Svoboda D. L., Fuscoe J. C., Deng Y., Shi L., Paules R. S., Auerbach S. S., Tong W. (2014) The concordance between RNA-seq and microarray data depends on chemical treatment and transcript abundance. Nat. Biotechnol. 32, 926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David M., Sahay D., Mege F., Descotes F., Leblanc R., Ribeiro J., Clézardin P., Peyruchaud O. (2014) Identification of heparin-binding EGF-like growth factor (HB-EGF) as a biomarker for lysophosphatidic acid receptor type 1 (LPA1) activation in human breast and prostate cancers. PLoS One 9, e97771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meijering E., Jacob M., Sarria J. C., Steiner P., Hirling H., Unser M. (2004) Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58, 167–176 [DOI] [PubMed] [Google Scholar]
- 24.Mythreye K., Knelson E. H., Gatza C. E., Gatza M. L., Blobe G. C. (2013) TβRIII/β-arrestin2 regulates integrin α5β1 trafficking, function, and localization in epithelial cells. Oncogene 32, 1416–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson D., Savage K., Reis-Filho J. S., Isacke C. M. (2008) Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol. 9, 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaneko M., Yang W., Matsumoto Y., Watt F., Funa K. (2006) Activity of a novel PDGF beta-receptor enhancer during the cell cycle and upon differentiation of neuroblastoma. Exp. Cell Res. 312, 2028–2039 [DOI] [PubMed] [Google Scholar]
- 27.Edsjö A., Holmquist L., Påhlman S. (2007) Neuroblastoma as an experimental model for neuronal differentiation and hypoxia-induced tumor cell dedifferentiation. Semin. Cancer Biol. 17, 248–256 [DOI] [PubMed] [Google Scholar]
- 28.Scarpa S., Coppa A., Ragano-Caracciolo M., Mincione G., Giuffrida A., Modesti A., Colletta G. (1996) Transforming growth factor beta regulates differentiation and proliferation of human neuroblastoma. Exp. Cell Res. 229, 147–154 [DOI] [PubMed] [Google Scholar]
- 29.Encinas M., Iglesias M., Liu Y., Wang H., Muhaisen A., Ceña V., Gallego C., Comella J. X. (2000) Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 75, 991–1003 [DOI] [PubMed] [Google Scholar]
- 30.Hahn C. K., Ross K. N., Warrington I. M., Mazitschek R., Kanegai C. M., Wright R. D., Kung A. L., Golub T. R., Stegmaier K. (2008) Expression-based screening identifies the combination of histone deacetylase inhibitors and retinoids for neuroblastoma differentiation. Proc. Natl. Acad. Sci. USA 105, 9751–9756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monaghan T. K., Mackenzie C. J., Plevin R., Lutz E. M. (2008) PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP-mediated activation of ERK and p38 MAP kinases. J. Neurochem. 104, 74–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henrich K. O., Bauer T., Schulte J., Ehemann V., Deubzer H., Gogolin S., Muth D., Fischer M., Benner A., König R., Schwab M., Westermann F. (2011) CAMTA1, a 1p36 tumor suppressor candidate, inhibits growth and activates differentiation programs in neuroblastoma cells. Cancer Res. 71, 3142–3151 [DOI] [PubMed] [Google Scholar]
- 33.Leli U., Cataldo A., Shea T. B., Nixon R. A., Hauser G. (1992) Distinct mechanisms of differentiation of SH-SY5Y neuroblastoma cells by protein kinase C activators and inhibitors. J. Neurochem. 58, 1191–1198 [DOI] [PubMed] [Google Scholar]
- 34.Ross R. A., Biedler J. L., Spengler B. A. (2003) A role for distinct cell types in determining malignancy in human neuroblastoma cell lines and tumors. Cancer Lett. 197, 35–39 [DOI] [PubMed] [Google Scholar]
- 35.Kelsh R. N. (2006) Sorting out Sox10 functions in neural crest development. BioEssays 28, 788–798 [DOI] [PubMed] [Google Scholar]
- 36.Gershon T. R., Oppenheimer O., Chin S. S., Gerald W. L. (2005) Temporally regulated neural crest transcription factors distinguish neuroectodermal tumors of varying malignancy and differentiation. Neoplasia 7, 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du W., Hozumi N., Sakamoto M., Hata J., Yamada T. (2008) Reconstitution of schwannian stroma in neuroblastomas using human bone marrow stromal cells. Am. J. Pathol. 173, 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higashiyama S., Abraham J. A., Klagsbrun M. (1993) Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migration: dependence on interactions with cell surface heparan sulfate. J. Cell Biol. 122, 933–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raab G., Klagsbrun M. (1997) Heparin-binding EGF-like growth factor. Biochim. Biophys. Acta 1333, F179–F199 [DOI] [PubMed] [Google Scholar]
- 40.Shishido Y., Sharma K. D., Higashiyama S., Klagsbrun M., Mekada E. (1995) Heparin-like molecules on the cell surface potentiate binding of diphtheria toxin to the diphtheria toxin receptor/membrane-anchored heparin-binding epidermal growth factor-like growth factor. J. Biol. Chem. 270, 29578–29585 [DOI] [PubMed] [Google Scholar]
- 41.Whitelock J. M., Iozzo R. V. (2005) Heparan sulfate: a complex polymer charged with biological activity. Chem. Rev. 105, 2745–2764 [DOI] [PubMed] [Google Scholar]
- 42.Ashikari-Hada S., Habuchi H., Kariya Y., Itoh N., Reddi A. H., Kimata K. (2004) Characterization of growth factor-binding structures in heparin/heparan sulfate using an octasaccharide library. J. Biol. Chem. 279, 12346–12354 [DOI] [PubMed] [Google Scholar]
- 43.Passiatore G., Gentilella A., Rom S., Pacifici M., Bergonzini V., Peruzzi F. (2011) Induction of Id-1 by FGF-2 involves activity of EGR-1 and sensitizes neuroblastoma cells to cell death. J. Cell. Physiol. 226, 1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu H., Yue X., Zhao Y., Li X., Wu L., Zhang C., Liu Z., Lin K., Xu-Monette Z. Y., Young K. H., Liu J., Shen Z., Feng Z., Hu W. (2014) LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nat. Commun. 5, 5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X., Wang Z., Yao Y., Li J., Zhang X., Li C., Cheng Y., Ding G., Liu L., Ding Z. (2011) Essential role of ERK activation in neurite outgrowth induced by α-lipoic acid. Biochim. Biophys. Acta 1813, 827–838 [DOI] [PubMed] [Google Scholar]
- 46.Qiao J., Paul P., Lee S., Qiao L., Josifi E., Tiao J. R., Chung D. H. (2012) PI3K/AKT and ERK regulate retinoic acid-induced neuroblastoma cellular differentiation. Biochem. Biophys. Res. Commun. 424, 421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciana P., Ghisletti S., Mussi P., Eberini I., Vegeto E., Maggi A. (2003) Estrogen receptor alpha, a molecular switch converting transforming growth factor-alpha-mediated proliferation into differentiation in neuroblastoma cells. J. Biol. Chem. 278, 31737–31744 [DOI] [PubMed] [Google Scholar]
- 48.Evangelopoulos M. E., Weis J., Krüttgen A. (2009) Mevastatin-induced neurite outgrowth of neuroblastoma cells via activation of EGFR. J. Neurosci. Res. 87, 2138–2144 [DOI] [PubMed] [Google Scholar]
- 49.Evangelopoulos M. E., Weis J., Krüttgen A. (2005) Signalling pathways leading to neuroblastoma differentiation after serum withdrawal: HDL blocks neuroblastoma differentiation by inhibition of EGFR. Oncogene 24, 3309–3318 [DOI] [PubMed] [Google Scholar]
- 50.Hienola A., Tumova S., Kulesskiy E., Rauvala H. (2006) N-syndecan deficiency impairs neural migration in brain. J. Cell Biol. 174, 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett K. L., Jackson D. G., Simon J. C., Tanczos E., Peach R., Modrell B., Stamenkovic I., Plowman G., Aruffo A. (1995) CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J. Cell Biol. 128, 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleeff J., Ishiwata T., Kumbasar A., Friess H., Büchler M. W., Lander A. D., Korc M. (1998) The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J. Clin. Invest. 102, 1662–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuda K., Maruyama H., Guo F., Kleeff J., Itakura J., Matsumoto Y., Lander A. D., Korc M. (2001) Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 61, 5562–5569 [PubMed] [Google Scholar]
- 54.Celie J. W., Katta K. K., Adepu S., Melenhorst W. B., Reijmers R. M., Slot E. M., Beelen R. H., Spaargaren M., Ploeg R. J., Navis G., van der Heide J. J., van Dijk M. C., van Goor H., van den Born J. (2012) Tubular epithelial syndecan-1 maintains renal function in murine ischemia/reperfusion and human transplantation. Kidney Int. 81, 651–661 [DOI] [PubMed] [Google Scholar]
- 55.Jackson D. G., Bell J. I., Dickinson R., Timans J., Shields J., Whittle N. (1995) Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J. Cell Biol. 128, 673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown T. A., Bouchard T., St John T., Wayner E., Carter W. G. (1991) Human keratinocytes express a new CD44 core protein (CD44E) as a heparan-sulfate intrinsic membrane proteoglycan with additional exons. J. Cell Biol. 113, 207–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cole C. L., Rushton G., Jayson G. C., Avizienyte E. (2014) Ovarian cancer cell heparan sulfate 6-O-sulfotransferases regulate an angiogenic program induced by heparin-binding epidermal growth factor (EGF)-like growth factor/EGF receptor signaling. J. Biol. Chem. 289, 10488–10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chu C. L., Goerges A. L., Nugent M. A. (2005) Identification of common and specific growth factor binding sites in heparan sulfate proteoglycans. Biochemistry 44, 12203–12213 [DOI] [PubMed] [Google Scholar]
- 59.Nam S. O., Yotsumoto F., Miyata K., Souzaki R., Taguchi T., Kuroki M., Miyamoto S. (2015) Validity of HB-EGF as target for human neuroblastoma therapy. Anticancer Res. 35, 4433–4440 [PubMed] [Google Scholar]
- 60.Richards K. N., Zweidler-McKay P. A., Van Roy N., Speleman F., Trevino J., Zage P. E., Hughes D. P. (2010) Signaling of ERBB receptor tyrosine kinases promotes neuroblastoma growth in vitro and in vivo. Cancer 116, 3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ho R., Minturn J. E., Hishiki T., Zhao H., Wang Q., Cnaan A., Maris J., Evans A. E., Brodeur G. M. (2005) Proliferation of human neuroblastomas mediated by the epidermal growth factor receptor. Cancer Res. 65, 9868–9875 [DOI] [PubMed] [Google Scholar]
- 62.Tamura S., Hosoi H., Kuwahara Y., Kikuchi K., Otabe O., Izumi M., Tsuchiya K., Iehara T., Gotoh T., Sugimoto T. (2007) Induction of apoptosis by an inhibitor of EGFR in neuroblastoma cells. Biochem. Biophys. Res. Commun. 358, 226–232 [DOI] [PubMed] [Google Scholar]
- 63.Miyata K., Yotsumoto F., Nam S. O., Odawara T., Manabe S., Ishikawa T., Itamochi H., Kigawa J., Takada S., Asahara H., Kuroki M., Miyamoto S. (2014) Contribution of transcription factor, SP1, to the promotion of HB-EGF expression in defense mechanism against the treatment of irinotecan in ovarian clear cell carcinoma. Cancer Med. 3, 1159–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edwards J. P., Zhang X., Mosser D. M. (2009) The expression of heparin-binding epidermal growth factor-like growth factor by regulatory macrophages. J. Immunol. 182, 1929–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan S., Li Z., Thiele C. J. (2013) Inhibition of STAT3 with orally active JAK inhibitor, AZD1480, decreases tumor growth in neuroblastoma and pediatric sarcomas in vitro and in vivo. Oncotarget 4, 433–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maris J. M., Morton C. L., Gorlick R., Kolb E. A., Lock R., Carol H., Keir S. T., Reynolds C. P., Kang M. H., Wu J., Smith M. A., Houghton P. J. (2010) Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP). Pediatr. Blood Cancer 55, 26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mosse Y. P., Lipsitz E., Fox E., Teachey D. T., Maris J. M., Weigel B., Adamson P. C., Ingle M. A., Ahern C. H., Blaney S. M. (2012) Pediatric phase I trial and pharmacokinetic study of MLN8237, an investigational oral selective small-molecule inhibitor of Aurora kinase A: a Children's Oncology Group Phase I Consortium study. Clin Cancer Res. 18, 6058–6064 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.