Abstract
Hyperglycemia is a major pathogenic factor that promotes diabetic nephropathy, but the underlying mechanism remains incompletely understood. Here, we show that high glucose induced cAMP response element-binding protein (CREB)-binding protein (CBP)–mediated H3K9/14 hyperacetylation in approximately 5000 gene promoters in glomerular mesangial cells, including those of Tgfb1, Tgfb3, and Ctgf, the major profibrotic factors that are known to drive diabetic renal fibrogenesis. In these promoters, H3K9/14 hyperacetylation was closely associated with NF-κB or CREB motifs. Chromatin immunoprecipitation assays confirmed that hyperglycemia promoted phospho-p65 or phospho-CREB and CBP bindings and RNA polymerase II recruitment to these promoters in mesangial cells as well as in glomeruli that were purified from type I and type II diabetic mice. Under hyperglycemia, cAMP production and PKA activity were markedly increased as a result of glucose transporter 1–mediated glucose influx that drives glucose metabolism and ATP production, which led to increased phosphorylation of p65 and CREB. Inhibition of adenylyl cyclase or PKA activity blocked p65 and CREB phosphorylation, CBP recruitment, and histone acetylation in these promoters. Collectively, these data demonstrate that the cAMP-PKA pathway plays a key role in epigenetic regulation of key profibrotic factors in diabetes.—Deb, D. K., Bao, R., Li, Y. C. Critical role of the cAMP-PKA pathway in hyperglycemia-induced epigenetic activation of fibrogenic program in the kidney.
Keywords: diabetic complication, renal fibrosis, histone acetylation, ChIP-Seq, protein kinase A
In the United States, 29.1 million people, or 9.3% of the population, have diabetes mellitus (1). Hyperglycemia is the most important pathogenic factor for long-term diabetic microvascular complications in both type I and type II diabetes. Diabetic nephropathy is the most common renal complication of diabetes and a leading cause of end-stage renal disease; thus, the growing incidence of diabetes has been accompanied by a parallel rise in chronic kidney disease and kidney failure. Glomerulosclerosis, which is characterized by mesangial expansion and excess deposition of extracellular matrix (ECM) in the glomeruli, is a hallmark feature of diabetic renal injury (2, 3). Induction of potent profibrotic factors, such as TGF-β and connective tissue growth factor (CTGF) under a hyperglycemic environment, which drive ECM production, is a major cause of glomerulosclerosis (4, 5). A number of pathways have been suggested to mediate renal injury in diabetes (2, 3), but how hyperglycemia induces profibrotic factors remains poorly understood.
In eukaryotic cells where DNA is packed into chromosomes, gene transcription requires chromatin remodeling for the transcription machinery to move in, and histone acetylation is one of the most common and critical modulators of chromatin remodeling (6–8). The status of histone acetylation is dynamically controlled by antagonistic actions of histone acetyl-transferases (HATs) and histone deacetylases. cAMP response element-binding protein (CREB)-binding protein (CBP) is a ubiquitous coactivator with potent HAT activity and is widely involved in gene transactivation via acetylation of H3 and H4 histones (9, 10). For example, H3K9/14Ac (HAC) and H4K5/8Ac—histone marks that are commonly found in active, protein-coding gene promoters (11, 12)—are known targets of CBP (10). CBP can be recruited to gene promoters by many transcription factors to modify histones and induce chromatin remodeling.
PKA is a cAMP-dependent kinase that plays a key role in cell metabolism (13). cAMP is converted from ATP by adenylyl cyclase. At least 2 families of cAMP-producing adenylyl cyclases have been identified in mammalian cells. One is soluble adenylyl cyclase, the other transmembrane adenylyl cyclase (14, 15). PKA is a holoenzyme that is composed of 2 regulatory and catalytic subunits each. PKA is activated by cAMP, which binds to regulatory subunits, triggering the release of the catalytic subunits, which move to the nucleus to phosphorylate specific transcription factors for gene transactivation (13). For example, PKA phosphorylates CREB at Ser133 (16) and NF-κB p65 at Ser276 (17). These phosphorylated transcription factors bind to specific cis-DNA elements in gene promoters and are able to recruit CBP/p300 for histone modifications.
In this report, we tested the hypothesis that glucose alters cellular activities by regulating the epigenome in complication-prone cells. We showed that hyperglycemia promotes genome-wide histone acetylation in mesangial cells (MCs) that involves diverse pathways that could alter cellular functions. Among hyperacetylated gene promoters are those of TGF-β1, TGF-β3, and CTGF, which are potent profibrotic factors that are well known to drive renal fibrosis. Our data demonstrate that hyperglycemia-induced histone hyperacetylation in these promoters is mediated by the cAMP-PKA pathway. This epigenetic activation of the fibrogenic program provides new insight into the molecular basis for hyperglycemia-induced renal injury.
MATERIALS AND METHODS
Cell culture
Primary MCs (within 5–10 passages), which were isolated from purified glomeruli with d-valine selection as described (18, 19), were routinely maintained in DMEM/F12 that contained ∼15 mM glucose and was supplemented with 10% heat-inactivated FBS, insulin, and antibiotics at 5% CO2 and 37°C. For high glucose (HG) stimulation, cells were synchronized in serum-free DMEM that contained 5 mM glucose for 24 h, then changed to DMEM that contained 5 mM glucose [low glucose (LG)] and 10% FBS or to DMEM that contained 25 mM glucose (HG) and 10% FBS, and were cultured for different hours as required before analysis. Mouse MC line SV40 MES 13 (American Type Culture Collection, Manassas, VA, USA) was cultured under similar conditions and used in some experiments. To inhibit the PKA pathway, cells were treated with KH7 (50 μM; Cayman Chemicals, Ann Arbor, MI, USA), 2′5′-dideoxyadenosine (50 μM; Sigma-Aldrich, St. Louis, MO, USA) or H89 (5 μM; Sigma-Aldrich).
Chromatin immunoprecipitation sequencing
Primary MCs were exposed to LG or HG medium for 16 h before being cross-linked with 1% formaldehyde. Nuclei were isolated and sonicated to shear the chromatin. Sonicated chromatin was incubated with anti-CBP Ab (sc-7300x; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-HAC Ab (06-599; Millipore, Billerica, MA, USA), or with nonimmune IgG as controls. After overnight incubation, the chromatin-Ab complex was precipitated with protein G agarose beads followed by proteinase K digestion. DNA was purified, from which a chromatin immunoprecipitation sequencing (ChIP-Seq) library was constructed using an Illumina TruSeq ChIP Sample Preparation Kit (Illumina, San Diego, CA, USA) according to manufacturer instructions. Sequencing data were generated on an Illumina HiSeq2500 instrument at the University of Chicago Functional Genomics Facility. For each sample, more than 17 million 50-bp single-end reads were obtained. Reads were mapped to the mouse reference genome assembly mm9 (NCBI build 37) by using Burrows-Wheeler Aligner (BWA v0.7.9a) (20). Uniquely mapped reads were kept for further analysis. Model-based analysis of ChIP-Seq (21, 22) version 2_ENREF_3 (MACS2, v2.0.9) was used to detect peaks in immunoprecipitation (IP) samples with the corresponding input genomic DNA (input) sample as control. Peaks were identified with parameters -g 2.3E9 -s 50–bw 200 -m 5.50 -q 0.001, and other settings as default. Scaled IP to input tag density ratio was calculated by ChIP-Seq processing pipeline (SPP, v1.11) (23) and adjusting for potential sequence content bias, where a tag indicates a sequence read. To identify HG-induced peaks in IP experiments, scaled IP to input tag density ratios were compared between LG and HG condition for each peak region using 2-sided Student’s t test, followed by multiple testing correction. Peaks with false discovery rate–adjusted to a value of P < 0.01 were carried on for the annotation of the closest promoter, transcription start site (TSS), and gene products using BEDTools (v2.17) (24) and Hypergeometric Optimization of Motif Enrichment (HOMER; http://homer.salk.edu/homer/). A promoter region was defined between 1.5 kb upstream and 100 bp downstream of TSS. Known transcription binding site motifs in the peaks were predicted by using HOMER. For CREB and NF-κB motifs, peak sequences in genes of interest were also analyzed by using position weight matrix from the JASPAR database (25). CBP and HAC ChIP-Seq peaks for specific genes were visualized on the University of California, Santa Cruz Genome Browser.
Animals
DBA/2J and ob/ob BTBR (BTBR.Cg-Lepob/WiscJ; 004824) mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Two-month-old DBA/2J mice were treated with streptozotocin (35 mg/kg dissolved in 10 mM citrate, pH 4.2, i.p.) for 5 consecutive days to induce diabetes as described in Zhang et al. (26). Nondiabetic mice served as controls. Mice were killed 6 wk after streptozotocin treatment. Ob/ob BTBR and control ob/+ BTBR mice were killed at age 2 mo. Kidneys were harvested immediately after death. For glomerular purification, mice were perfused with Dynabeads M-450 tosylactivated (Thermo Fisher Scientific, Waltham, MA, USA) dissolved in PBS, and glomeruli were isolated by using a magnet as described in Takemoto et al. (27). All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of The University of Chicago. All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, D.C., USA).
ChIP assays
ChIP assays were performed as described in Yuan et al. (28). In brief, MCs or purified glomeruli were cross-linked with 1% formaldehyde. After sonication, the sonicated chromatin was incubated with ChIP-validated Abs or nonimmune IgG as controls. Abs used were as follows: HAC (Millipore), CBP (Santa Cruz Biotechnology), phospho-CREB(S133) (17-10131; Millipore), phospho-p65(S276) (3037; Cell Signaling Technology, Danvers, MA, USA), and RNA Pol II (17-672; Millipore). The chromatin-Ab complex was precipitated with protein G agarose beads, followed by proteinase K digestion to remove proteins. DNA isolated from the complex was subject to real time PCR quantification using primers listed in Supplemental Table S1 DNA was normalized with input DNA and expressed as the ratio.
Western blotting
MCs and purified glomeruli were lysed in RIPA buffer that contained a cocktail of proteinase inhibitors (Roche, Basel, Switzerland). Protein concentrations were determined by using a bicinchoninic acid protein assay reagent kit (Pierce, Rockford, IL, USA). Proteins were separated by SDS-PAGE, and Western blotting was carried out as previously described (29) using the following Abs: p65 (sc-109; Santa Cruz Biotechnology), phospho-p65(S276) (Cell Signaling Technology), phospho-p65(S536) (ab28856; Abcam, Cambridge, MA, USA), CREB (06-863; Millipore), phospho-CREB(S133) (Millipore), TGF-β1 (ab92486; Abcam), Glut-1 (ab652; Abcam), fibronectin (F6140; Sigma-Aldrich), and β-actin (A00702; GenScript, Piscataway, NJ, USA). Secondary Ab was horseradish peroxidase–conjugated anti-rabbit IgG (Pierce), and signals were detected by using SuperSignal West Dura Extended Duration Substrate (Pierce).
RT-PCR
Total RNA was extracted by using Trizol reagents (Thermo Fisher Scientific). First-strand cDNA was synthesized by using a ThermoScript RT kit (Thermo Fisher Scientific). Conventional PCR was carried out in a Bio-Rad DNA Engine (Bio-Rad, Hercules, CA, USA). Real-time PCR was performed in a Roche 480 Real-Time PCR System using SensiFast SYBR No-Rox kits (Bioline, London, United Kingdom). The relative number of transcripts was calculated by using the 2−ΔΔCt formula (30) normalized to β-2-microglobulin transcript as an internal control. PCR primers used in the study are listed in Supplemental Table S1.
Glucose uptake
MCs grown in LG or HG medium were incubated with [3H]-2-deoxy-d-glucose, and glucose uptake was measured with scintillation counting according to a previously described method (31).
cAMP assays
Cell lysate cAMP concentration was quantified by using a Cyclic AMP EIA Kit from Cayman Chemicals according to manufacturer instructions.
PKA activity
Cell lysate PKA enzymatic activity was assayed according to a published method (32).
Electrophoretic mobility shift assay
Nuclear extract preparation and electrophoretic mobility shift assays were performed according to previously published methods (28). Oligonucleotide probes used in these assays are listed in Supplemental Table S1. Supershift assays were performed by using Abs against CREB or p65 as indicated.
Coimmunoprecipitation
Co-IP experiments were performed on the basis of previously described methods (33).
Statistical analysis
Data are presented as means ± sd. Statistical comparisons were carried out by using unpaired 2-tailed Student’s t test for 2 group comparisons, and for 3 or more group comparisons, a 2-way ANOVA with a Student-Newman-Keuls post hoc test was used. A value of P < 0.05 was considered statistically significant.
RESULTS
HG induces genome-wide histone hyperacetylation in MCs
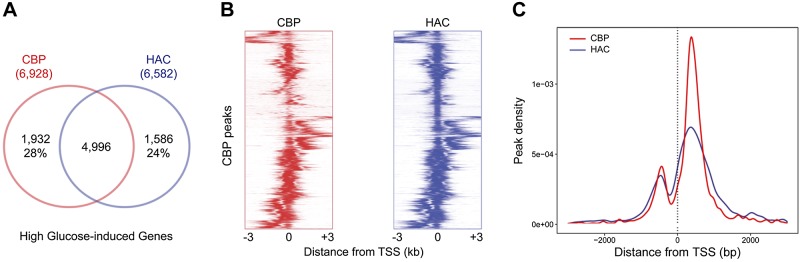
MCs are the main contributor to diabetic glomerulosclerosis in diabetes; therefore, we used MCs as a model to assess the impact of hyperglycemia on histone acetylation. We performed ChIP-Seq experiments in primary MCs exposed to LG (5 mM) or HG (25 mM) medium using anti-CBP and anti-HAC Abs. ChIP-Seq analyses identified more than 15,000 and 17,000 CBP peaks and more than 25,000 and 24,000 HAC peaks in the genome under LG and HG conditions, respectively. The majority of these CBP and HAC peaks are distributed in gene promoters (28–43%), introns (31–42%), and intergenic regions (9–21%) across the genome (Supplemental Fig. S1A). HG-induced CBP and HAC peaks, however, were highly enriched in 5′-UTR and promoter regions in the genome (Supplemental Fig. S1B). Compared with LG, 6928 and 6582 genes in the genome were identified as having significant HG-induced CBP and HAC peaks (false discovery rate–adjusted, P < 0.01) in their promoter regions (defined as 1.5 kb upstream or 100 bp downstream of TSS), respectively, and 4,996 of these genes had CBP and HAC peaks colocalized in the promoters, representing 72 and 76% of CBP and HAC modified genes, respectively (Fig. 1A). Analysis of the ChIP-Seq signal distribution showed that in both assays, CBP and HAC peaks were enriched within ±2 kb of the TSS, which indicated that the most frequent modifications occurred in the promoter, 5′-UTR, and first exon/intron regions (Fig. 1B, C). Of interest, the CBP and HAC peak profiles showed an almost completely overlapping bimodal distribution, with 2 proximal enrichments at ∼0.5 kb upstream and 0.3 kb downstream of the TSS, which suggested that HG-induced CBP and HAC marks are largely colocalized in the promoter and 5′-UTR regions of genes (Fig. 1C). Similar bimodal distributions of HAC peaks around the TSS were reported previously in active genes in human and mouse embryonic stem cell genomes (11, 12).
Figure 1.
HG induces histone acetylation in gene promoters across the genome. ChIP-Seq was performed on MCs that were exposed to LG or HG by using Abs against CBP and HAC. A) Number and overlap of genes associated with HG-induced CBP (red) and HAC (blue) peaks in the promoter regions. B) Density heatmap showing colocalization of HG-induced CBP and HAC peaks across a 6-kb region centered at TSS (±3 kb). C) Distribution of HG-induced CBP and HAC peak signals proximal to the TSS (±3 kb).
Ingenuity pathway analysis suggested that HG-induced CBP and HAC marks were associated with genes involved in multiple canonical pathways, such as cell-cycle regulation, mitochondrial dysfunction, oxidative stress response, PKA signaling, TGF-β signaling, NF-κB activation, oxidative phosphorylation, and tricarboxylic acid (TCA) cycle (Table 1 and Supplemental Table S2; note that 33–59% of the genes in these pathways are modified). It is conceivable that changes in these pathways may dramatically alter cellular functions. For example, altered cell-cycle pathways could lead to MC proliferation and hypertrophy, inducing mesangial expansion, whereas activation of PKA, TGF-β, and NF-κB signaling pathways would promote renal fibrosis and inflammation. These are the main features of diabetic renal injury. Indeed, the leading enriched pathways that are associated with nephrotoxicity include renal necrosis, renal proliferation, renal hypertrophy, and glomerular injury (Table 2). Therefore, these data collectively support the supposition that hyperglycemia can drive glomerular injury by modifying the epigenetic landscape of the genome in MCs.
TABLE 1.
Representative ingenuity pathways enriched in genes with HG-induced CBP and HAC peaks
| Canonical pathway | -log(P) | Count | Ratio |
|---|---|---|---|
| EIF2 signaling | 14.50 | 88/183 | 0.481 |
| Regulation of eIF4 and p70S6K signaling | 12.40 | 71/145 | 0.490 |
| PI3K/AKT signaling | 11.00 | 61/123 | 0.496 |
| mTOR signaling | 10.20 | 80/186 | 0.430 |
| Cell cycle: G1/S checkpoint regulation | 10.10 | 38/64 | 0.594 |
| Insulin receptor signaling | 9.42 | 61/132 | 0.462 |
| ERK/MAPK signaling | 7.19 | 73/187 | 0.390 |
| Mitochondrial dysfunction | 7.11 | 68/171 | 0.398 |
| PKA signaling | 6.87 | 128/386 | 0.332 |
| NRF2-mediated oxidative stress response | 6.13 | 68/180 | 0.378 |
| Cyclins and cell-cycle regulation | 5.83 | 36/78 | 0.462 |
| Cell cycle: G2/M DNA damage checkpoint regulation | 5.79 | 26/49 | 0.531 |
| Apoptosis signaling | 5.11 | 38/89 | 0.427 |
| ErbB2-ErbB3 signaling | 4.27 | 26/57 | 0.456 |
| Oxidative phosphorylation | 4.27 | 42/109 | 0.385 |
| TGF-β signaling | 4.10 | 35/87 | 0.402 |
| TCA cycle II (eukaryotic) | 3.52 | 13/23 | 0.565 |
| STAT3 pathway | 3.01 | 28/73 | 0.384 |
| NF-κB activation by viruses | 2.66 | 27/73 | 0.370 |
EIF2, eukaryotic initiation factor 2; mTOR, mammalian target of rapamycin; NRF2, nuclear factor erythroid 2-related factor 2; STAT, signal transducers and activators of transcription.
TABLE 2.
Top enriched nephrotoxicity pathways
| Toxicity function | P | Molecules (n) |
|---|---|---|
| Renal necrosis/cell death | 5.74E-01 to 1.35E-07 | 152 |
| Renal proliferation | 5.22E-01 to 4.29E-05 | 63 |
| Renal hypertrophy | 3.89E-01 to 7.48E-04 | 14 |
| Glomerular injury (hypertrophy of MCs) | 1.00E00 to 1.07E-03 | 39 |
| Nephrosis | 5.22E-01 to 7.73E-03 | 24 |
HG induces chromatin remodeling in fibrogenic genes
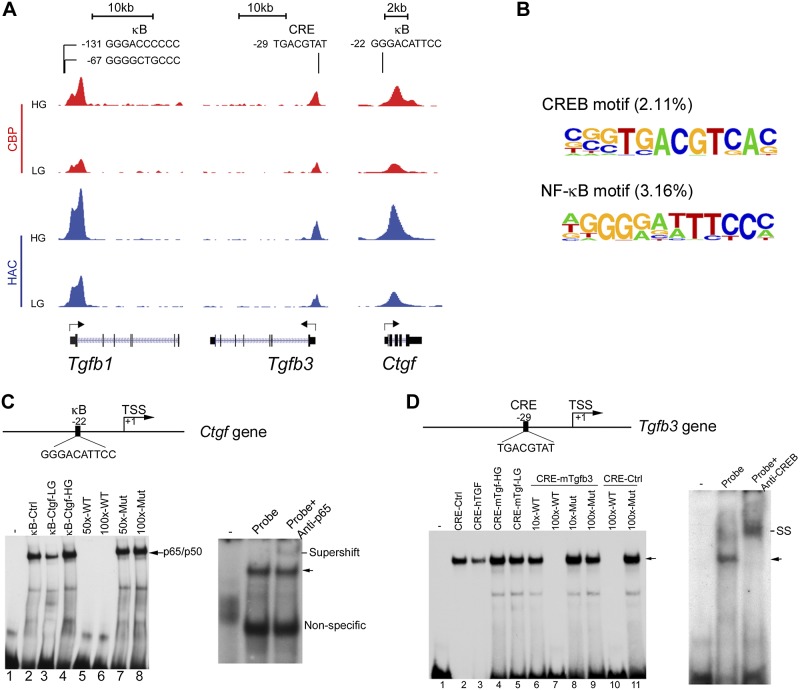
Glomerular fibrosis is a major manifestation of diabetic nephropathy. To understand how hyperglycemia causes fibrogenesis, we focused on Tgfb1, Tgfb3, and Ctgf genes, because they are the most potent and well-known profibrotic factors that promote glomerulosclerosis (2, 5, 34). It is known that all TGF-β isoforms are profibrotic (35), and TGF-β3 is a potent inducer of TGF-β1 (36). Examination of these gene loci revealed overlapping CBP and HAC peaks around the TSS region of each gene, and these peaks were all markedly enhanced under HG conditions (Fig. 2A). Of interest, κB or CRE motifs were found around these peaks in these gene promoters (Fig. 2A), which suggested that CBP was recruited by NF-κB or CREB to these regions for histone modification. Tgfb1 and Ctgf gene promoters contain κB sites, whereas Tgfb3 promoter contains a CRE site. Analysis of ChIP-Seq data showed that in the genome, conserved CREB and NF-κB motifs account for 2.11 and 3.16% of the more than 200 known cis-DNA motifs that have been identified around HG-induced CBP and HAC peaks (Fig. 2B).
Figure 2.
HG induces histone acetylation in profibrotic factor gene promoters. A) ChIP-Seq tracks showing CBP (red) and HAC (blue) signal peaks in Tgfb1, Tgfb3, and Ctgf genes. CBP and HAC peaks overlap around the κB and CRE motifs in the gene promoters, and these peaks are elevated by HG exposure. Sequences for κB and CRE motifs are presented at the top. B) Identified nucleotide sequences of CREB and NF-κB motifs associated with HG-induced CBP and HAC peaks. C, D) Electrophoretic mobility shift assays (EMSAs) to validate κB and CRE cis-DNA motifs in fibrogenic gene promoters. EMSAs were carried out by incubating LG- and HG-treated MC nuclear extracts and oligonucleotide probes that contained the putative κB or CRE site in Ctgf (C) or Tgfb3 (D) gene. HG increases nuclear protein binding to Ctgf-κB site and Tgfb3-CRE site, and the binding is competed off by unlabeled probe but not by mutant probe. Binding of p65 to the κB site (C) or of CREB to the CRE site (D) was confirmed by supershift assays using Abs against p65 or CREB. SS, supershift band; WT, wild-type.
To validate the authenticity of the NF-κB and CREB motifs, we performed electrophoretic mobility shift assays. Binding of nuclear proteins to the κB and CRE sites was enhanced by HG treatment, and binding was competed off by unlabeled probes, but by not mutant probes. Supershift assays using anti-p65 or anti-CREB Abs confirmed the binding of p65 or CREB to these κB and CRE sites, respectively (Fig. 2C, D).
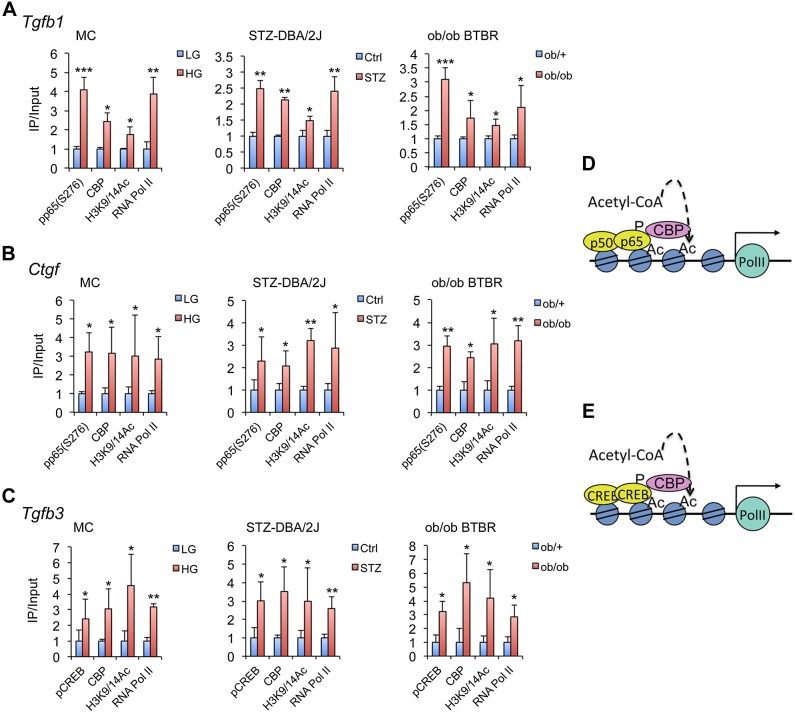
We further performed ChIP assays to assess the molecular events induced by HG surrounding these κB and CRE sites in Tgfb1, Ctgf, and Tgfb3 gene promoters. ChIP data showed that HG increased phospho-(p)p65(S276) or pCREB(S133) binding, CBP recruitment, and H3K9/14 acetylation in these regions and induced RNA polymerase (Pol) II occupation at the TSS location of these genes in MCs (Fig. 3). To assess the in vivo relevance of these findings, we also ChIP-analyzed these promoters in glomeruli that were purified from streptozotocin-induced diabetic DBA/2J mice and diabetic ob/ob BTBR mice, which are models of type I and type II diabetes mellitus that are highly susceptible to diabetic nephropathy (37–39). Consistently, increased pp65(S275) or pCREB(S133) binding, CBP recruitment, H3K9/14 acetylation, and RNA Pol II occupation in these promoters were also detected in these mice compared with their nondiabetic control (Fig. 3A–C). As purified glomeruli contain MCs, podocytes, and endothelial cells, these chromatin-remodeling events could occur in all these cells in vivo under hyperglycemia.
Figure 3.
HG induces chromatin remodeling in gene promoters of profibrotic factors. A–C) ChIP assays showing that HG induces pCREB(S133) or pp65(S276) binding, CBP recruitment, H3K9/14 hyperacetylation, and RNA Pol II occupation in the TSS region in Tgfb1 (A), Ctgf (B), and Tgfb3 (C) promoters in MCs, as well as in purified glomeruli from streptozotocin (STZ)-induced diabetic DBA/2J and ob/ob BTBR mice, as indicated. D, E) Illustration of the chromatin remodeling events around NF-κB motif and CREB motif in Tgfb1and Ctgf promoters (D) or Tgfb3 (E) promoter. *P < 0.05; **P < 0.01; ***P < 0.001 vs. corresponding controls (LG, Ctrl, or ob/+); n ≥ 3 in each assay.
cAMP-PKA pathway is required for chromatin remodeling in fibrogenic genes
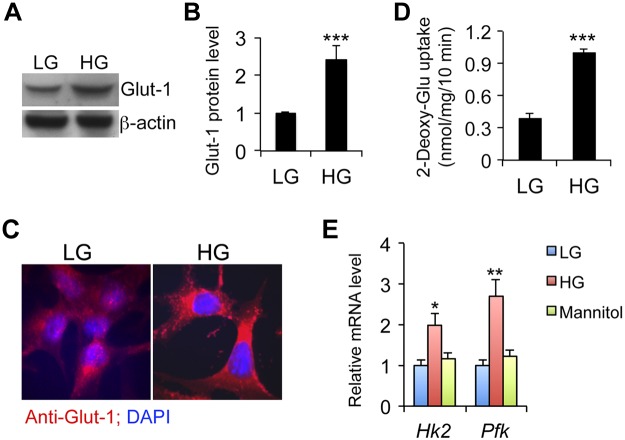
As an HAT, CBP recruitment to the gene promoter is crucial for histone modification. As CBP is recruited by pp65 or pCREB to the κB or CRE motif in Tgfb1, Tgfb3, and Ctgf promoters, the important question is, how does hyperglycemia regulate the phosphorylation of these transcription factors? As shown in Fig. 4, HG induced glucose transporter 1 (Glut-1) expression in MCs (Fig. 4A–C), and glucose uptake assays that used radiolabeled 2-deoxy-d-glucose confirmed increased glucose influx under HG conditions (Fig. 4D). Intracellular hyperglycemia increased glycolysis, as suggested by the induction of hexose kinase-2 (Hk2) and phospho-fructose kinase (Pfk), which are rate-limiting enzymes in the glycolytic pathway (Fig. 4E).
Figure 4.
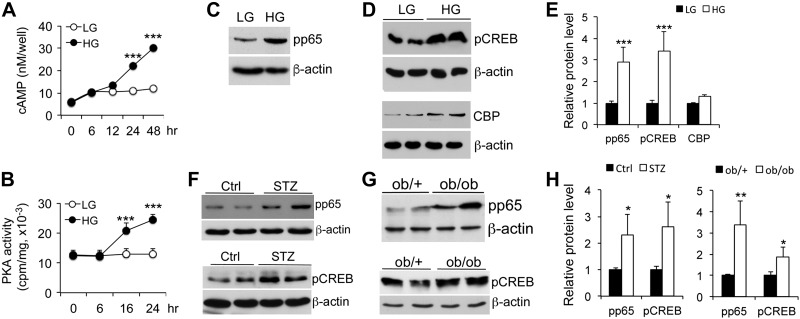
HG promotes Glut-1–mediated glucose influx in MCs. A, B) Western blot analysis (A) and densitometric quantitation (B) of Glut-1 protein levels at 24 h after HG exposure. C) Glut-1 expression (red) measured by immunostaining at 24 h. Nuclei are stained with DAPI (blue). D) [3H]-2-Deoxy-d-glucose uptake assays. E) Real time RT-PCR quantitation of hexose kinase 2 (Hk2) and phospho-fructose kinase (Pfk) gene transcripts in MCs exposed to LG, HG, or high mannitol for 24 h. *P < 0.05; **P < 0.01; ***P < 0.001 vs. LG.
We reasoned that heightened glycolysis should lead to increased TCA cycle and oxidative phosphorylation, as shown in ingenuity pathway analysis (Table 1 and Supplemental Table S2), which is expected to raise intracellular concentration of ATP that can then be converted to cAMP; therefore, we examined the effect of HG on intracellular cAMP generation and PKA activity. Indeed, intracellular cAMP levels increased over time after HG exposure (Fig. 5A), and PKA activity was also escalated in a time-dependent manner (Fig. 5B), which was consistent with the ChIP-Seq result that suggested that the PKA signaling pathway is activated (Table 1 and Supplemental Table S2). Consistent with the increases in cAMP and PKA, p65 Ser276 and CREB Ser133 phosphorylation—known targets of PKA—was markedly induced in HG-treated MCs (Fig. 5C–E) as well as in purified glomeruli from diabetic DBA/2J mice and ob/ob BTBR mice compared with relevant controls (Fig. 5F–H). These glomerular data confirm a stimulatory effect of hyperglycemia on p65 and CREB phosphorylation in vivo.
Figure 5.
HG activates the cAMP-PKA signaling pathway. A) Time course of HG-induced intracellular cAMP accumulation in MCs. B) Time course of HG-induced PKA activity in MCs. ***P < 0.001 vs. corresponding LG. C–E) Western blot analysis of p65(S276) phosphorylation (C), CREB(S133) phosporylation and CBP levels (D), and the densitometric quantitation (E) in MCs exposed to LG and HG for 24 h. F–H) Western blot analyses of purified glomerular lysates from nondiabetic controls and streptozotocin (STZ)-diabetic DBA/2J mice at 6 wk after STZ treatment (F), and from 2-mo-old ob/+ and ob/ob BTBR mice (G), and densitometric quantitation (H) for phospho-p65(S276) and phospho-CREB(S133) levels as indicated. *P < 0.05; **P < 0.01; ***P < 0.001 vs. corresponding controls (LG, Ctrl or ob/+); n ≥ 3 in each assay.
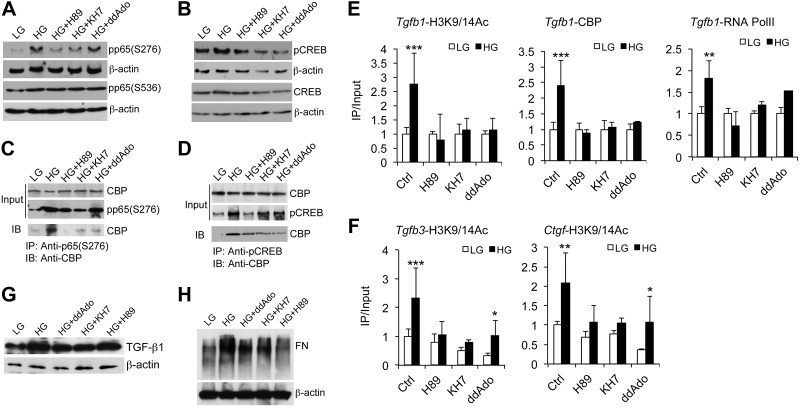
To assess the role of the cAMP-PKA pathway in chromatin remodeling of fibrogenic gene promoters, we blocked adenylyl cyclase and PKA activities with specific inhibitors. Of the 2 families of cAMP-producing adenylyl cyclase in mammalian cells, KH7 inhibits the soluble adenylyl cyclase, whereas 2′5′-dideoxyadenosine inhibits the transmembrane adenylyl cyclase (40). H89 is a PKA catalytic subunit inhibitor. When MCs were individually treated with these inhibitors, HG-induced PKA activity was significantly attenuated in 16–24 h (Supplemental Fig. S2). Consequently, HG-induced phosphorylation of p65(S276), not S536, and CREB(S133) was markedly inhibited (Fig. 6A, B), and HG-induced recruitment of CBP by pp65(S276) or pCREB(S133) was also blocked, as revealed by coimmunoprecipitation assays (Fig. 6C, D). As expected, HG-driven H3K9/14 hyperacetylation at the κB and CRE sites in Tgfb1, Tgfb3, and Ctgf promoters was markedly suppressed (Fig. 6E, F). Also attenuated was recruitment of CBP and RNA Pol II to the promoters, such as Tgfb1 promoter (Fig. 6E). Moreover, HG induction of TGF-β1 and its downstream target, fibronectin, were ameliorated under these inhibitory conditions (Fig. 6G, H). Similar results were observed in Tgfb3 promoter when the PKA pathway was inhibited by H89 (Supplemental Fig. S3). Collectively, these data establish an essential role for the cAMP-PKA pathway in hyperglycemia-driven histone acetylation and epigenetic control of fibrogenic genes.
Figure 6.
Essential roles of the cAMP-PKA pathway in HG-induced histone acetylation and chromatin remodeling in fibrogenic gene promoters. MCs were exposed to LG and HG conditions in the presence or absence of H89, KH7, or 2′5′-dideoxyadenosine (ddAdo) inhibitor. A, B) Western blots analyzing phospho-p65(S276) and phospho-p65(S536) (A) or phospho-CREB(S133) and total CREB (B) in cell lysates. C, D) Coimmiunoprecipitation assays measuring the recruitment of CBP by phospho-p65(S276) (C) or phospho-CREB(S133) (D) in these lysates. E) ChIP assays measuring HG-induced H3K9/14 acetylation, CBP recruitment, and RNA Pol II occupation in Tgfb1 promoter. F) ChIP assays assessing HG-induced H3K9/14 acetylation in Tgfb3 and Ctgf promoters. G, H) Western blots analyzing TGF-β1 (G) and fibronectin (H) proteins in these cell lysates. IB, immunoblot. FN, fibronectin. *P < 0.05; **P < 0.01; ***P < 0.001 vs. corresponding LG; n = 3.
DISCUSSION
Hyperglycemia is a major pathogenic factor for diabetic microvascular complications, but how glucose as a nutrient causes tissue injury in diabetes is only partially understood. For cells, such as MCs, that are unable to down-regulate their glucose transporters in the setting of extracellular hyperglycemia, an increase in intracellular glucose concentration is expected. It has been long held that such intracellular hyperglycemia induces overproduction of superoxide by the mitochondrial electron-transport chain, which, via inhibition of glyceraldehyde 3-phosphate dehydrogenase, diverts glycolysis to the polyol and hexosamine pathways and promotes the activation of PKC and formation of advanced glycosylation end products (41). These mechanisms are believed to ultimately lead to up-regulation of profibrotic factors, such as TGF-β, and increased oxidative stresses that drive diabetic complications (3).
One intriguing feature of diabetic complications is the long-lasting hyperglycemic memory, as reflected in the persistence or progression of hyperglycemia-induced complications long after glucose homeostasis is normalized. This is well illustrated in the Diabetes Control and Complications Trial (DCCT) (42) and the follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study (43), in which patients who were on intensive glycemic control during the DCCT and the EDIC follow-up had significantly lower incidents of microvascular complications compared with patients with diabetes who received conventional glycemic control in the DCCT and reverted to intensive control during the EDIC phase, even though there were no longer significant differences in HbA1c levels between the two groups several years into the EDIC period (44). In fact, patients with poor glycemic control in the DCCT continued to develop nephropathy at an increased rate even after 25 yr of excellent glycemic control (45). The phenomenon of hyperglycemic memory has been explored from an epigenetic perspective, which has emerged as novel a mechanism that drives diabetic complications (44, 46). Prior studies showed that a transient HG exposure could cause persistent H3K4m1 hypermethylation in NF-κB p65 gene promoter and drive p65 expression in aortic endothelial cells (47, 48). Increased H3K9m2 methylation was seen in lymphocytes from patients with type I diabetes (49, 50), and differential genome-wide changes in H3K4m2 and H3K9m2 methylation were reported in a human monocyte cell line that was exposed to HG (51); however, the molecular basis for glucose to alter these histone marks remains unclear. In MCs, TGF-β1 was shown to promote ECM gene expression by increasing H3K4m1/2/3 and repressing H3K9m2/3, apparently via up-regulation and recruitment of H3K4 methyltransferase SET7/9 (52). Hence, the current prevailing theory is that hyperglycemia indirectly influences the epigenomic landscape via profibrotic and pathogenic mediators—TGF-β1 and angiotensin II—in diabetic nephropathy (46, 53).
In this study, we investigated the global impact of hyperglycemia on histone acetylation in MCs, a major contributor to diabetic glomerulosclerosis. MCs express Glut-1 and Glut-4 (54). Hyperglycemia suppresses Glut-4 (55), an insulin-sensitive glucose transporter, but induces insulin-insensitive Glut-1 (31, 56), which we confirmed. Glut-1 mediates glucose influx when cells are exposed to a hyperglycemic environment. We showed that one major consequence of increased glucose influx is a genome-wide increase in H3K9/14 acetylation, and the majority (76%) of histone acetylation is mediated by CBP. Hyperglycemia-induced H3K9/14 hyperacetylation occurs predominately in the promoter regions of approximately 5000 genes across the genome, and these genes are involved in diverse metabolic and regulatory pathways that can radically change the function of cells, eventually causing renal injury. As an important regulatory code for gene transcription, histone acetylation seems to be an important mechanism that couples extracellular glucose with the genome to alter cellular functions.
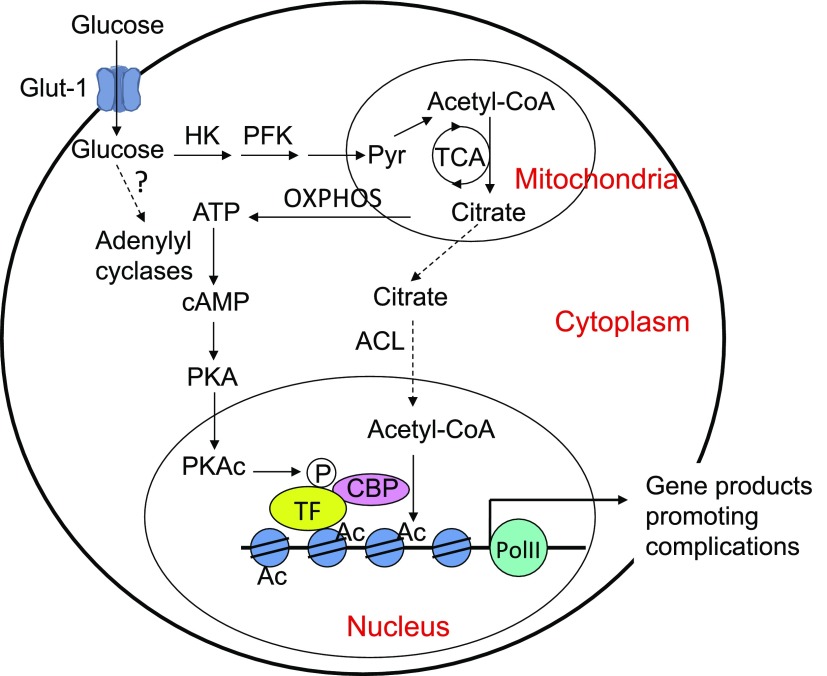
Profibrotic factors play a key role in diabetic renal fibrosis. Here, we demonstrate that the cAMP-PKA pathway is essential for hyperglycemia-induced epigenetic activation of these factors. We showed that HG exposure promotes glucose influx and glycolysis, and that intracellular cAMP concentration and PKA activity are markedly elevated. Of note, ChIP-Seq data revealed that 33% of the genes involved in the PKA signaling pathway are hyperacetylated (Table 1), which enforces the notion that hyperglycemia activates the PKA pathway. In fact, ChIP-Seq data also show that many gene promoters that are involved in the TCA cycle—56.5% of the involved genes—and oxidative phosphorylation—38.5% of the involved genes—become hyperacetylated (Table 1). Up-regulation of these biochemical processes is expected to accumulate ATP within cells via mitochondrial oxidative phosphorylation, which provides the source for cAMP production. Our data show that both membrane-associated and soluble adenylyl cyclases are involved in the synthesis of cAMP, although the mechanism that is involved in the activation of these enzymes remains unknown. PKA activation initiates a cascade of events that lead to histone hyperacetylation in Tgfb1, Tgfb3, and Ctgf promoters. PKA phosphorylates CREB and p65 to recruit CBP to these promoters to acetylate histones (Fig. 7). ChIP assays confirm increased H3K9/14 acetylation and RNA Pol II recruitment to these gene promoters in MCs as well as in glomeruli that are isolated from mouse models reflecting both type I and type II diabetes. Blockade of cAMP synthesis or PKA activation markedly attenuates these processes. These data uncover the importance of the cAMP-PKA pathway in epigenetic regulation of renal fibrogenesis in diabetes. Acetyl-CoA is the required substrate for histone acetylation. We speculate that ATP-citrate lyase, an enzyme that converts citrate to acetyl-CoA (57), may play an important role in linking glucose metabolism to histone acetylation in MCs (Fig. 7). This theory needs to be further investigated in future studies.
Figure 7.
Pathways that mediate glucose effects on histone acetylation in MCs. Dotted lines are speculative. Ac, acetyl group; ACL, ATP-citrate lyase; HK, hexose kinase; oxphos, oxidative phosphorylation; PFK, phospho-fructose kinase; PKAc, catalytic unit of PKA; PolII, RNA polymerase II; Pyr, pyruvate; TF, transcription factor.
CBP is a ubiquitous coactivator that is recruited by numerous transcription factors in transcriptional regulation. ChIP-Seq data reveal more than 200 cis-DNA motifs associated with CBP-driven H3K9/14 hyperacetylation in the genome. As PKA phosphorylates various transcription factors to recruit CBP, it is postulated that the cAMP-PKA pathway is not limited to the regulation of histone modification of the fibrogenic genes described here; however, it is unlikely that all H3K9/14 hyperacetylation sites across the genome are regulated by PKA—other mediators must be involved. Despite the limitation, the mechanism defined for the regulation of fibrogenic genes establishes a principle that links glucose metabolism to epigenomic modifications in diabetic renal complications, which provides new molecular insights into the development of diabetic nephropathy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by a Genzyme Renal Innovations Program research grant from the Genzyme Corp., U.S. National Institutes of Health (NIH) Clinical and Translational Science Award Grant CTSA-NIH UL1 TR000430, the Center for Research Informatics of The University of Chicago Biological Science Division, and the NIH Institute for Translational Medicine Grant CTSA-NIH UL1 RR024999. The authors declare no conflicts of interest.
Glossary
- CBP
cAMP response element-binding protein-binding protein
- ChIP
chromatin immunoprecipitation
- ChIP-Seq
chromatin immunoprecipitation sequencing
- CREB
cAMP response element-binding protein
- CTGF
connective tissue growth factor
- DCCT
Diabetes Control and Complications Trial
- ECM
extracellular matrix
- EDIC
Epidemiology of Diabetes Interventions and Complications
- Glut-1
glucose transporter 1
- HAC
H3K9/14Ac
- HAT
histone acetyl-transferase
- HG
high glucose
- IP
immunoprecipitation
- LG
low glucose
- MC
mesangial cell
- TCA
tricarboxylic acid
- TSS
transcription start site
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. C. Li formulated and designed the research; D. K. Deb performed the research; D. K. Deb, R. Bao, and Y. C. Li analyzed the data; R. Bao and Y. C. Li wrote the manuscript; and Y. C. Li acquired the funding and supervised the research.
REFERENCES
- 1. Center for Disease Control and Prevention (2014) 2014 National Diabetes Statistics Report. Available at: http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html.
- 2.Cooper M. E. (1998) Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 352, 213–219 [DOI] [PubMed] [Google Scholar]
- 3.Reidy K., Kang H. M., Hostetter T., Susztak K. (2014) Molecular mechanisms of diabetic kidney disease. J. Clin. Invest. 124, 2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziyadeh F. N., Sharma K., Ericksen M., Wolf G. (1994) Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J. Clin. Invest. 93, 536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta S., Clarkson M. R., Duggan J., Brady H. R. (2000) Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int. 58, 1389–1399 [DOI] [PubMed] [Google Scholar]
- 6.Eberharter A., Becker P. B. (2002) Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 3, 224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahbazian M. D., Grunstein M. (2007) Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 76, 75–100 [DOI] [PubMed] [Google Scholar]
- 8.Kouzarides T. (2007) Chromatin modifications and their function. Cell 128, 693–705 [DOI] [PubMed] [Google Scholar]
- 9.Bedford D. C., Kasper L. H., Fukuyama T., Brindle P. K. (2010) Target gene context influences the transcriptional requirement for the KAT3 family of CBP and p300 histone acetyltransferases. Epigenetics 5, 9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiltz R. L., Mizzen C. A., Vassilev A., Cook R. G., Allis C. D., Nakatani Y. (1999) Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. J. Biol. Chem. 274, 1189–1192 [DOI] [PubMed] [Google Scholar]
- 11.Guenther M. G., Levine S. S., Boyer L. A., Jaenisch R., Young R. A. (2007) A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130, 77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karmodiya K., Krebs A. R., Oulad-Abdelghani M., Kimura H., Tora L. (2012) H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics 13, 424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor S. S., Ilouz R., Zhang P., Kornev A. P. (2012) Assembly of allosteric macromolecular switches: lessons from PKA. Nat. Rev. Mol. Cell Biol. 13, 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buck J., Sinclair M. L., Schapal L., Cann M. J., Levin L. R. (1999) Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. USA 96, 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamenetsky M., Middelhaufe S., Bank E. M., Levin L. R., Buck J., Steegborn C. (2006) Molecular details of cAMP generation in mammalian cells: a tale of two systems. J. Mol. Biol. 362, 623–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montminy M. (1997) Transcriptional regulation by cyclic AMP. Annu. Rev. Biochem. 66, 807–822 [DOI] [PubMed] [Google Scholar]
- 17.Zhong H., Voll R. E., Ghosh S. (1998) Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1, 661–671 [DOI] [PubMed] [Google Scholar]
- 18.Gilbert S. F., Migeon B. R. (1975) D-valine as a selective agent for normal human and rodent epithelial cells in culture. Cell 5, 11–17 [DOI] [PubMed] [Google Scholar]
- 19.Kurtz A., Jelkmann W., Sinowatz F., Bauer C. (1983) Renal mesangial cell cultures as a model for study of erythropoietin production. Proc. Natl. Acad. Sci. USA 80, 4008–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Durbin R. (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Liu T., Meyer C. A., Eeckhoute J., Johnson D. S., Bernstein B. E., Nusbaum C., Myers R. M., Brown M., Li W., Liu X. S. (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T. (2014) Use model-based analysis of ChIP-Seq (MACS) to analyze short reads generated by sequencing protein-DNA interactions in embryonic stem cells. Methods Mol. Biol. 1150, 81–95 [DOI] [PubMed] [Google Scholar]
- 23.Kharchenko P. V., Tolstorukov M. Y., Park P. J. (2008) Design and analysis of ChIP-seq experiments for DNA-binding proteins. Nat. Biotechnol. 26, 1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinlan A. R., Hall I. M. (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathelier A., Zhao X., Zhang A. W., Parcy F., Worsley-Hunt R., Arenillas D. J., Buchman S., Chen C. Y., Chou A., Ienasescu H., Lim J., Shyr C., Tan G., Zhou M., Lenhard B., Sandelin A., Wasserman W. W. (2014) JASPAR 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res. 42, D142–D147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z., Sun L., Wang Y., Ning G., Minto A. W., Kong J., Quigg R. J., Li Y. C. (2008) Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 73, 163–171 [DOI] [PubMed] [Google Scholar]
- 27.Takemoto M., Asker N., Gerhardt H., Lundkvist A., Johansson B. R., Saito Y., Betsholtz C. (2002) A new method for large scale isolation of kidney glomeruli from mice. Am. J. Pathol. 161, 799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan W., Pan W., Kong J., Zheng W., Szeto F. L., Wong K. E., Cohen R., Klopot A., Zhang Z., Li Y. C. (2007) 1,25-Dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J. Biol. Chem. 282, 29821–29830 [DOI] [PubMed] [Google Scholar]
- 29.Li Y. C., Bolt M. J. G., Cao L.-P., Sitrin M. D. (2001) Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am. J. Physiol. Endocrinol. Metab. 281, E558–E564 [DOI] [PubMed] [Google Scholar]
- 30.Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- 31.Heilig C. W., Liu Y., England R. L., Freytag S. O., Gilbert J. D., Heilig K. O., Zhu M., Concepcion L. A., Brosius F. C. III (1997) D-glucose stimulates mesangial cell GLUT1 expression and basal and IGF-I-sensitive glucose uptake in rat mesangial cells: implications for diabetic nephropathy. Diabetes 46, 1030–1039 [DOI] [PubMed] [Google Scholar]
- 32.Birukova A. A., Liu F., Garcia J. G., Verin A. D. (2004) Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L86–L93 [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Zhang J., Ge X., Du J., Deb D. K., Li Y. C. (2013) Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J. Biol. Chem. 288, 19450–19458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziyadeh F. N. (2004) Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J. Am. Soc. Nephrol. 15, S55–S57 [DOI] [PubMed] [Google Scholar]
- 35.Yu L., Border W. A., Huang Y., Noble N. A. (2003) TGF-beta isoforms in renal fibrogenesis. Kidney Int. 64, 844–856 [DOI] [PubMed] [Google Scholar]
- 36.Murata H., Zhou L., Ochoa S., Hasan A., Badiavas E., Falanga V. (1997) TGF-beta3 stimulates and regulates collagen synthesis through TGF-beta1-dependent and independent mechanisms. J. Invest. Dermatol. 108, 258–262 [DOI] [PubMed] [Google Scholar]
- 37.Qi Z., Fujita H., Jin J., Davis L. S., Wang Y., Fogo A. B., Breyer M. D. (2005) Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54, 2628–2637 [DOI] [PubMed] [Google Scholar]
- 38.Hudkins K. L., Pichaiwong W., Wietecha T., Kowalewska J., Banas M. C., Spencer M. W., Mühlfeld A., Koelling M., Pippin J. W., Shankland S. J., Askari B., Rabaglia M. E., Keller M. P., Attie A. D., Alpers C. E. (2010) BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J. Am. Soc. Nephrol. 21, 1533–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pichaiwong W., Hudkins K. L., Wietecha T., Nguyen T. Q., Tachaudomdach C., Li W., Askari B., Kobayashi T., O’Brien K. D., Pippin J. W., Shankland S. J., Alpers C. E. (2013) Reversibility of structural and functional damage in a model of advanced diabetic nephropathy. J. Am. Soc. Nephrol. 24, 1088–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos L. S., Zippin J. H., Kamenetsky M., Buck J., Levin L. R. (2008) Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J. Gen. Physiol. 132, 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brownlee M. (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 [DOI] [PubMed] [Google Scholar]
- 42.Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 [DOI] [PubMed] [Google Scholar]
- 43.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group (2003) Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290, 2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pirola L., Balcerczyk A., Okabe J., El-Osta A. (2010) Epigenetic phenomena linked to diabetic complications. Nat. Rev. Endocrinol. 6, 665–675 [DOI] [PubMed] [Google Scholar]
- 45.De Boer I. H., Rue T. C., Cleary P. A., Lachin J. M., Molitch M. E., Steffes M. W., Sun W., Zinman B., Brunzell J. D., White N. H., Danis R. P., Davis M. D., Hainsworth D., Hubbard L. D., Nathan D. M.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group (2011) Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch. Intern. Med. 171, 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato M., Natarajan R. (2014) Diabetic nephropathy--emerging epigenetic mechanisms. Nat. Rev. Nephrol. 10, 517–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Osta A., Brasacchio D., Yao D., Pocai A., Jones P. L., Roeder R. G., Cooper M. E., Brownlee M. (2008) Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J. Exp. Med. 205, 2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brasacchio D., Okabe J., Tikellis C., Balcerczyk A., George P., Baker E. K., Calkin A. C., Brownlee M., Cooper M. E., El-Osta A. (2009) Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 58, 1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miao F., Smith D. D., Zhang L., Min A., Feng W., Natarajan R. (2008) Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation: an epigenetic study in diabetes. Diabetes 57, 3189–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miao F., Wu X., Zhang L., Riggs A. D., Natarajan R. (2008) Histone methylation patterns are cell-type specific in human monocytes and lymphocytes and well maintained at core genes. J. Immunol. 180, 2264–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miao F., Wu X., Zhang L., Yuan Y. C., Riggs A. D., Natarajan R. (2007) Genome-wide analysis of histone lysine methylation variations caused by diabetic conditions in human monocytes. J. Biol. Chem. 282, 13854–13863 [DOI] [PubMed] [Google Scholar]
- 52.Sun G., Reddy M. A., Yuan H., Lanting L., Kato M., Natarajan R. (2010) Epigenetic histone methylation modulates fibrotic gene expression. J. Am. Soc. Nephrol. 21, 2069–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy M. A., Zhang E., Natarajan R. (2015) Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia 58, 443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heilig C., Zaloga C., Lee M., Zhao X., Riser B., Brosius F., Cortes P. (1995) Immunogold localization of high-affinity glucose transporter isoforms in normal rat kidney. Lab. Invest. 73, 674–684 [PubMed] [Google Scholar]
- 55.Marcus R. G., England R., Nguyen K., Charron M. J., Briggs J. P., Brosius F. C. III (1994) Altered renal expression of the insulin-responsive glucose transporter GLUT4 in experimental diabetes mellitus. Am. J. Physiol. 267, F816–F824 [DOI] [PubMed] [Google Scholar]
- 56.D'Agord Schaan B., Lacchini S., Bertoluci M. C., Irigoyen M. C., Machado U. F., Schmid H. (2001) Increased renal GLUT1 abundance and urinary TGF-beta 1 in streptozotocin-induced diabetic rats: implications for the development of nephropathy complicating diabetes. Horm. Metab. Res. 33, 664–669 [DOI] [PubMed] [Google Scholar]
- 57.Wellen K. E., Hatzivassiliou G., Sachdeva U. M., Bui T. V., Cross J. R., Thompson C. B. (2009) ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.