Abstract
Background
Periconception maternal nutrition and folate in particular are important factors influencing the incidence of Neural Tube Defects (NTDs). Many but not all NTDs are prevented by folic acid supplementation and there is a pressing need for additional strategies to prevent these birth defects. Other micronutrients such as iron are potential candidates, yet a clear role for iron deficiency in contributing to NTDs is lacking. Our previous studies with the flatiron (ffe) mouse model of Ferroportin1 (Fpn1) deficiency suggest that iron is required for neural tube closure and forebrain development raising the possibility that iron supplementation could prevent NTDs.
Methods
We determined the effect of periconception iron and/or folic acid supplementation on the penetrance of NTDs in the Fpn1ffe mouse model. Concurrently, measurements of folate and iron were made to ensure supplementation had the intended effects.
Results
High levels of iron supplementation significantly reduced the incidence of NTDs in Fpn1ffe mutants. Fpn1 deficiency resulted in reduced folate levels in both pregnant dams and embryos. Yet folic acid supplementation did not prevent NTDs in the Fpn1ffe model. Similarly, forebrain truncations were rescued with iron. Surprisingly, the high levels of iron supplementation used in this study caused folate deficiency in wildtype dams and embryos.
Conclusions
Our results demonstrate that iron supplementation can prevent NTDs and forebrain truncations in the Fpn1ffe model. Surprisingly, high levels of iron supplementation and iron overload can cause folate deficiency. If iron is essential for neural tube closure, it is possible that iron deficiency might contribute to NTDs.
Keywords: Neural tube defects, spina bifida, exencephaly, iron deficiency, folic acid supplementation
Introduction
Neural tube defects are among the most common structural birth defects in humans affecting anywhere from 1 in 100 to 6 in 10,000 live births (Li et al., 2006; Liu et al., 2016; Parker et al., 2010; Zohn, 2012, 2014). NTDs such as anencephaly and spina bifida occur when neural tube closure fails in the anterior and posterior ends of the neural tube, respectively. The causes of NTDs are complex and involve both genetic and environmental factors (Zohn, 2012, 2014; Zohn and Sarkar, 2008). Multiple studies implicate periconception maternal nutrition as an important factor influencing the occurrence of NTDs and folic acid has emerged as an important micronutrient (Blom et al., 2006; Czeizel, 2009; Obican et al., 2010; Scott et al., 1990; Smithells et al., 1976). Furthermore, human and animal studies demonstrate a clear benefit of folic acid supplementation for the prevention of NTDs (Czeizel, 2009; Gray and Ross, 2009; Harris, 2009; Obican et al., 2010). To improve folate levels in women of childbearing age, wheat flour is now fortified with folic acid in many countries and is associated with significant reductions in the incidence of NTDs (Crider et al., 2011; Eichholzer et al., 2006; Obican et al., 2010). However, fortification has only reduced NTDs rates to certain levels (Crider et al., 2011). Similarly, NTDs in many mouse models are not prevented by folic acid supplementation (Gray and Ross, 2009; Harris, 2009). Together these observations suggest that not all NTDs can be prevented by folic acid supplementation. Consequently, NTDs still represent a significant proportion of birth defects and there is a pressing need for additional strategies for prevention.
Other nutrients have emerged from retrospective studies as potential factors to influence the incidence of NTDs (Czeizel, 2009; Czeizel and Banhidy, 2011; Kappen, 2013; Scott et al., 1990). Iron deficiency is one of the most common micronutrient deficiencies in women of childbearing age (Lopez et al., 2016). Iron and folate deficiencies often occur simultaneously and iron and folate metabolism are linked in many ways (Herbig and Stover, 2002). However, unlike the wealth of data supporting the importance of folate in prevention of NTDs, only a handful of studies directly investigated the impact of iron and with mixed results (Felkner et al., 2005; Groenen et al., 2004; Molloy et al., 2014; Weekes et al., 1992). Mouse models with disruption of iron homeostasis have not provided clarity due to early embryonic lethality or redundancy (De Domenico et al., 2008).
Our previous studies suggested iron might be required for neural tube closure (Mao et al., 2010; Zohn et al., 2007). In the ENU-induced flatiron (ffe) mouse line, we identified a hypomorphic mutation in the iron exporter Fpn1 resulting in NTDs. During neurulation Fpn1 is expressed in tissues essential for delivery of nutrients to the embryo (Donovan et al., 2000; Donovan et al., 2005). Conditional deletion studies demonstrate that Fpn1 expression in the visceral endoderm and visceral endoderm-derived lineages of the yolk sac is critical for neural development (Mao et al., 2010). Multiple transporters are localized to the apical surface of the visceral endoderm to mediate iron uptake from the maternal environment, but Fpn1 is the only transporter on the basal surface responsible for export of iron out of the visceral endoderm to the developing embryo (Donovan et al., 2005). Thus mutation of Fpn1 is expected to result in iron overload in the visceral endoderm along with iron deficiency in the embryo proper.
The visceral endoderm not only provides nutrients to the embryo, but also functions as a specialized signaling center necessary for induction of the anterior neural tube (Srinivas, 2006; Stower and Srinivas, 2014). Mutations that affect formation and/or function of the anterior visceral endoderm (AVE) result in a spectrum of phenotypes ranging from mild anterior truncations to headless embryos (Acampora et al., 1998; Kimura et al., 2000; Thomas and Beddington, 1996). The AVE initially forms at the distal end of the embryo and migrates to the anterior region to overlie the nascent anterior neural plate (Rodriguez et al., 2001; Srinivas et al., 2004; Thomas and Beddington, 1996). In addition to neural tube closure defects, Fpn1 mutants show forebrain truncations that are also dependent on expression of Fpn1 in the visceral endoderm lineage (Mao et al., 2010). Since forebrain truncations can be phenocopied by culture of wildtype embryos with iron chelators, iron deficiency is likely responsible for these defects (Mao et al., 2010). On the other hand, migration of the AVE is impaired in Fpn1ffe/null trans-heterozygous embryos indicating that iron overload in the visceral endoderm might also have a negative impact on embryonic development (Mao et al., 2010). While a sizable domain of anterior forebrain is initially induced in Fpn1 mutants, this anterior neural tissue is not maintained and by E8.5 the forebrain is severely truncated. Thus, defects in Fpn1 mutants could be due to iron overload in the visceral endoderm, iron deficiency in the embryo proper or a combination of the two. To begin to distinguish between these possibilities, in this study we supplemented Fpn1ffe mice with a relatively high levels of iron. Because of the hypomorphic nature of the Fpn1ffe mutation, this is predicted to increase iron overload in the visceral endoderm but at the same time, increase iron transport to the embryo. Our data demonstrate that a periconceptional iron supplementation reduced the incidence of NTDs in Fpn1 mutants. While additional experiments will be necessary to definitively demonstrate this, our data support the idea that NTDs might be due to iron deficiency rather than iron overload in the visceral endoderm. Surprisingly, we found that Fpn1 mutation results in folate deficiency in both Fpn1ffe/+ dams and mutant embryos. Yet folic acid supplementation, while improving folate status, did not prevent NTDs in the Fpn1ffe model.
Materials and Methods
Mouse Lines and Diet supplementation
The Fpn1ffe mouse line was described previously (Zohn et al., 2007) and crossed onto a C3H background (C3H/HeNcrl, Charles River Laboratories) for at least 10 generations before analysis. The diets used in this study are based on the AIN-76A rodent diet and were manufactured by Research Diets, Inc (New Brunswick, NJ). High iron diets have added 0.5% carbonyl iron (Sigma) and folic acid supplementation with 10 ppm folic acid compared to 2 ppm in the control diet. A different color dye was added to each diet for ease of identification. Wildtype or Fpn1ffe/+ females from crosses between wildtype females and Fpn1ffe/+ males were switched from standard rodent chow (Tekland Global #2918 with 200 mg/kg iron and 4 mg/kg folate) to the four diets at weaning for approximately 4 weeks before mating.
Mating Experiments and Phenotypic Analysis
Females were mated with wildtype or Fpn1ffe/+ males and copulation verified by the presence of a vaginal plug 0.5 days post coitum (dpc). Pregnant females were kept on diets until sacrificed at 9.5 or 11.5 dpc. Upon sacrifice, maternal blood was retrieved by cardiac puncture. Blood was allocated to heparin-coated tubes for analysis of folate levels in whole blood, uncoated Eppendorf tubes for serum separation and analysis of serum ferritin levels or EDTA coated tubes for complete blood counts (CBC). Embryos were dissected and exencephaly assessed by visual inspection. Yolk sacs were used to genotype embryos as described previously (Mao et al., 2010; Zohn et al., 2007). A proportion of embryos dissected at 9.5 dpc were subjected to in situ hybridization analysis with a digoxigenin-labeled antisense probe targeting Six3 (Mao et al., 2010). These embryos were also used to measure the size of the forebrain, crown-rump length and somite numbers. Embryos dissected at 11.5 dpc were used for analysis of folate levels.
Analysis of Ferritin and Folate in dams and embryos
Serum ferritin levels were determined by ELISA according to manufactures instructions (Abnova Ferritin (Mouse) ELISA Kit #KA1941). Folate levels were determined by the Microbiological method using Enterococcus hirae (ATCC 8043) as described (Horne and Patterson, 1988; Molloy and Scott, 1997). For determination of folate content in embryos, 11.5 dpc embryos were processed as described (Kur et al., 2014) then folate levels determined by the Microbiological assay. Blood samples were sent to Charles River Laboratories, Inc (USA) for CBC analysis.
Statistical methods
Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc. La Jolla, CA). All results are reported as mean ±SE. The Fisher's Exact test was used to determine significance of reductions in NTD frequency. The significance of the effect of diets on nutrient levels and CBC analyses were determined using 2-factor ANOVA with post hoc analysis by Sidak's multiple comparisons or Tukey tests as indicated. Significance of changes in forebrain size, embryo weight and crown rump length were determined by the unpaired t-test.
Results
Iron Supplementation reduces the incidence of NTD in Fpn1ffe/ffe mutant embryos
To determine if iron supplementation could reduce the incidence of NTD in Fpn1ffe/ffe mutant embryos, Fpn1ffe/+ females were fed either a standard synthetic control diet or the identical diet supplemented with 0.5% carbonyl iron for four weeks beginning at weaning. Supplemented females were mated to Fpn1ffe/+ males and timed pregnancies recorded. For these studies, a relatively high dosage of supplemental iron (0.5% carbonyl iron) was used. Previous work demonstrated that the Fpn1ffe mutation results in greatly reduced activity of the Fpn1 iron transporter (Zohn et al., 2007). Thus, we reasoned a high dosage of iron would be needed to allow for sufficient iron transport on this hypomorphic mutant background.
While Fpn1ffe/ffe mutants do show both exencephaly and spina bifida (Mao et al., 2010), spina bifida is difficult to assess at earlier stages of development and only exencephaly was scored in this study. At 9.5 dpc, exencephaly was counted in 13-24 somite-staged embryos by visual inspection when the neural folds failed to transform from the convex to midline convergent morphology. In embryos dissected at 11.5 dpc, exencephaly was scored when the brain exhibited the “cauliflower like” morphology typical of exencephaly. As shown in Figure 1, the frequency of NTDs in embryos from dams fed the control diet was approximately 75% (n=48). Supplementation with 0.5% carbonyl iron significantly reduced the incidence of NTDs to 40% (p=0.0002). No difference was observed in the frequency of NTDs between embryos analyzed at 9.5 and 11.5 dpc (Supplemental Figure S1). These data demonstrates that NTDs in Fpn1ffe mutants can be prevented by periconceptional iron supplementation.
Figure 1. Periconceptional supplementation with a high iron diet but not folic acid prevents NTDs in the Fpn1ffe mouse line.
A-A”. A. Normal neural tube closure in a 9.5 dpc Fpn1ffe/+ embryo compared to A’ exencephaly in an Fpn1ffe/ffe mutant from a dam fed the control diet. A” normal morphology and neural tube closure in an Fpn1ffe/ffe mutant from a dam fed the high iron diet. Size bar = 1 mm. B. Frequency of NTDs in Fpn1ffe/ffe mutant embryos from dams supplemented with control (yellow bar), high folic acid (10 ppm, orange bar), high iron (0.5% carbonyl iron, blue bar) or high folic acid and iron (purple bar) diets for 4 weeks before mating. Statistical significance was determined by the Fisher's Exact test and p-values: =0.0002 ***, ≤0.0001 **** or non significant (ns). The number of samples represented in each group is indicated.
At 9.5 dpc, crown-rump measurements indicate that Fpn1ffe mutant embryos were smaller than wildtype littermates on both the control (p=0.06) and high iron (p≤0.01) diets (Figure 2A). While smaller, mutant embryos dissected at 9.5 dpc were not developmentally delayed compared to wildtype littermates as indicated by somite numbers (Figure 2B). Similarly, developmental stage was essentially the same in embryos dissected at 9.5 dpc from dams fed the control versus high iron diets (Figure 2B). Weights of wildtype versus mutant embryos dissected at 11.5 dpc from dams fed either control or high folic acid diets were similar (p>0.05). However, weights of mutant and wildtype embryos from dams fed the high iron diet were smaller than embryos from dams fed the control diets (p≤0.005, Figure 2C).
Figure 2. Effects of iron and folate supplementation on embryo size and developmental stage.
A. Comparison of crown to rump length (A) and somite numbers (B) of embryos from Fpn1ffe/ffe mutant embryos (White Bars in A) and wildtype littermates (Colored Bars in A) dissected at 9.5 dpc from dams supplemented with control (yellow), high folic acid (10 ppm, orange), high iron (0.5% carbonyl iron, blue) or high folic acid and iron (purple) diets for 4 weeks before mating. C. Comparison of weights of Fpn1ffe/ffe mutant embryos and wildtype littermates dissected at 11.5 dpc from dams fed the various diets for 4 weeks before mating. Statistical significance was determined by unpaired t-test in A and C, and by 2- factor ANOVA with post-hoc Sidak's multiple comparisons test in B. P-values: ≤0.05 *, ≤0.01 ** or non significant (ns).
NTDs in the Fpn1ffe/ffe line are not prevented by folate supplementation
To determine if folic acid supplementation can prevent NTDs in the Fpn1ffe model, heterozygous females were a fed diet containing 10 or 2 ppm folic acid (high folic acid and control diets, respectively) for four weeks before mating. This protocol prevents NTDs in some mouse lines (Carter et al., 1999; Marean et al., 2011), but in others has a negative impact on embryonic development (Marean et al., 2011). While no obvious adverse effects on development were observed on the Fpn1ffe background, folic acid supplementation did not reduce the frequency of NTDs in Fpn1ffe/ffe mutants (Figure 1B, 82 versus 75% p>0.05). To determine if dual supplementation with folic acid and iron could further reduce the incidence of NTDs, Fpn1ffe/+ females were supplemented with a diet that contains both 10 ppm folic acid and 0.5% carbonyl iron. Dual supplementation did not reduce the frequency of NTDs beyond the reduction seen with iron supplementation alone (31 versus 40%, p>0.05). These results demonstrate that NTDs in the Fpn1ffe/ffe mutant line are not prevented by folate supplementation.
Folate supplementation alone had no effect on the size of 9.5 dpc mutant embryos, but dual supplementation improved crown-rump length of mutant embryos (p≤0.05, Figure 2A). On the other hand, somite/developmental stage was not altered by maternal diet (p>0.05, Figure 2B). Weights of embryos dissected at 11.5 dpc were similar between wildtype and mutant embryos from folate or dual supplemented dams; but dual supplementation restored the reduction in embryos weight observed with iron supplementation (Figure 2C).
Iron Supplementation increases the iron status of wildtype and Fpn1ffe/+ dams
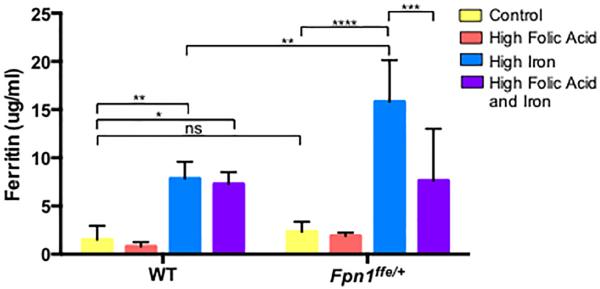
The effect of supplementation on iron status of wildtype and Fpn1ffe/+ dams was determined. Measurements of ferritin levels in the serum of pregnant dams served as a proxy of stored iron (Figure 3). Maternal ferritin levels were increased with iron supplementation in both wildtype dams (1.51±0.45 versus 7.85±1.00 μg ferritin/ml, p≤0.001) and to a greater degree in Fpn1ffe/+ dams (2.32±0.46 versus 15.81±1.94 μg ferritin/ml, p≤0.0001). This enhanced increase in ferritin was expected since the Fpn1ffe is a model of the iron overload disorder Hemochromatosis Type IV (HFE4) (Zohn et al., 2007). Folic acid supplementation did not alter the iron status of neither wildtype (1.51±0.45 versus 0.79±0.27 μg ferritin/ml, p>0.05) nor Fpn1ffe/+ dams (2.32±0.46 versus 1.81±0.19 μg ferritin/ml, p>0.05). Dual supplementation with iron and folic acid had no further effect on elevated iron status in wildtype dams (7.87±1.00 versus 7.29±0.71 μg ferritin/ml, p>0.05). In Fpn1ffe/+ dams, dual supplementation reduced the iron overload observed with iron supplementation alone (7.63±2.41 versus 15.81±1.94 μg ferritin/ml, p≤0.001).
Figure 3. Supplementation with a high iron diet increases iron stores in wildtype and Fpn1ffe/+ dams.
Serum was obtained from pregnant dams upon dissection of embryos at 9.5 or 11.5 dpc. Dams were supplemented with control (yellow bar), high folic acid (10 ppm, orange bar), high iron (0.5% carbonyl iron, blue bar) or high folic acid and iron (purple bar) diets for 4 weeks before mating. Ferritin levels were determined by ELISA and served as a proxy for stored iron levels. Maternal serum ferritin was measured in 3 samples in the wildtype (WT) group and 5 in the Fpn1ffe/+ group. Statistical significance was determined by 2-factor ANOVA with post-hoc Sidak's multiple comparisons test. P-values ≤0.05 *, ≤0.01 **, ≤0.001 ***, ≤0.0001 **** or non significant (ns).
High dose Iron supplementation influences folate status
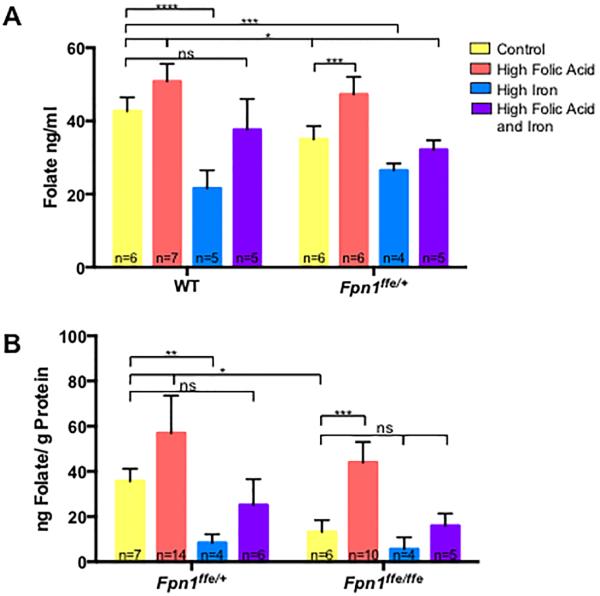
To determine if folic acid supplementation increased folate status of dams, the folate content of whole maternal blood was compared between wildtype and Fpn1ffe dams (Figure 4A). Folate levels were lower in blood from pregnant Fpn1ffe/+ females than wildtype dams fed a control diet (34.99±1.48 versus 42.69±1.55 ng folate/ml, p≤0.05), indicating that Fpn1 deficiency has some impact on folate status. Folate levels increased with folic acid supplementation in both wildtype (42.69±1.55 versus 50.83±1.82 ng folate/ml, p≤0.05) and Fpn1ffe/+ (34.99±1.48 versus 47.29±1.95 ng folate/ml, p≤0.001) dams. Surprisingly, supplementation with 0.5% carbonyl iron significantly reduced maternal folate levels in wildtype dams (42.69±1.55 versus 21.60±2.20 ng folate/ml, p≤0.0001), which was ameliorated by dual supplementation (42.69±1.55 versus 37.63±3.75 ng folate/ml, p>0.05). Folate levels were also reduced in Fpn1ffe/+ dams on the high iron diet (42.69±1.55 versus 26.49±0.95 ng folate/ml, p≤0.001) and improved with dual supplementation (42.69±1.55 versus 32.14±1.15 ng folate/ml, p≤0.05).
Figure 4. Folate levels in dams and embryos.
A. Determination of red blood cell folate levels in pregnant dams. Whole blood was obtained from pregnant wildtype (WT) or Fpn1ffe/+ dams at 9.5 or 11.5 dpc. Dams were supplemented with control (yellow bar), high folic acid (10 ppm, orange bar), high iron (0.5% carbonyl iron, blue bar) or high folic acid and iron (purple bar) diets for 4 weeks before mating. B. Determination of folate levels in 11.5 dpc wildtype (Fpn1+/+) and Fpn1ffe/ffe embryos from dams fed the various diets. The number of samples represented in each group is indicated. The Sidak's test was used to determine significance of multiple comparisons within a genotype and the Tukey test across genotypes. P-values: ≤0.05 *, ≤0.01**, ≤0.001 ***, ≤0.0001 **** or non significant (ns). The number of samples represented in each group is indicated.
Folate levels were also measured in embryos dissected at 11.5 dpc (Figure 4B). Fpn1ffe/ffe mutant embryos showed reduced folate levels compared to wildtype littermates (35.69±3.17 versus 13.17±2.62 ng folate/gm protein, p≤0.05). Folate levels did not correlate with NTDs when comparison was made between Fpn1 mutants with or without NTDs from dams fed the control diet (16.71±7.76 versus 23.08±8.52 ng folate/gm protein, p>0.05, n=3, not shown). Folate levels increased with folic acid supplementation in both Fpn1ffe/ffe mutant embryos (13.17±2.62 versus 43.97±4.05 ng folate/gm protein, p≤0.001) and wildtype littermates (35.69±3.17 versus 56.92±8.32 ng folate/gm protein, p≤0.05). Supplementation with 0.5% carbonyl iron greatly reduced folate levels in wildtype littermates (35.69±3.17 versus 8.37±1.70 ng folate/gm protein, p≤0.01), which was restored with dual supplementation with iron and folic acid in wildtype embryos. Iron supplementation slightly but not significantly reduce folate levels in Fpn1ffe/ffe mutant embryos (13.17±2.62 versus 5.63±3.00 ng folate/gm protein, p=0.12), which improved to control levels with dual supplementation (13.17±2.62 versus 15.92±3.14 ng folate/gm protein, p>0.05).
High dose iron supplementation results in macrocytic anemia typical of folate deficiency
Folate and iron status can affect the red blood cell composition as measured by complete blood count (CBC) analysis. Thus the effect of iron and folic acid supplementation on hematologic parameters was determined (Table 1). There was no significant difference in total red blood cells (RBC), hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) or red blood cell distribution width (RDW) between wildtype or Fpn1ffe/+ dams on the control diet or with folate supplementation. The high iron diet reduced RBC numbers and increased both the MCV and MCH in both the wildtype and Fpn1ffe/+ dams (Table 1). These hematological findings are consistent with the macrocytic anemia that occurs with folate deficiency. Dual supplementation with iron and folic acid reverted these changes in the wildtype, but not Fpn1ffe/+ dams.
Table 1. Mild macrocytic anemia occurs with high iron diet.
Hematological findings from CBC analyses of whole blood obtained from wildtype (WT) or Fpn1ffe/+ females fed control, high folic acid (10 ppm), high iron (0.5% carbonyl iron) or high folic acid and iron diets for 6 weeks. Each group represents values from 3 samples and significance was determined by 2-factor ANOVA.
| Control | High Folic Acid | High Iron | High Folic Acid and High Iron | 2- Factor Anova P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Wildtype | Fpn1ffe/+ | Wildtype | Fpn1ffe/+ | Wildtype | Fpn1ffe/+ | Wildtype | Fpn1ffe/+ | Genotype | Diet | Interaction |
| Red blood cell count, ×106 cells/μL | 9.43 ± 0.31 | 9.54 ± 0.10 | 9.55 ± 0.24 | 9.93 ± 0.41 | 8.90 ± 0.13 | 9.01 ± 0.08 | 9.21 ± 0.07 | 8.97 ± 0.07 | 0.58 | 0.01 | 0.57 |
| Hemoglobin, g/dL | 15.23 ± 0.37 | 15.25 ± 0.03 | 15.33 ± 0.45 | 15.33 ± 0.40 | 15.20 ±0.50 | 15.45 ± 0.09 | 15.73 ± 0.27 | 15.35 ± 0.03 | 0.69 | 0.65 | 0.55 |
| Hematocrit, % | 51.37 ± 1.38 | 51.15 ± 0.03 | 52.13 ± 1.24 | 53.83 ± 1.34 | 50.90 ± 0.92 | 52.15 ±0.61 | 53.33 ± 0.38 | 51.80 ± 0.12 | 0.65 | 0.22 | 0.31 |
| Mean corpuscular volume, fL | 54.47 ± 0.38 | 53.65 ± 0.61 | 54.57 ± 0.33 | 54.27 ± 0.98 | 56.27 ± 1.10 | 57.90 ± 0.17 | 57.93 ± 0.15 | 57.75 ± 0.61 | 0.85 | <0.0001 | 0.27 |
| Mean corpuscular hemoglobin, pg | 16.20 ± 0.15 | 16.00 ± 0.17 | 16.03 ± 0.18 | 15.97 ± 0.32 | 17.17 ± 0.19 | 17.20 ± 0.06 | 17.07 ± 0.24 | 17.15 ± 0.09 | 0.78 | <0.0001 | 0.88 |
| Red cell distribution width, % | 12.73 ± 0.18 | 13.65 ± 0.55 | 12.87 ± 0.15 | 13.17 ± 0.09 | 13.23 ± 0.55 | 12.20 ± 0.06 | 13.03 ± 0.13 | 15.30 ± 0.58 | 0.03 | 0.005 | 0.003 |
Iron supplementation prevents forebrain truncations in Fpn1ffe/ffe mutant embryos
Our previous studies demonstrate that Fpn1ffe/ffe mutant embryos show forebrain truncations (Mao et al., 2010). To determine if iron, folate or combined supplementation can rescue forebrain defects in Fpn1ffe/ffe mutants, the size of the forebrain was measured in 9.5 dpc embryos from pregnant dams fed the various diets. Measurements were taken from the most rostral point of the eye vesicle to the most rostral point on the forebrain (Figure 5A). As previously demonstrated in embryos from dams fed standard mouse chow (Mao et al., 2010), the forebrain was significantly smaller in Fpn1ffe/ffe mutant embryos compared to wildtype littermates from dams fed the control diet (0.44±0.02 versus 0.28±0.007 mm, p≤0.0005). Interestingly, Fpn1ffe/ffe mutant embryos without NTDs had normal sized forebrains regardless of diet (data not shown), thus all measurements were done on embryos with NTDs. The high folic acid diet had no significant effect on the reduced forebrain size of Fpn1ffe/ffe mutants (0.44±0.002 versus 0.26±0.01 mm, p≤0.0005) but iron supplementation alone or dual supplementation restored forebrain size to wildtype levels in mutants with NTDs (0.44±0.02 versus 0.38±0.03 mm, p>0.05) and (0.44±0.02 versus 0.32±0.05 mm, p>0.05), respectively.
Figure 5. Forebrain truncations in Fpn1ffe/ffe mutant embryos are rescued by supplementation with a high iron diet.
A. In situ hybridization to detect Six3 expression in 9.5 dpc embryos. The forebrain was measured from the rostral point of the optic vesicle (stained by Six3) to the most rostral point of the forebrain as indicated by white line. B. Forebrain measurements in 9.5 dpc wildtype (Fpn1+/+) and Fpn1ffe/ffe embryos from dams fed control (yellow bar), high folic acid (10 ppm, orange bar), high iron (0.5% carbonyl iron, blue bar) or high folic acid and iron (purple bar) diets for 4 weeks before mating. Statistical significance was determined by the unpaired t-test. P-values: ≤0.0005 *** or non significant (ns). The number of samples represented in each group is indicated.
Discussion
Our previous studies of the Fpn1ffe mouse line suggested that NTDs and forebrain truncations could be due to either iron deficiency in the embryo or iron overload in the visceral endoderm (Mao et al., 2010). In this study, we supplemented Fpn1ffe pregnancies with a relatively high iron diet and determined the effect on the incidence of NTDs and forebrain truncations. We predicted that iron supplementation would improve iron deficiency but also worsen iron overload in the visceral endoderm. Our data demonstrate that both NTDs and forebrain truncations are prevented by iron supplementation suggesting that these defects are likely due to iron deficiency. However, future experiments to measure iron levels in embryos and the visceral endoderm under these conditions are needed to definitively prove this assumption. Our data also suggest that NTDs in the Fpn1ffe model are folate resistant. While the Fpn1ffe mutant embryos have lower folate levels than wildtype littermates, folic acid supplementation did not prevent NTDs. These findings are not entirely surprising as folate deficiency alone is not sufficient to cause NTDs in the absence of additional factors (Burgoon et al., 2002; Burren et al., 2008; Burren et al., 2010). Thus our data indicate that NTDs in the Fpn1 mouse line are iron responsive but folate resistant.
Interaction of iron supplementation and folate deficiency
Our data highlight an important interaction between high levels of iron supplementation and folate status. While supplementation with relatively high levels of iron did prevent NTDs in the Fpn1 mutant mouse line, it had negative effects on folate status in wildtype dams and embryos. Wildtype dams with high levels of iron supplementation showed signs of macrocytic anemia consistent with folate deficiency. This was further supported by improvement of anemia with the addition of folic acid supplementation. High levels of iron supplementation also resulted in reduced weight of embryo dissected at 11.5 dpc that were restored with dual supplementation. Human data support this negative interaction between high levels or iron supplementation/iron overload and folate status. For example, macrocytic anemia has been reported in individuals with the iron overload disorder hemochromatosis (see (Arakawa et al., 1965; Granville and Dameshek, 1958; Koszewski, 1952; Toghill, 1965) for examples).
In our experiments we used a relatively high level of iron supplementation to overcome the reduced iron transport activity of the Fpn1 transporter in the Fpn1ffe model. While these dosages are not likely given to pregnant women, this level of supplementation is within the range of carbonyl iron dosages given in humans with severe anemia. Recommendations for iron supplementation in human populations range from 16 mg iron per day in Canada to 60 mg iron per day by the World Health Organization (Cockell et al., 2009; Stoltzfus and Dreyfuss, 1998). In the United States, the average multivitamin has 18 mg iron and prenatal vitamins contain 30 mg carbonyl iron. However, for severe iron deficiency anemia, dosages of 120-360 mg/day carbonyl iron is given (7-20 fold increase) and 90-150 mg/day (5-8.3 fold increase) is typically prescribed during pregnancy. To compare to guidelines in rodents, the National Research Council recommends 35 mg/kg iron in the average rodent diet and twice this amount during pregnancy (Nutrition, 1995). Thus the addition of 0.5% (500 ppm) carbonyl iron used in this study represents an approximate 15-fold increase over the recommended supplementation levels for rodents but is well within the range of dosages recommended for patients with severe anemia. Future studies will determine if iron supplementation with equivalent dosages used during human pregnancy would also have a similar effect on folate status of mouse dams and embryos.
Iron and folate share many commonalities (Herbig and Stover, 2002). Simultaneous deficiencies are common especially during pregnancy and result in complications including increased risk of anemia, low birth weight, premature birth and mortality. Both iron and folate serve as cofactors for enzymatic reactions involved in a variety of metabolic processes including DNA repair and synthesis. Iron supplementation might influence folate status at multiple levels. For example, Ferritin catabolizes folate into inactive metabolites (Suh et al., 2001; Suh et al., 2000). Thus the high levels of ferritin in the serum of dams supplemented with iron could potentially cause or otherwise contribute to folate deficiency by catabolism of folate. Another molecular link is the regulation of cytoplasmic serine hydroxymethyltransferase (cSHMT) levels by iron (Oppenheim et al., 2001; Oppenheim et al., 2000). On the other hand, sites of iron and folate absorption and hemostasis in the mother and fetus overlap significantly and iron overload in these tissues could potentially interfere with folate absorption and/or metabolism. The primary site of both folate and iron absorption from the diet occurs in the enterocytes of the small intestine with common and distinct transporters (Lipinski et al., 2013; Visentin et al., 2014). Once absorbed, iron and folate are delivered to the liver for storage and/or mobilization to the circulation (Gambling et al., 2011; Lipinski et al., 2013; Visentin et al., 2014). Iron overload occurs in both intestinal enterocytes and liver macrophages with mutation of Fpn1 (Donovan et al., 2005; Zohn et al., 2007). With the relatively high levels of iron given in this study, both sites likely are overloaded with iron potentially interfering with folate absorption and/or metabolism. Similarly, delivery of iron and folate to the embryo during neurulation depends upon the visceral endoderm of the yolk sac (Zohn and Sarkar, 2010) and this tissue also likely becomes overloaded in Fpn1ffe/ffe mutant embryos with high levels of iron supplementation. This could further reduce transport of folate to the embryo.
Role of Fpn1 in transport of other metals
Fpn1 also transports other metals and Fpn1ffe/+ mice show reduced manganese and zinc levels (Madejczyk and Ballatori, 2012; Seo et al., 2016; Yin et al., 2010). Deficiencies of both of these is implicated in increased NTD risk (Buamah et al., 1984; Cavdar et al., 1980; Chandler et al., 2012; Scott et al., 1990; Sever and Emanuel, 1973; Soltan and Jenkins, 1982; Vats et al., 2011; Velie et al., 1999). However, there is an inverse relationship between iron absorption and absorption of zinc and manganese (Erikson et al., 2002; Erikson et al., 2004; Garcia et al., 2007) and iron supplementation competes with Fpn1-mediated transport of these and other metals (Davis et al., 1992; Hansen et al., 2009; O'Brien et al., 2000; Thompson et al., 2006; Zhang et al., 2016). Thus our data that iron supplementation prevents NTDs in this mouse line argues against the possibility that zinc or manganese deficiency are responsible for NTDs in this model. However, additional experiments will be necessary to definitively rule out the involvement of other metals to NTDs in the Fpn1ffe model.
Conclusions
It is well established that iron deficiency during pregnancy results in increased risk of complications such as premature birth, reduced birth weight and intellectual disability (Gambling et al., 2011). Because of the increased iron requirement during pregnancy and the difficulty of replenishing stores under these conditions, it is important that sufficient iron stores are present before conception (Bothwell, 2000). Our results presented here and in our previous studies (Mao et al., 2010; Zohn et al., 2007) make a strong case that sufficient iron stores at conception are also important for successful neural tube closure. This study provides additional support for the possibility that iron deficiency could play a role in NTDs in humans and periconception iron supplementation might prevent some folate resistant NTDs.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the Board of Visitors to the Children's National Medical Center, R21-HD076202 from Eunice Kennedy Shriver National Institute of Child Health & Human Development and Award Number UL1RR031988 from the National Center for Research Resources. Microscopic analysis was carried out at the Children's Research Institute (CRI) Light Microscopy and Image Analysis Core supported by CRI and NIH grant P30HD040677. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Literature Cited
- Acampora D, Avantaggiato V, Tuorto F, Briata P, Corte G, Simeone A. Visceral endoderm-restricted translation of Otx1 mediates recovery of Otx2 requirements for specification of anterior neural plate and normal gastrulation. Development. 1998;125:5091–5104. doi: 10.1242/dev.125.24.5091. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Ohara K, Kakizaki R, Takahashi Y, Hirata K, Fujii M, Konno T, Morikawa T, Chiba F, Chiba R. Folic acid deficiency in hemochromatosis: probably due to a defective storage of folic acid in the liver. The Tohoku journal of experimental medicine. 1965;86:301–306. doi: 10.1620/tjem.86.301. [DOI] [PubMed] [Google Scholar]
- Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nature reviews. 2006;7:724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. The American journal of clinical nutrition. 2000;72:257S–264S. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- Buamah PK, Russell M, Bates G, Ward AM, Skillen AW. Maternal zinc status: a determination of central nervous system malformation. British journal of obstetrics and gynaecology. 1984;91:788–790. doi: 10.1111/j.1471-0528.1984.tb04851.x. [DOI] [PubMed] [Google Scholar]
- Burgoon JM, Selhub J, Nadeau M, Sadler TW. Investigation of the effects of folate deficiency on embryonic development through the establishment of a folate deficient mouse model. Teratology. 2002;65:219–227. doi: 10.1002/tera.10040. [DOI] [PubMed] [Google Scholar]
- Burren KA, Savery D, Massa V, Kok RM, Scott JM, Blom HJ, Copp AJ, Greene ND. Gene-environment interactions in the causation of neural tube defects: folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum Mol Genet. 2008;17:3675–3685. doi: 10.1093/hmg/ddn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burren KA, Scott JM, Copp AJ, Greene ND. The genetic background of the curly tail strain confers susceptibility to folate-deficiency-induced exencephaly. Birth Defects Res A Clin Mol Teratol. 2010;88:76–83. doi: 10.1002/bdra.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M, Ulrich S, Oofuji Y, Williams DA, Ross ME. Crooked tail (Cd) models human folate-responsive neural tube defects. Hum Mol Genet. 1999;8:2199–2204. doi: 10.1093/hmg/8.12.2199. [DOI] [PubMed] [Google Scholar]
- Cavdar AO, Arcasoy A, Baycu T, Himmetoglu O. Zinc deficiency and anencephaly in Turkey. Teratology. 1980;22:141. doi: 10.1002/tera.1420220116. [DOI] [PubMed] [Google Scholar]
- Chandler AL, Hobbs CA, Mosley BS, Berry RJ, Canfield MA, Qi YP, Siega-Riz AM, Shaw GM, National Birth Defects Prevention, S. Neural tube defects and maternal intake of micronutrients related to one-carbon metabolism or antioxidant activity. Birth Defects Res A Clin Mol Teratol. 2012;94:864–874. doi: 10.1002/bdra.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell KA, Miller DC, Lowell H. Application of the Dietary Reference Intakes in developing a recommendation for pregnancy iron supplements in Canada. The American journal of clinical nutrition. 2009;90:1023–1028. doi: 10.3945/ajcn.2009.27561. [DOI] [PubMed] [Google Scholar]
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011;3:370–384. doi: 10.3390/nu3030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE. Periconceptional folic acid and multivitamin supplementation for the prevention of neural tube defects and other congenital abnormalities. Birth Defects Res A Clin Mol Teratol. 2009;85:260–268. doi: 10.1002/bdra.20563. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Banhidy F. Vitamin supply in pregnancy for prevention of congenital birth defects. Current opinion in clinical nutrition and metabolic care. 2011;14:291–296. doi: 10.1097/MCO.0b013e328344b288. [DOI] [PubMed] [Google Scholar]
- Davis CD, Malecki EA, Greger JL. Interactions among dietary manganese, heme iron, and nonheme iron in women. The American journal of clinical nutrition. 1992;56:926–932. doi: 10.1093/ajcn/56.5.926. [DOI] [PubMed] [Google Scholar]
- De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Eichholzer M, Tonz O, Zimmermann R. Folic acid: a public-health challenge. Lancet. 2006;367:1352–1361. doi: 10.1016/S0140-6736(06)68582-6. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biological trace element research. 2002;87:143–156. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Steinnes E, Aschner M. Globus pallidus: a target brain region for divalent metal accumulation associated with dietary iron deficiency. The Journal of nutritional biochemistry. 2004;15:335–341. doi: 10.1016/j.jnutbio.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Felkner MM, Suarez L, Brender J, Scaife B, Hendricks K. Iron status indicators in women with prior neural tube defect-affected pregnancies. Maternal and child health journal. 2005;9:421–428. doi: 10.1007/s10995-005-0017-3. [DOI] [PubMed] [Google Scholar]
- Gambling L, Lang C, McArdle HJ. Fetal regulation of iron transport during pregnancy. The American journal of clinical nutrition. 2011;94:1903S–1907S. doi: 10.3945/ajcn.110.000885. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M. Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicological sciences : an official journal of the Society of Toxicology. 2007;95:205–214. doi: 10.1093/toxsci/kfl139. [DOI] [PubMed] [Google Scholar]
- Granville N, Dameshek W. Hemochromatosis with megaloblastic anemia responding to folic acid; report of a case. The New England journal of medicine. 1958;258:586–589. doi: 10.1056/NEJM195803202581204. [DOI] [PubMed] [Google Scholar]
- Gray JD, Ross ME. Mechanistic insights into folate supplementation from Crooked tail and other NTD-prone mutant mice. Birth Defects Res A Clin Mol Teratol. 2009;85:314–321. doi: 10.1002/bdra.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenen PM, van Rooij IA, Peer PG, Ocke MC, Zielhuis GA, Steegers-Theunissen RP. Low maternal dietary intakes of iron, magnesium, and niacin are associated with spina bifida in the offspring. J Nutr. 2004;134:1516–1522. doi: 10.1093/jn/134.6.1516. [DOI] [PubMed] [Google Scholar]
- Hansen SL, Trakooljul N, Liu HC, Moeser AJ, Spears JW. Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. J Nutr. 2009;139:1474–1479. doi: 10.3945/jn.109.105866. [DOI] [PubMed] [Google Scholar]
- Harris MJ. Insights into prevention of human neural tube defects by folic acid arising from consideration of mouse mutants. Birth Defects Res A Clin Mol Teratol. 2009;85:331–339. doi: 10.1002/bdra.20552. [DOI] [PubMed] [Google Scholar]
- Herbig AK, Stover PJ. Regulation of folate metabolism by iron. In: Massaro EJ, Rogers JM, editors. Folate and Human Development. Humana Press; Totowa, NJ: 2002. pp. 241–262. [Google Scholar]
- Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clinical chemistry. 1988;34:2357–2359. [PubMed] [Google Scholar]
- Kappen C. Modeling anterior development in mice: diet as modulator of risk for neural tube defects. American journal of medical genetics. 2013;163C:333–356. doi: 10.1002/ajmg.c.31380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura C, Yoshinaga K, Tian E, Suzuki M, Aizawa S, Matsuo I. Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Dev Biol. 2000;225:304–321. doi: 10.1006/dbio.2000.9835. [DOI] [PubMed] [Google Scholar]
- Koszewski BJ. The occurrence of megaloblastic erythropoiesis in patients with hemochromatosis. Blood. 1952;7:1182–1195. [PubMed] [Google Scholar]
- Kur E, Mecklenburg N, Cabrera RM, Willnow TE, Hammes A. LRP2 mediates folate uptake in the developing neural tube. Journal of cell science. 2014;127:2261–2268. doi: 10.1242/jcs.140145. [DOI] [PubMed] [Google Scholar]
- Li Z, Ren A, Zhang L, Ye R, Li S, Zheng J, Hong S, Wang T, Li Z. Extremely high prevalence of neural tube defects in a 4-county area in Shanxi Province, China. Birth Defects Res A Clin Mol Teratol. 2006;76:237–240. doi: 10.1002/bdra.20248. [DOI] [PubMed] [Google Scholar]
- Lipinski P, Stys A, Starzynski RR. Molecular insights into the regulation of iron metabolism during the prenatal and early postnatal periods. Cellular and molecular life sciences : CMLS. 2013;70:23–38. doi: 10.1007/s00018-012-1018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang L, Li Z, Jin L, Zhang Y, Ye R, Liu J, Ren A. Prevalence and trend of neural tube defects in five counties in Shanxi province of Northern China, 2000 to 2014. Birth Defects Res A Clin Mol Teratol. 2016 doi: 10.1002/bdra.23486. [DOI] [PubMed] [Google Scholar]
- Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–916. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- Madejczyk MS, Ballatori N. The iron transporter ferroportin can also function as a manganese exporter. Biochimica et biophysica acta. 2012;1818:651–657. doi: 10.1016/j.bbamem.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, McKean DM, Warrier S, Corbin JG, Niswander L, Zohn IE. The iron exporter ferroportin 1 is essential for development of the mouse embryo, forebrain patterning and neural tube closure. Development. 2010;137:3079–3088. doi: 10.1242/dev.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marean A, Graf A, Zhang Y, Niswander L. Folic acid supplementation can adversely affect murine neural tube closure and embryonic survival. Hum Mol Genet. 2011;20:3678–3683. doi: 10.1093/hmg/ddr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy AM, Einri CN, Jain D, Laird E, Fan R, Wang Y, Scott JM, Shane B, Brody LC, Kirke PN, Mills JL. Is low iron status a risk factor for neural tube defects? Birth Defects Res A Clin Mol Teratol. 2014;100:100–106. doi: 10.1002/bdra.23223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. doi: 10.1016/s0076-6879(97)81007-5. [DOI] [PubMed] [Google Scholar]
- Nutrition S.o.L.A. In: Nutrient Requirements of Laboratory Animals. Council NR, editor. NATIONAL ACADEMY PRESS; Washington, D.C.: 1995. [Google Scholar]
- O'Brien KO, Zavaleta N, Caulfield LE, Wen J, Abrams SA. Prenatal iron supplements impair zinc absorption in pregnant Peruvian women. J Nutr. 2000;130:2251–2255. doi: 10.1093/jn/130.9.2251. [DOI] [PubMed] [Google Scholar]
- Obican SG, Finnell RH, Mills JL, Shaw GM, Scialli AR. Folic acid in early pregnancy: a public health success story. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:4167–4174. doi: 10.1096/fj.10-165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim EW, Adelman C, Liu X, Stover PJ. Heavy chain ferritin enhances serine hydroxymethyltransferase expression and de novo thymidine biosynthesis. J Biol Chem. 2001;276:19855–19861. doi: 10.1074/jbc.M100039200. [DOI] [PubMed] [Google Scholar]
- Oppenheim EW, Nasrallah IM, Mastri MG, Stover PJ. Mimosine is a cell-specific antagonist of folate metabolism. J Biol Chem. 2000;275:19268–19274. doi: 10.1074/jbc.M001610200. [DOI] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- Rodriguez TA, Casey ES, Harland RM, Smith JC, Beddington RS. Distinct enhancer elements control Hex expression during gastrulation and early organogenesis. Dev Biol. 2001;234:304–316. doi: 10.1006/dbio.2001.0265. [DOI] [PubMed] [Google Scholar]
- Scott JM, Kirke PN, Weir DG. The role of nutrition in neural tube defects. Annual review of nutrition. 1990;10:277–295. doi: 10.1146/annurev.nu.10.070190.001425. [DOI] [PubMed] [Google Scholar]
- Seo YA, Elkhader JA, Wessling-Resnick M. Distribution of manganese and other biometals in flatiron mice. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2016;29:147–155. doi: 10.1007/s10534-015-9904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever LE, Emanuel I. Is there a connection between maternal zinc deficiency and congenital malformations of the central nervous system in man? Teratology. 1973;7:117. doi: 10.1002/tera.1420070116. [DOI] [PubMed] [Google Scholar]
- Smithells RW, Sheppard S, Schorah CJ. Vitamin dificiencies and neural tube defects. Arch Dis Child. 1976;51:944–950. doi: 10.1136/adc.51.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltan MH, Jenkins DM. Maternal and fetal plasma zinc concentration and fetal abnormality. British journal of obstetrics and gynaecology. 1982;89:56–58. doi: 10.1111/j.1471-0528.1982.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Srinivas S. The anterior visceral endoderm-turning heads. Genesis. 2006;44:565–572. doi: 10.1002/dvg.20249. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Rodriguez T, Clements M, Smith JC, Beddington RS. Active cell migration drives the unilateral movements of the anterior visceral endoderm. Development. 2004;131:1157–1164. doi: 10.1242/dev.01005. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Dreyfuss ML. In: Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. (INACG), I.N.A.C.G., (WHO), W.H.O., (UNICEF), U.N.C.F., editor. ILSI PRESS; Washington, DC.: 1998. [Google Scholar]
- Stower MJ, Srinivas S. Heading forwards: anterior visceral endoderm migration in patterning the mouse embryo. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2014;369 doi: 10.1098/rstb.2013.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JR, Herbig AK, Stover PJ. New perspectives on folate catabolism. Annual review of nutrition. 2001;21:255–282. doi: 10.1146/annurev.nutr.21.1.255. [DOI] [PubMed] [Google Scholar]
- Suh JR, Oppenheim EW, Girgis S, Stover PJ. Purification and properties of a folate-catabolizing enzyme. J Biol Chem. 2000;275:35646–35655. doi: 10.1074/jbc.M005864200. [DOI] [PubMed] [Google Scholar]
- Thomas P, Beddington R. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol. 1996;6:1487–1496. doi: 10.1016/s0960-9822(96)00753-1. [DOI] [PubMed] [Google Scholar]
- Thompson K, Molina R, Donaghey T, Brain JD, Wessling-Resnick M. The influence of high iron diet on rat lung manganese absorption. Toxicology and applied pharmacology. 2006;210:17–23. doi: 10.1016/j.taap.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Toghill PJ. Megaloblastic Anaemia in Haemochromatosis. Postgraduate medical journal. 1965;41:86–88. doi: 10.1136/pgmj.41.472.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats R, Sharma RK, Sharma A. Copper and Manganese Concentration in Neural Tube Defects. Our Nature. 2011;9:173–175. [Google Scholar]
- Velie EM, Block G, Shaw GM, Samuels SJ, Schaffer DM, Kulldorff M. Maternal supplemental and dietary zinc intake and the occurrence of neural tube defects in California. American journal of epidemiology. 1999;150:605–616. doi: 10.1093/oxfordjournals.aje.a010059. [DOI] [PubMed] [Google Scholar]
- Visentin M, Diop-Bove N, Zhao R, Goldman ID. The intestinal absorption of folates. Annual review of physiology. 2014;76:251–274. doi: 10.1146/annurev-physiol-020911-153251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weekes EW, Tamura T, Davis RO, Birch R, Vaughn WH, Franklin JC, Barganier C, Cosper P, Finley SC, Finley WH. Nutrient levels in amniotic fluid from women with normal and neural tube defect pregnancies. Biology of the neonate. 1992;61:226–231. doi: 10.1159/000243747. [DOI] [PubMed] [Google Scholar]
- Yin Z, Jiang H, Lee ES, Ni M, Erikson KM, Milatovic D, Bowman AB, Aschner M. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. Journal of neurochemistry. 2010;112:1190–1198. doi: 10.1111/j.1471-4159.2009.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gilbert ER, Pan S, Zhang K, Ding X, Wang J, Zeng Q, Bai S. Dietary iron concentration influences serum concentrations of manganese in rats consuming organic or inorganic sources of manganese. The British journal of nutrition. 2016;115:585–593. doi: 10.1017/S0007114515004900. [DOI] [PubMed] [Google Scholar]
- Zohn IE. Mouse as a model for multifactorial inheritance of neural tube defects. Birth Defects Res C Embryo Today. 2012;96:193–205. doi: 10.1002/bdrc.21011. [DOI] [PubMed] [Google Scholar]
- Zohn IE. Neural Tube Defects. Principles of Developmental Genetics 2nd Edition. 2014 [Google Scholar]
- Zohn IE, De Domenico I, Pollock A, Ward DM, Goodman JF, Liang X, Sanchez AJ, Niswander L, Kaplan J. The flatiron mutation in mouse ferroportin acts as a dominant negative to cause ferroportin disease. Blood. 2007;109:4174–4180. doi: 10.1182/blood-2007-01-066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohn IE, Sarkar AA. Modeling neural tube defects in the mouse. Current topics in developmental biology. 2008;84:1–35. doi: 10.1016/S0070-2153(08)00601-7. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Sarkar AA. The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birth Defects Res A Clin Mol Teratol. 2010;88:593–600. doi: 10.1002/bdra.20705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.