Abstract
Background
Neural tube defects (NTDs) are among the most common structural birth defects in humans and are caused by the complex interaction of genetic and environmental factors. Periconceptional supplementation with folic acid can prevent NTDs in both mouse models and human populations. A better understanding of how genes and environment interact is critical towards development of rational strategies to prevent NTDs. Low density lipoprotein-related protein 2 (Lrp2) is involved in endocytosis of the folic acid receptor among numerous other nutrients and ligands.
Methods
We determined the effect of iron and/or folic acid supplementation on the penetrance of NTDs in the Lrp2null mouse model. The effects of supplementation on folate and iron status were measured in embryos and dams.
Results
Periconceptional dietary supplementation with folic acid did not prevent NTDs in Lrp2 mutant embryos whereas high levels of folic acid supplementation by intraperitoneal injection did. Importantly, Lrp2null/+ dams had reduced blood folate levels that improved with daily intraperitoneal injections of folate but not dietary supplementation. On the contrary, iron supplementation had no effect on the penetrance of NTDs in Lrp2 mutant embryos and negated the preventative effect of folic acid supplementation in Lrp2null/null mutants.
Conclusions
Lrp2 is required for folate homeostasis in heterozygous dams and high levels of supplementation prevent NTDs. Furthermore, high levels of dietary iron supplementation interfered with folic acid supplementation negating the positive effects of supplementation in this model.
Keywords: Neural tube defects, exencephaly, iron deficiency, folic acid supplementation
Introduction
Neural tube defects (NTDs) are some of the most common structural birth defects affecting humans and occur in approximately 6 in 10,000 live births (Li et al., 2006; Liu et al., 2016; Parker et al., 2010; Zohn, 2012, 2014). NTDs such as anencephaly and spina bifida occur when neural tube closure fails in the anterior and posterior ends of the neural tube, respectively. These structural birth defects are caused by complex interactions of genetic and environmental factors (Zohn, 2012, 2014; Zohn and Sarkar, 2008). Periconception maternal nutrition is an important environmental factor and folic acid has emerged as a critical micronutrient (Blom et al., 2006; Czeizel, 2009; Obican et al., 2010; Scott et al., 1990; Smithells et al., 1976). Abnormalities in folate homeostasis, metabolism and uptake are linked to increased risk for NTDs and folic acid supplementation can prevent many NTDs (Czeizel, 2009; Gray and Ross, 2009; Harris, 2009; Obican et al., 2010). The recommendation that women of childbearing age maintain adequate folate intakes, along with food fortification programs, resulted in significant reductions in the incidence of NTDs (Crider et al., 2011; Eichholzer et al., 2006; Obican et al., 2010).
Folic acid is absorbed by two parallel transport systems (Taparia et al., 2007). The reduced folate carrier (RFC1/Slc19a1) is widely expressed and is the prime transporter involved in uptake of folate from the diet (Qiu et al., 2006; Wang et al., 2001). The folate binding protein (FBP) also known as folate receptor (Folr1) in mice or folate receptor alpha (FRα) in humans binds folate mediating cellular uptake via endocytic mechanisms (Birn, 2006; Zhao and Goldman, 2013). The association of increased autoantibodies against FRα in the serum of women with NTD affected pregnancies and miscarriages illustrate the importance of this receptor for human development (Rothenberg et al., 2004). Uptake of folate bound to Folr1 requires the multifunctional endocytic receptor complex consisting of Low density lipoprotein-related protein 2 (Lrp2, also known as Megalin), Cubulin (Cubn) and/or Amnionless (Amn) (Birn, 2006; Christensen et al., 2013). This receptor complex mediates transport of multiple nutrients, hormones and steroids in the intestine and kidney in addition to the visceral endoderm that mediates nutrient uptake during neurulation of the mouse embryo (Christensen and Birn, 2002; Zohn and Sarkar, 2010). Lrp2 in particular is critical for uptake of folate into the developing neural tube, reabsorption in the renal proximal tubule and intestinal absorption in pups from breast milk (Birn et al., 2005a; (Birn et al., 2005b; Kur et al., 2014; Vazquez-Carretero et al., 2014). While only a single LRP2 polymorphism is implicated in NTDs in humans (Pangilinan et al., 2012), multiple CUBN polymorphisms are associated with altered NTD risk (Franke et al., 2009; Pangilinan et al., 2012). On the other hand, mutation of Lrp2 in mouse results in NTDs along with forebrain truncations (Willnow et al., 1996). While the mechanisms leading to NTDs in Lrp2 mutant embryos are poorly understood, forebrain truncations are linked to the function of Lrp2 as clearance and/or co-receptors for BMP and Shh ligands (Christ et al., 2012; Kur et al., 2014; Spoelgen et al., 2005). Interestingly, reduced levels of folate are found in Lrp2 mutant embryos (Kur et al., 2014), suggesting that folic acid deficiency might contribute to NTDs in this model.
Lrp2 is also a transporter for iron, mediating uptake of transferrin bound iron in the proximal tubule of the adult and visceral endoderm of the embryo (Kozyraki et al., 2001). Iron might also influence the incidence of NTDs (Stokes et al., In Press; Zohn and Sarkar, 2010). Iron deficiency is one of the most common micronutrient deficiencies in women of childbearing age (Lopez et al., 2016). Iron and folate deficiencies often occur simultaneously and iron and folate metabolism and homeostasis are intertwined (Herbig and Stover, 2002). Our recent experiments demonstrate that iron supplementation can prevent NTDs in a mouse model with severe embryonic iron deficiency (Mao et al., 2010; Stokes et al., In Press; Zohn et al., 2007).
Here we demonstrate that high levels of folic acid supplementation by intraperitoneal injection but not dietary supplementation prevent NTDs in Lrp2 mutant embryos. We found that females heterozygous for mutant Lrp2 have reduced blood folate levels, which like NTDs, did not improved with dietary folic acid supplementation. Folate levels did increase in heterozygous dams with high levels of folate supplementation by intraperitoneal injection. On the contrary, iron supplementation had no effect on the penetrance of NTDs in Lrp2 mutant embryos.
Materials and Methods
MOUSE LINES AND DIET SUPPLEMENTATION
The B6;129S7-Lrp2tm1Her (Lrp2null) mouse line (Willnow et al., 1996) was obtained from the Mutant Mouse Resource and Research Center (Stock#029592-Mu) and crossed onto a C3H/HeNcrl background (C3H; Charles River Laboratories) for 5 generations before analysis. The diets used in this study are based on the AIN-76A rodent diet and were manufactured by Research Diets, Inc (New Brunswick, NJ). High iron diets have added 0.5% carbonyl iron (Sigma) and folic acid supplementation with 10 ppm folic acid compared to 2 ppm in the control diet as described (Stokes et al., In Press). Wild type or Lrp2null/+ females from crosses between wild type females and Lrp2null/+ males were switched from standard rodent chow (Tekland Global #2918) that contains 200 mg/kg iron and 4 mg/kg folate) to one of four experimental diets at weaning for approximately 4 weeks before mating. Folic acid (Sigma #F8758) was delivered by daily intraperitoneal injection at a concentration of 25 mg folic acid per kg body weight between 5.5 and 9.5 dpc.
MATING EXPERIMENTS AND PHENOTYPIC ANALYSIS
Lrp2null/+ females fed experimental diets since weaning were mated with Lrp2null/+ males and copulation verified by the presence of a vaginal plug 0.5 days post coitum (dpc). Pregnant females were kept on diets until sacrificed at 9.5 or 11.5 dpc. Upon sacrifice, maternal blood was retrieved by cardiac puncture and allocated to heparin-coated tubes for analysis of folate levels in whole blood or uncoated Eppendorf tubes for serum separation and analysis of serum ferritin levels. Embryos were dissected and exencephaly assessed by visual inspection. Yolk sacs were used to genotype embryos as described (Willnow and Herz, 1994; Willnow et al., 1996). Measurements of forebrain size, crown-rump length and somite numbers were made on E9.5 embryos as described (Stokes et al., In Press). Embryos dissected at 11.5 dpc were used for analysis of folate levels.
ANALYSIS OF FERRITIN AND FOLATE IN DAMS AND EMBRYOS
Folate levels in whole blood and embryos were determined by the Microbiological method using Enterococcus hirae (ATCC 8043) as described (Horne and Patterson, 1988; Molloy and Scott, 1997; Stokes et al., In Press). 11.5 dpc embryos were processed as described for determination of folate content (Kur et al., 2014; Stokes et al., In Press). Serum ferritin levels were determined by an enzyme-linked immunosorbent assay (ELISA; Abnova Ferritin (Mouse) ELISA Kit #KA1941) according to manufactures instructions.
STATISTICAL METHODS
Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc. La Jolla, CA). All results are reported as mean ± Standard Error. The Fisher’s exact test was used to determine significant changes in NTD frequency. The significance of the effect of diets on nutrient levels was determined using two-factor ANOVA with post-hoc analysis using Tukey’s multiple comparisons tests as indicated. The effect of diets on embryo size was determined using the unpaired t-test.
Results
INCREASED PENETRANCE OF NTDS IN LRP2NULL/NULL MUTANT EMBRYOS ON THE C3H GENETIC BACKGROUND
Initial studies reported a relatively low penetrance (~10%) of NTDs in Lrp2null/null embryos on a hybrid 129SvEmcTcr × C57BL/6N background (Willnow et al., 1996). Our previous work with an iron deficient mouse line indicates a higher penetrance of NTDs when the mutation was crossed onto the C3H versus 129 genetic backgrounds (Mao et al., 2010; Stokes et al., In Press; Zohn et al., 2007). Thus we tested whether penetrance of NTDs in the Lrp2null line would similarly increase on the C3H background by crossing the Lrp2null/+ mutation onto the C3H background for five generations. This is predicted to result in approximately 95% of the genome to be of C3H origin (Silver, 1995). 8/12=66.7% (n=12) of N(5)C3H;B6;129S7-Lrp2null/null mutants dissected at E9.5 (17–22 somite stages) from dams fed a control diet showed exencephaly where the neural folds failed to transform from the convex to midline convergent morphology (Fig. 1A and B). At 11.5 dpc anterior NTDs were confirmed when the brain exhibited the typical “cauliflower like” morphology of exencephaly (Fig. 1C and D) and occurred with similar penetrance as in E9.5 embryos (12/22=54.5, n=22; p=0.7170, Fisher’s exact test). Spina bifida was not observed in E11.5 Lrp2null/null mutants.
FIGURE 1.
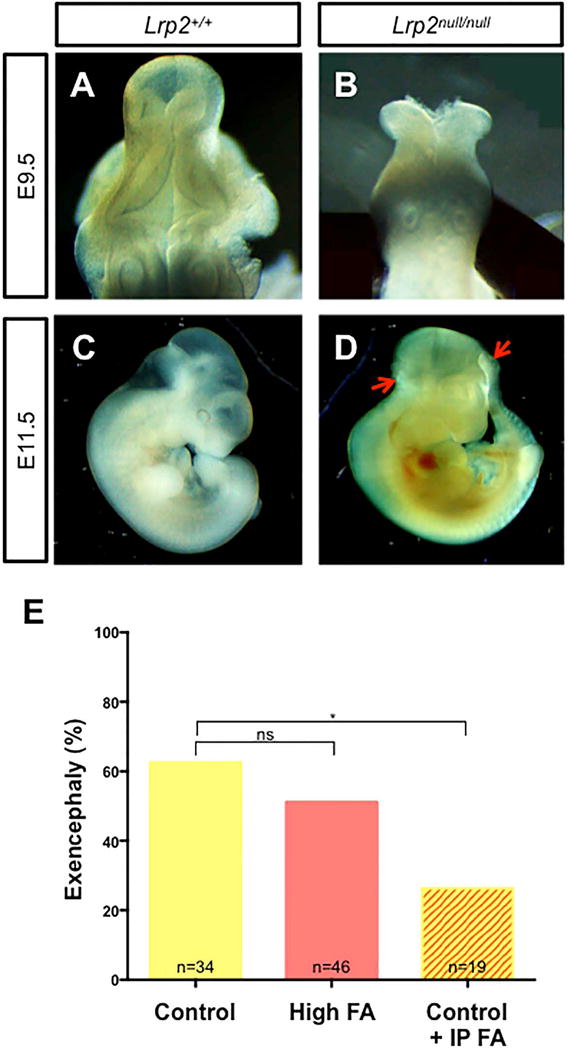
Prevention of NTDs in the Lrp2null mouse line by folic acid supplementation delivered by intraperitoneal injection not in the diet. A–D: Normal neural tube closure in 9.5 dpc (A, B; dorsal view) or 11.5 (C, D; lateral view) wild type (Lrp2+/+) littermates (A, C) compared to exencephaly in Lrp2null/null embryos (B, between arrows in D) from Lrp2null/+ dams fed the control diet. E: Frequency of NTDs in Lrp2null/null mutant embryos from Lrp2null/+ dams fed control (yellow) or folic acid (FA; orange) supplemented diets for 4 weeks before mating or the control diet along with daily intraperitoneal injections of 25 mg/kg folic acid between 5.5 to 9.5 dpc (control + IP FA; yellow/orange stripes). Statistical significance between treatments was determined by the Fisher’s exact test. p-values: ≤0.05 (*), or non significant (ns). The number of samples in each group is indicated.
NTDS IN LRP2NULL/NULL MUTANT EMBRYOS ARE NOT PREVENTED BY PERICONCEPTIONAL DIETARY SUPPLEMENTATION WITH FOLIC ACID
Lrp2 is a key mediator of folate transport during neurulation and Lrp2null/null embryos show reduced folate levels compared to wild type (Lrp2+/+ and Lrp2null/+) littermates (Kur et al., 2014). To determine if folate supplementation could reduce the incidence of NTDs in Lrp2null/null mutant embryos, Lrp2null/+ females were fed either a control diet containing 2 ppm folic acid or a folic acid supplemented diet with 10 ppm folic acid for four weeks before mating. This protocol prevents NTDs in some mouse lines (Carter et al., 1999; Marean et al., 2011). However, folic acid supplementation did not reduce the incidence of NTDs in Lrp2null/null mutant embryos (Fig. 1E; 58.8% on control diet versus 50.0% on the folic acid supplemented diet; p=0.36, Fisher’s exact test).
FOLIC ACID SUPPLEMENTATION IMPROVES GROWTH OF LRP2NULL/NULL MUTANT EMBRYOS
While folic acid supplementation prevents NTDs in some mouse models, supplementation negatively affects development of others (Carter et al., 1999; Marean et al., 2011; Mikael et al., 2013). The severity of developmental defects in Lrp2null/null embryos appeared similar among mutants from dams reared on the different diets (not shown). To quantitate the effect of folate supplementation on development of mutant embryos, crown-rump measurements and somite counts were compared between E9.5 Lrp2null/null mutants from dams fed the different diets. E9.5 Lrp2null/null mutants are similar in size and developmental age to their wild type littermates and each other across the diets (Fig. 2A, B). Similarly, the weight of Lrp2null/null mutant embryos and wild type littermates did not differ at E11.5 on either the control or folic acid supplemented diets (Fig. 2C). On the other hand, folic acid supplementation did result in a slight increase in weight of both Lrp2null/null mutant embryos and wild type littermates compared to embryos from dams fed the control diet (0.03±0.002 versus 0.04±0.004 gm, p=0.0238, Fig. 2C). These results indicate that folic acid supplementation does not have a negative impact on development of the Lrp2null/null mutant embryo but rather positively effects growth of the mid-gestation embryo.
FIGURE 2.
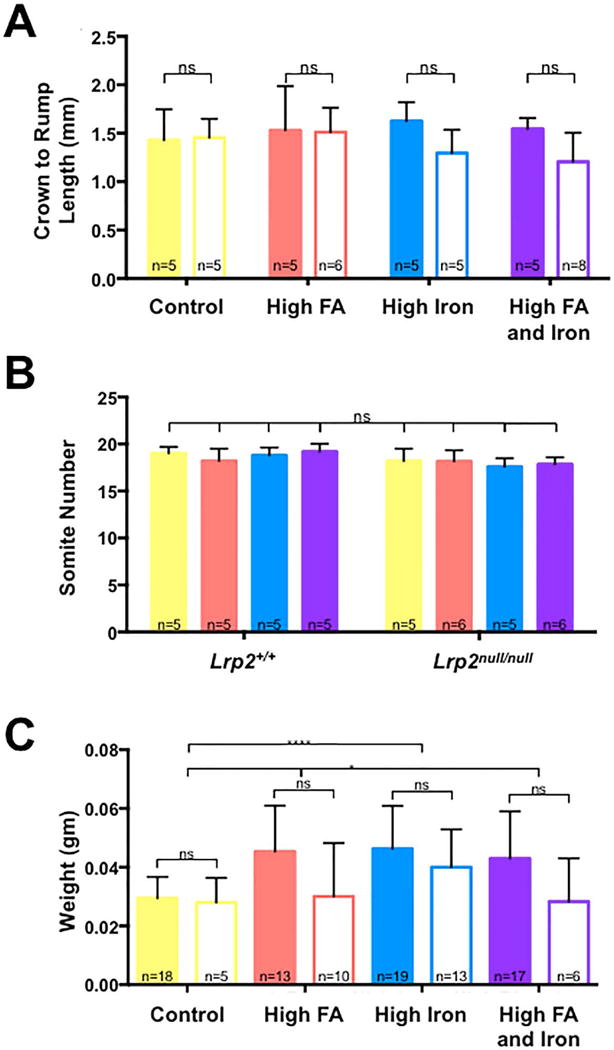
Effects of high iron and folic acid supplementation on growth of embryos. A: Comparison of crown to rump length (A) and somite numbers (B) between Lrp2null/null mutant embryos (no fill) and wild type (Lrp2+/+) littermates (filled) dissected at 9.5 dpc from Lrp2null/+ dams fed control (yellow), folic acid (FA; orange), high iron (blue) or high iron and folic acid (purple) supplemented diets. C: Comparison of weights of Lrp2null/null mutant embryos and wild type littermates dissected at 11.5 dpc from dams fed the indicated diets. Statistical significance was determined by two-factor ANOVA with post-hoc Tukey’s multiple comparisons test. p-values: ≤0.05 (*), ≤0.0001 (****) or non significant (ns). An additional unpaired t-test was performed across the diets in (C). P-values are indicated as 0.012 (*), 0.0238 (*), or ≤0.0001 (****). The number of samples in each group is indicated.
Lrp2null/+ DAMS SHOW REDUCED WHOLE BLOOD FOLATE LEVELS THAT DO NOT IMPROVE WITH DIETARY SUPPLEMENTATION
Since the folic acid supplemented diet appeared to have no effect on the incidence of NTDs in Lrp2null/null mutant embryos, the effect of diets on folate levels in dams and embryos was determined. For these experiments, folate levels were measured by the microbiological method in whole blood from dams or E11.5 embryos. Unexpectedly, heterozygous Lrp2null dams fed the control diet showed significantly lower blood folate levels than wild type dams (26.08±0.82 versus 42.69±1.55 ng folate/ml, p≤0.0001), demonstrating that Lrp2 deficiency has an impact on maternal folate status. Interestingly, while the folic acid supplemented diet increased blood folate levels in wild type dams (42.69±1.55 versus 50.83±1.82 ng folate/ml, p≤0.05) levels in heterozygous Lrp2null/+ dams were not improved (26.08±0.82 versus 23.11±2.9 ng folate/ml, p>0.05). Folate levels were similarly reduced in E11.5 Lrp2null/null mutant embryos compared to wild type littermates (21.36±2.27 versus 42.51±3.55 ng folate/g protein, p≤0.01). Folate levels did improve in Lrp2null/null mutant embryos from Lrp2null/+ dams fed the high folate diets (21.36±2.27 versus 42.51±3.55 ng folate/g protein, p≤0.001) but results did not reach statistical significance in wild type littermates (p>0.05; Fig. 3B). Thus, while reduced blood folate levels in Lrp2null/+ dams did not improve with dietary supplementation, folic acid levels in Lrp2null/null mutant embryos did.
FIGURE 3.
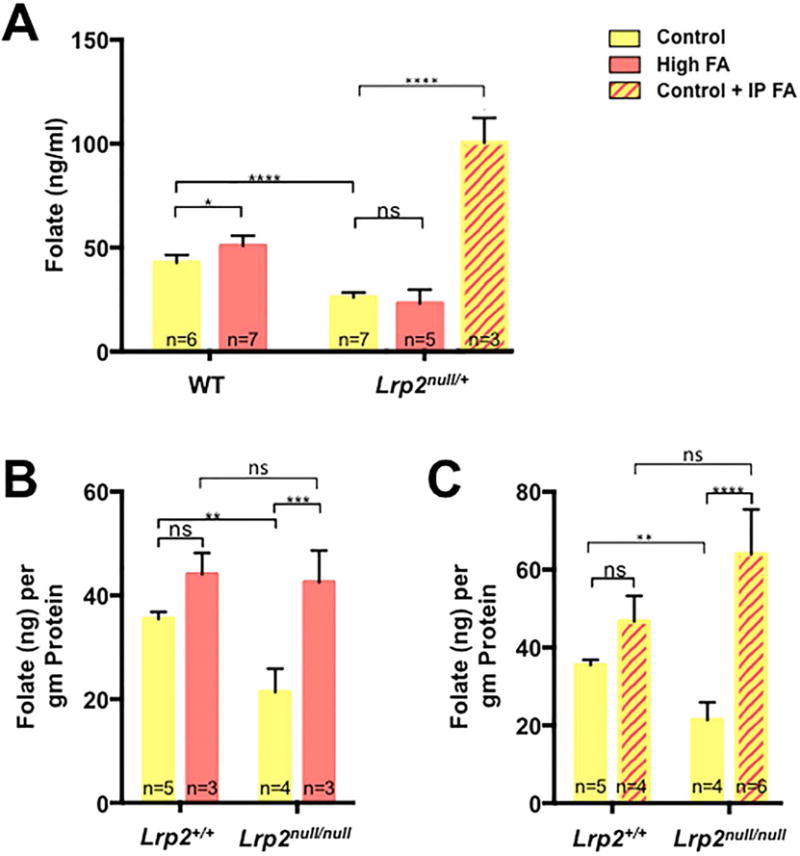
Folate levels in dams and embryos with folic acid supplementation. A: Determination of folate levels in whole blood from wild type (WT) or Lrp2null/+ dams. Dams were fed control (yellow) or folic acid (FA; orange) supplemented diets for 4 weeks before mating or control diet plus daily injections of 25 mg/kg folic acid from E5.5 to 9.5 (control + IP FA; yellow/orange stripes). Maternal blood was collected at 9.5 or 11.5 dpc. B: Determination of folate levels in 11.5 dpc Lrp2null/null mutant embryos and wild type (Lrp2+/+) littermates from Lrp2null/+ dams fed the indicated diets. Statistical significance was determined by two-factor ANOVA with post-hoc Tukey’s multiple comparisons test. p-values: ≤0.05 (*), ≤0.01 (**), ≤0.001 (***), ≤0.0001 (****) or non significant (ns). The number of samples represented in each group is indicated.
INTRAPERITONEAL INJECTION OF HIGH DOSES OF FOLIC ACID PREVENTS NTDS IN LRP2NULL/NULL MUTANT EMBRYOS
Since dietary folic acid supplementation had no effect on NTD incidence in the Lrp2null/null embryo or the folate status of Lrp2null/+ dams, we wanted to determine if delivery of higher levels of folic acid by daily intraperitoneal injections during a critical window of neurulation could prevent NTDs. Lrp2null/+ dams fed the control diet were mated and 25 mg folic acid per kg body weight was administered by daily intraperitoneal injections between 5.5–9.5 dpc. This supplementation protocol significantly reduced the incidence of NTDs in Lrp2null/null mutant embryos from 58.9% on the control diet to 26.3% (Fig. 1E, p≤0.05). NTD prevention correlated with higher folate levels in dams (Fig. 3A, 100.30±6.93 versus 26.08±0.82 ng folate/ml, p≤0.0001) and Lrp2null/null mutant embryos (Fig. 3C, 64.06±4.66 versus 21.36±2.27 ng folate/g protein, p≤0.0001). Folate levels did not significantly increase in wild type littermates (33.02±2.52 versus 46.67±3.31 ng folate/g protein p>0.05). These results demonstrate that folic acid supplementation can prevent NTDs in the Lrp2null line, although a higher dose and altered mode of delivery is required.
SUPPLEMENTATION WITH A HIGH IRON DIET DOES NOT PREVENT NTDS IN LRP2NULL/NULL MUTANT EMBRYOS
In addition to folate, Lrp2 can mediate the cellular uptake of iron (Kozyraki et al., 2001). Our previous studies indicate that severe iron deficiency in the Fpn1ffe mouse model results in NTDs that are prevented by high levels of periconceptional dietary iron supplementation (Mao et al., 2010; Stokes et al., In Press; Zohn et al., 2007). Thus, we tested whether iron supplementation could also prevent NTDs in Lrp2null/null mutant embryos. Lrp2null/+ dams were fed a diet supplemented with 0.5% carbonyl iron (High iron) or 0.5% carbonyl iron and 10 ppm folic acid (High iron and folic acid supplemented diet). Though NTD rates were slightly reduced in mutant embryos from dams supplemented with the high iron (40.7%, n=27) compared to control diets (58.9%, n=34), results did not reach significance (Fig. 4, p=0.2016, Fisher’s exact test). Dual supplementation with high iron and folic acid had no effect on the incidence of NTDs in Lrp2null/null mutant embryos (Fig. 3, 53.2%, n=47, p=0.6560). Unexpectedly, supplementation with the high iron diet negated the preventative effect of daily injections of folic acid on the incidence of NTDs in Lrp2null/null mutant embryos (42.1%, n=19, p=0.2671).
FiGURE 4.
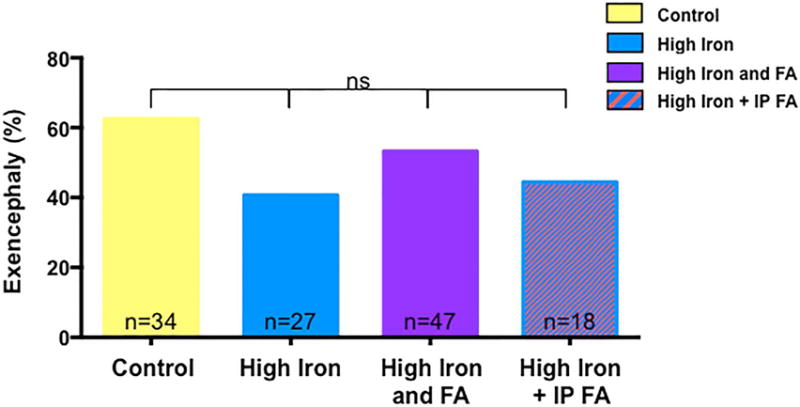
Supplementation with a high iron diet does not prevent NTDs in Lrp2null/null mutant embryos. Frequency of NTDs in Lrp2null/null mutant embryos from dams fed control (yellow), high iron (blue) or high folic acid and iron (high FA and Iron; purple) diets for 4 weeks before mating or high iron diet with daily injections of folic acid (high iron + IP FA; blue/orange stripes). Statistical significance was determined by the Fisher’s Exact test and p-values were non significant (ns) for all treatments. The number of samples represented in each group is indicated.
SUPPLEMENTATION WITH A HIGH IRON DIET INFLUENCES FOLATE LEVELS
The level of iron in our high iron diet corresponds to a high-end dose given to humans, but not typically during pregnancy (Stokes et al., In Press). Furthermore, this diet has a negative impact on folate levels in wild type dams and embryos (Stokes et al., In Press). Since iron supplementation did not prevent NTDs in the Lrp2null line and even had a negative effect on prevention of NTDs by folic acid injection, we determined if the effect of high levels of iron supplementation on folate levels. Lrp2null/+ dams supplemented with 0.5% carbonyl iron showed reduced folate level compared to dams fed the control diet (Fig. 5A; 9.46±0.55 versus 26.08±0.82 ng folate/ml, p≤0.001), which was improved by combined folic acid and iron supplementation (18.20±3.15 ng folate/ml, p>0.05). Folate levels were greatly increased in Lrp2null/+ dams fed the high iron diet that also received daily injections of folic acid (109.16±9.01 ng folate/ml, p≤0.0001).
FIGURE 5.
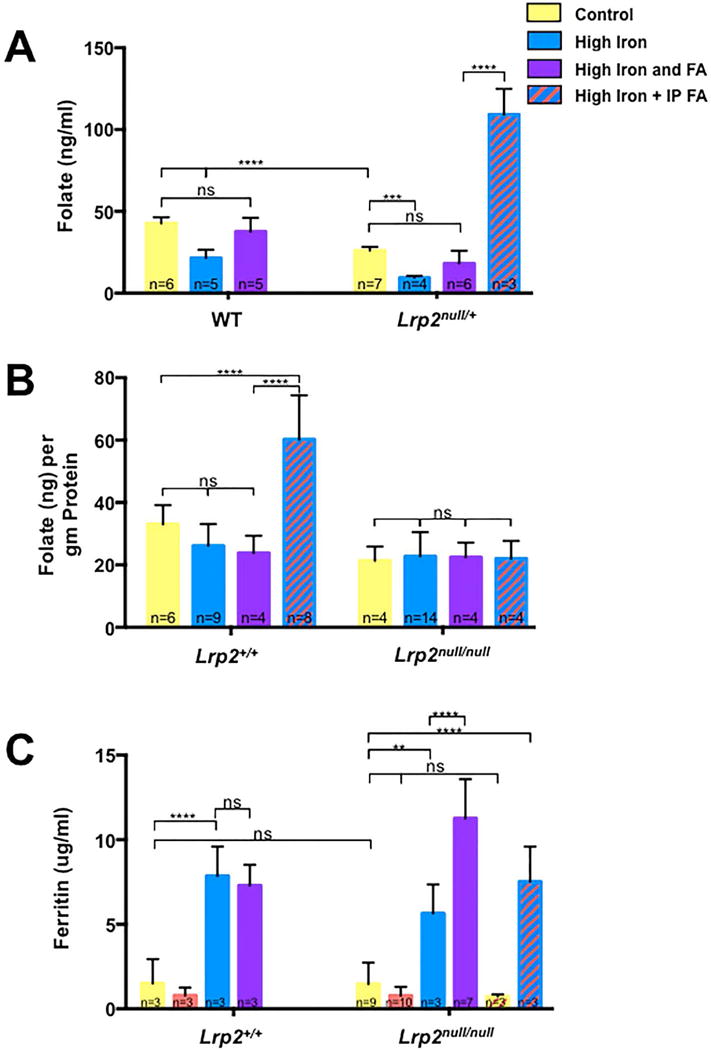
Folate and ferritin levels in dams and embryos with high iron and/or folic acid supplementation. A: Determination of folate levels in whole blood from pregnant wild type (WT) or Lrp2null/+ dams at 9.5 or 11.5 dpc. Dams were fed control (yellow), folic acid (FA; orange), high iron (blue) or high iron and folic acid (purple) diets for 4 weeks before mating or the high iron diet along with folic acid supplemented by daily injection of 25 mg/kg folic acid between 5.5 to 9.5 dpc (high iron + IP FA; blue/orange stripes). B: Determination of folate levels in 11.5 dpc Lrp2null/null mutant embryos and wild type (Lrp2+/+) littermates from Lrp2null/+ dams subjected to the various treatments. C: Ferritin levels in serum from pregnant dams fed the indicated diets was determined by ELISA and served as a proxy for stored iron levels. Statistical significance was determined by two-factor ANOVA with post-hoc Tukey’s multiple comparisons test. p-values: ≤0.01 (**), ≤0.001 (***), ≤0.0001 (****) or non significant (ns). The number of samples represented in each group is indicated.
Unlike our previous results with the Fpn1ffe mouse line (Stokes et al., In Press), supplementation of Lrp2null/+ dams with iron did not significantly reduce folate levels in either Lrp2null/null mutant embryos or wild type littermates (Fig. 5B). While dietary folic acid supplementation did improve folate levels in Lrp2null/null mutant embryos (Fig. 3B; 21.36±2.27 versus 42.51±3.55 ng folate/g protein, p≤0.001), this improvement was not observed with combined folate/iron supplementation (Fig. 5B; 21.36±2.27 versus 22.42±2.38 ng folate/g protein, p≤0.05) or daily injection of folic acid in dams fed the high iron diet (21.36±2.27 versus 22.00±2.87 ng folate/g protein, p≤0.05). On the other hand, daily folic acid injections did significantly increase folate levels in wild type littermates from Lrp2null/+ dams fed the high iron diet (33.02±2.52 versus 60.23±5.00 ng folate/g protein, p≤0.0001).
SUPPLEMENTATION WITH A HIGH IRON DIET INCREASES IRON STORES INDEPENDENTLY OF FOLIC ACID
Iron status of Lrp2null/+ dams was evaluated to determine if levels were altered compared to wild type dams and if iron supplementation had the intended effects on maternal iron status. For this analysis, Ferritin levels in the serum of pregnant dams served as a proxy of iron status. Lrp2null/+ dams fed the control diet were not iron deficient in comparison to wild type dams (1.47±0.42 versus 1.51±0.45 μg ferritin/ml, p>0.05). Supplementation with iron increased maternal ferritin levels in both the wild type (1.51±0.45 versus 7.85±1.0 μg ferritin/ml, p≤0.0001) and Lrp2null/+ dams (1.47±0.42 versus 5.64±1.0 μg ferritin/ml, p≤0.001). Folic acid supplementation alone did not alter the iron status of wild type (1.5±0.45 versus 0.8±0.27 μg ferritin/ml, p>0.05) or Lrp2null/+ dams (1.47±0.42 versus 0.78±0.17 μg ferritin/ml, p>0.05) but dual supplementation increased ferritin levels to a greater degree than the high iron diet alone (5.64±1.0 versus 11.26±0.88 μg ferritin/ml, p≤0.0001). Supplementation of folic acid by daily injections had no effect on ferritin levels in either Lrp2null/+ dams fed the control diet or the high iron diet.
Discussion
Lrp2 acts as clearance/co-receptor for multiple ligands and endocytosis of numerous nutrients (Christensen and Birn, 2002). Furthermore, a previous study demonstrated reduced levels of folate in Lrp2null/null mutant embryos (Kur et al., 2014), suggesting that folate deficiency might be involved in development of NTDs in this mouse line. In the present study, we demonstrate that NTDs in Lrp2null/null mutants can be prevented by high dose supplementation with folic acid by injection but not dietary supplementation. For our studies we crossed the Lrp2null mutation onto the C3H genetic background, which increased the penetrance of NTDs. Thus the genetic background is an important contributor to the incidence of NTDs with Lrp2 mutations, a finding consistent with many other mouse models of NTDs (Greene et al., 2009; Harris and Juriloff, 2010). Since our previous findings demonstrate that iron supplementation can prevent NTDs in a mouse model of iron deficiency (Stokes et al., In Press) and Lrp2 is involved in the uptake of iron (Kozyraki et al., 2001), we tested if NTDs Lrp2null/null mutants might also be prevented by high dosages of iron supplementation. However, high levels of iron supplementation did not prevent NTDs in the Lrp2null/null model and even negated the preventative effects of folic acid supplementation.
ALTERED FOLATE STATUS IN LRP2NULL MUTANTS
One surprising and significant result of this study is that pregnant heterozygous Lrp2null/+ dams have reduced whole blood folate levels compared to wild types. Furthermore, this folate deficiency was not improved with dietary folate supplementation. Lrp2 is involved in the cellular uptake of folate via endocytic mechanisms involving Folr1 and a complex of Lrp2, Cubn and/or Amn (Birn, 2006; Birn et al., 2005a; Birn et al., 2005b; Christensen et al., 2013). Lrp2 is expressed in both the distal small intestine and renal proximal tubule (Christensen and Birn, 2002). While absorption of dietary folate via the Folr1/Lrp2 complex in the ileum likely plays a significant role in suckling young, absorption of folates occurs primarily in the proximal small intestine and is mediated by PCFT/Slc46a1 and RFC1/Slc19a1 (Birn et al., 2005a; Birn et al., 2005b; Qiu et al., 2006; Vazquez-Carretero et al., 2014; Visentin et al., 2014). A physiological role in folate reuptake in the kidney by endocytic mechanisms involving Folr1 and Lrp2 is demonstrated in mouse models (Birn et al., 2005a). Thus it is likely that reduced folate levels in the blood of pregnant dams reflects reduced reuptake of folate in the kidney rather than dietary folate from the diet. Importantly, resorption via this mechanism is particularly important under conditions of lower folate levels (Birn et al., 2005a). This is consistent with our finding that folate levels can be replenished by high doses of folate delivered by daily injections.
Our finding of reduced folate levels in Lrp2null/+ dams has implications for human NTDs. While folate deficiency is not reported with mutation LRP2 in Donnai–Barrow/Faciooculoacousticorenal (DBS/FOAR) syndrome (Pober et al., 2009), a single polymorphism of LRP2 is associated with increased NTD risk in human populations (Pangilinan et al., 2012). Lrp2 and Cubn interact to mediate endocytosis of the Folr1 receptor in the kidney (Birn, 2006; Birn et al., 2005a; Birn et al., 2005b; Christensen et al., 2013). Mutations in CUBN cause autosomal recessive megaloblastic anemia (Imerslund–Gräsbeck syndrome) and clinically relevant Vitamin B12 deficiency (Kozyraki and Cases, 2013). Importantly, altered red blood cell folate levels are associated with certain polymorphisms in CUBN (Franke et al., 2009; Zinck et al., 2015) and multiple polymorphisms of CUBN are associated with altered NTD risk (Franke et al., 2009; Pangilinan et al., 2012). Interestingly, an effect of maternal genotype was found in one of these studies (Pangilinan et al., 2012). Thus, our data that folate levels are reduced in Lrp2null/+ dams suggests that reduced CUBN function might also lead to reduced folate levels, possibly explaining the maternal effect of genetic variants in CUBN on NTD risk.
IRON SUPPLEMENTATION DOES NOT PREVENT NTDS IN LRP2NULL/NULL MUTANT EMBRYOS
Lrp2 is also involved in iron absorption and phenotypes of NTDs combined with forebrain truncations in Lrp2nul/nulll mutants are reminiscent of those observed with mutation of the iron transporter Fpn1 (Christ et al., 2012; Kozyraki et al., 2001; Mao et al., 2010; Spoelgen et al., 2005; Willnow et al., 1996). Furthermore, our previous studies demonstrate NTDs in Fpn1ffe mutants can be prevented by dietary supplementation with relatively high doses of carbonyl iron within the range given to humans with severe anemia, but not typically to pregnant women (Stokes et al., In Press). Unexpectedly, iron supplementation did not prevent NTDs in Lrp2null/null mutants and even negated the preventative effect of high levels of folic acid supplementation by daily injections. Our previous study demonstrated that this level of iron supplementation resulted in reduced folate status of wild type dams and embryos that was restored with dual dietary supplementation of iron and folate (Stokes et al., In Press). Here we replicate this result but also show that while whole blood folate levels did increase in Lrp2null/+ dams and with dual supplementation (dietary iron plus injected folate) and in wild type littermates, folate levels did not increase in Lrp2nul/null mutant embryos. Together these findings provide evidence that high levels of iron supplementation not only impacts folate homeostasis in the dam but also at the level of the conceptus.
During neural tube closure, the embryo absorbs nutrients by histotrophic mechanisms involving endocytic uptake of uterine secretions by the endodermal layer of the visceral yolk sac (visceral endoderm) (Zohn and Sarkar, 2010). This is opposed to haemotrophic mechanisms involving the placenta, which do not contribute significantly until after neural tube closure at E10.5 in the mouse embryo or approximately 10 weeks of human development (Burton, 2001). During neurulation Lrp2 is expressed in both the embryo proper and the visceral endoderm (Christensen and Birn, 2002). In the embryo proper Lrp2 plays an essential role in uptake of folates (Kur et al., 2014). Furthermore, conditional knockout of Lrp2 in the embryo lineages but not the visceral endoderm resulted in both NTDs and forebrain truncations (Spoelgen et al., 2005). However, whether the penetrance and severity of NTDs in the conditional mutants is the same as in the traditional knockout was not determined (Spoelgen et al., 2005). Thus while Lrp2 clearly plays an essential role in the embryo proper for uptake of folate in neural tissues, Lrp2 might also be involved in delivery of folate to the embryo through histotrophic mechanisms in the neurulating embryo.
Both Lrp2 and RFC1/Slc19a1 are expressed in the visceral endoderm of the yolk sac where they mediate uptake of folic acid (Christensen and Birn, 2002; Maddox et al., 2003). Importantly, expression of Lrp2 and Cubn are increased in RFC1/Slc19a1 mutant embryos as a compensatory pathway to maintain folate uptake (Gelineau-van Waes et al., 2008). Conversely, RFC1/Slc19a1 expression is upregulated in Lrp2null/null mutants; yet this is not sufficient to restore folate levels or rescue NTDs (Kur et al., 2014). Our data demonstrate that this alternate pathway for folate absorption can be sufficient with high levels of folic acid supplementation. Furthermore, high levels of iron supplementation might somehow impact this compensatory mechanism.
TRANSPORT OF OTHER NUTRIENTS AND FUNCTIONS OF LRP2 LIKELY CONTRIBUTE TO NTDS
Folic acid deficiency alone is not sufficient to cause NTDs (Heid et al., 1992). In addition to mediating folate uptake, Lrp2 is required for uptake of other nutrients that could contribute to NTDs including Vitamin A, B12, Cholesterol and iron (Christensen and Birn, 2002). Lrp2 also acts as clearance/co-receptor for BMP and Shh pathways implicated in NTDs (Christ et al., 2012; Spoelgen et al., 2005). Thus Lrp2 mutants potentially have multiple nutrient deficiencies that combined with altered signaling of key pathways for neural tube development could result in a NTD. Importantly, our data demonstrates that restoration of folate levels by supplementation can overcome these deficiencies resulting in normal neural tube closure.
Acknowledgments
This work was supported in part by R21-HD076202 from Eunice Kennedy Shriver National Institute of Child Health & Human Development. Microscopic analysis was carried out at the Children’s Research Institute (CRI) Light Microscopy and Image Analysis Core supported by CRI and NIH grant P30HD040677. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- Birn H. The kidney in vitamin B12 and folate homeostasis: characterization of receptors for tubular uptake of vitamins and carrier proteins. American journal of physiology. Renal physiology. 2006;291:F22–36. doi: 10.1152/ajprenal.00385.2005. [DOI] [PubMed] [Google Scholar]
- Birn H, Spiegelstein O, Christensen EI, Finnell RH. Renal tubular reabsorption of folate mediated by folate binding protein 1. J Am Soc Nephrol. 2005a;16:608–615. doi: 10.1681/ASN.2004080711. [DOI] [PubMed] [Google Scholar]
- Birn H, Zhai X, Holm J, Hansen SI, Jacobsen C, Christensen EI, Moestrup SK. Megalin binds and mediates cellular internalization of folate binding protein. The FEBS journal. 2005b;272:4423–4430. doi: 10.1111/j.1742-4658.2005.04857.x. [DOI] [PubMed] [Google Scholar]
- Blom HJ, Shaw GM, den Heijer M, Finnell RH. Neural tube defects and folate: case far from closed. Nature reviews. 2006;7:724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M, Ulrich S, Oofuji Y, Williams DA, Ross ME. Crooked tail (Cd) models human folate-responsive neural tube defects. Hum Mol Genet. 1999;8:2199–2204. doi: 10.1093/hmg/8.12.2199. [DOI] [PubMed] [Google Scholar]
- Christ A, Christa A, Kur E, Lioubinski O, Bachmann S, Willnow TE, Hammes A. LRP2 is an auxiliary SHH receptor required to condition the forebrain ventral midline for inductive signals. Dev Cell. 2012;22:268–278. doi: 10.1016/j.devcel.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Nielsen R, Birn H. From bowel to kidneys: the role of cubilin in physiology and disease. Nephrol Dial Transplant. 2013;28:274–281. doi: 10.1093/ndt/gfs565. [DOI] [PubMed] [Google Scholar]
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011;3:370–384. doi: 10.3390/nu3030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE. Periconceptional folic acid and multivitamin supplementation for the prevention of neural tube defects and other congenital abnormalities. Birth Defects Res A Clin Mol Teratol. 2009;85:260–268. doi: 10.1002/bdra.20563. [DOI] [PubMed] [Google Scholar]
- Eichholzer M, Tonz O, Zimmermann R. Folic acid: a public-health challenge. Lancet. 2006;367:1352–1361. doi: 10.1016/S0140-6736(06)68582-6. [DOI] [PubMed] [Google Scholar]
- Franke B, Vermeulen SH, Steegers-Theunissen RP, Coenen MJ, Schijvenaars MM, Scheffer H, den Heijer M, Blom HJ. An association study of 45 folate-related genes in spina bifida: Involvement of cubilin (CUBN) and tRNA aspartic acid methyltransferase 1 (TRDMT1) Birth Defects Res A Clin Mol Teratol. 2009;85:216–226. doi: 10.1002/bdra.20556. [DOI] [PubMed] [Google Scholar]
- Gelineau-van Waes J, Maddox JR, Smith LM, van Waes M, Wilberding J, Eudy JD, Bauer LK, Finnell RH. Microarray analysis of E9.5 reduced folate carrier (RFC1; Slc19a1) knockout embryos reveals altered expression of genes in the cubilin-megalin multiligand endocytic receptor complex. BMC genomics. 2008;9:156. doi: 10.1186/1471-2164-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JD, Ross ME. Mechanistic insights into folate supplementation from Crooked tail and other NTD-prone mutant mice. Birth Defects Res A Clin Mol Teratol. 2009;85:314–321. doi: 10.1002/bdra.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene ND, Massa V, Copp AJ. Understanding the causes and prevention of neural tube defects: Insights from the splotch mouse model. Birth Defects Res A Clin Mol Teratol. 2009;85:322–330. doi: 10.1002/bdra.20539. [DOI] [PubMed] [Google Scholar]
- Harris MJ. Insights into prevention of human neural tube defects by folic acid arising from consideration of mouse mutants. Birth Defects Res A Clin Mol Teratol. 2009;85:331–339. doi: 10.1002/bdra.20552. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A Clin Mol Teratol. 2010;88:653–669. doi: 10.1002/bdra.20676. [DOI] [PubMed] [Google Scholar]
- Heid MK, Bills ND, Hinrichs SH, Clifford AJ. Folate deficiency alone does not produce neural tube defects in mice. J Nutr. 1992;122:888–894. doi: 10.1093/jn/122.4.888. [DOI] [PubMed] [Google Scholar]
- Herbig AK, Stover PJ. Regulation of folate metabolism by iron. In: Massaro EJ, Rogers JM, editors. Folate and Human Development. Humana Press; Totowa, NJ: 2002. pp. 241–262. [Google Scholar]
- Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clinical chemistry. 1988;34:2357–2359. [PubMed] [Google Scholar]
- Kozyraki R, Cases O. Vitamin B12 absorption: mammalian physiology and acquired and inherited disorders. Biochimie. 2013;95:1002–1007. doi: 10.1016/j.biochi.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Kozyraki R, Fyfe J, Verroust PJ, Jacobsen C, Dautry-Varsat A, Gburek J, Willnow TE, Christensen EI, Moestrup SK. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci U S A. 2001;98:12491–12496. doi: 10.1073/pnas.211291398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kur E, Mecklenburg N, Cabrera RM, Willnow TE, Hammes A. LRP2 mediates folate uptake in the developing neural tube. Journal of cell science. 2014;127:2261–2268. doi: 10.1242/jcs.140145. [DOI] [PubMed] [Google Scholar]
- Li Z, Ren A, Zhang L, Ye R, Li S, Zheng J, Hong S, Wang T, Li Z. Extremely high prevalence of neural tube defects in a 4-county area in Shanxi Province, China. Birth Defects Res A Clin Mol Teratol. 2006;76:237–240. doi: 10.1002/bdra.20248. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang L, Li Z, Jin L, Zhang Y, Ye R, Liu J, Ren A. Prevalence and trend of neural tube defects in five counties in Shanxi province of Northern China, 2000 to 2014. Birth Defects Res A Clin Mol Teratol. 2016 doi: 10.1002/bdra.23486. [DOI] [PubMed] [Google Scholar]
- Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–916. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- Maddox DM, Manlapat A, Roon P, Prasad P, Ganapathy V, Smith SB. Reduced-folate carrier (RFC) is expressed in placenta and yolk sac, as well as in cells of the developing forebrain, hindbrain, neural tube, craniofacial region, eye, limb buds and heart. BMC developmental biology. 2003;3:6. doi: 10.1186/1471-213X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, McKean DM, Warrier S, Corbin JG, Niswander L, Zohn IE. The iron exporter ferroportin 1 is essential for development of the mouse embryo, forebrain patterning and neural tube closure. Development. 2010;137:3079–3088. doi: 10.1242/dev.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marean A, Graf A, Zhang Y, Niswander L. Folic acid supplementation can adversely affect murine neural tube closure and embryonic survival. Hum Mol Genet. 2011;20:3678–3683. doi: 10.1093/hmg/ddr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikael LG, Deng L, Paul L, Selhub J, Rozen R. Moderately high intake of folic acid has a negative impact on mouse embryonic development. Birth Defects Res A Clin Mol Teratol. 2013;97:47–52. doi: 10.1002/bdra.23092. [DOI] [PubMed] [Google Scholar]
- Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. doi: 10.1016/s0076-6879(97)81007-5. [DOI] [PubMed] [Google Scholar]
- Obican SG, Finnell RH, Mills JL, Shaw GM, Scialli AR. Folic acid in early pregnancy: a public health success story. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:4167–4174. doi: 10.1096/fj.10-165084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangilinan F, Molloy AM, Mills JL, Troendle JF, Parle-McDermott A, Signore C, O’Leary VB, Chines P, Seay JM, Geiler-Samerotte K, Mitchell A, VanderMeer JE, Krebs KM, Sanchez A, Cornman-Homonoff J, Stone N, Conley M, Kirke PN, Shane B, Scott JM, Brody LC. Evaluation of common genetic variants in 82 candidate genes as risk factors for neural tube defects. BMC medical genetics. 2012;13:62. doi: 10.1186/1471-2350-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- Pober BR, Longoni M, Noonan KM. A review of Donnai-Barrow and facio-oculo-acoustico-renal (DB/FOAR) syndrome: clinical features and differential diagnosis. Birth Defects Res A Clin Mol Teratol. 2009;85:76–81. doi: 10.1002/bdra.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Rothenberg SP, da Costa MP, Sequeira JM, Cracco J, Roberts JL, Weedon J, Quadros EV. Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. The New England journal of medicine. 2004;350:134–142. doi: 10.1056/NEJMoa031145. [DOI] [PubMed] [Google Scholar]
- Scott JM, Kirke PN, Weir DG. The role of nutrition in neural tube defects. Annual review of nutrition. 1990;10:277–295. doi: 10.1146/annurev.nu.10.070190.001425. [DOI] [PubMed] [Google Scholar]
- Silver LM. Mouse genetics: concepts and applications. Oxford University Press; New York: 1995. [Google Scholar]
- Smithells RW, Sheppard S, Schorah CJ. Vitamin dificiencies and neural tube defects. Arch Dis Child. 1976;51:944–950. doi: 10.1136/adc.51.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelgen R, Hammes A, Anzenberger U, Zechner D, Andersen OM, Jerchow B, Willnow TE. LRP2/megalin is required for patterning of the ventral telencephalon. Development. 2005;132:405–414. doi: 10.1242/dev.01580. [DOI] [PubMed] [Google Scholar]
- Stokes BA, Sabatino JA, Zohn IE. High Levels of Iron Supplementation Prevents Neural Tube Defects in the Fpn1ffe Mouse Model. Birth Defects Research Part A: Clinical and Molecular Teratology. doi: 10.1002/bdra.23542. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taparia S, Gelineau-van Waes J, Rosenquist TH, Finnell RH. Importance of folate-homocysteine homeostasis during early embryonic development. Clin Chem Lab Med. 2007;45:1717–1727. doi: 10.1515/CCLM.2007.345. [DOI] [PubMed] [Google Scholar]
- Vazquez-Carretero MD, Palomo M, Garcia-Miranda P, Sanchez-Aguayo I, Peral MJ, Calonge ML, Ilundain AA. Dab2, megalin, cubilin and amnionless receptor complex might mediate intestinal endocytosis in the suckling rat. Journal of cellular biochemistry. 2014;115:510–522. doi: 10.1002/jcb.24685. [DOI] [PubMed] [Google Scholar]
- Visentin M, Diop-Bove N, Zhao R, Goldman ID. The intestinal absorption of folates. Annual review of physiology. 2014;76:251–274. doi: 10.1146/annurev-physiol-020911-153251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochimica et biophysica acta. 2001;1513:49–54. doi: 10.1016/s0005-2736(01)00340-6. [DOI] [PubMed] [Google Scholar]
- Willnow TE, Herz J. Homologous recombination for gene replacement in mouse cell lines. Methods in cell biology. 1994;43(Pt A):305–334. doi: 10.1016/s0091-679x(08)60610-x. [DOI] [PubMed] [Google Scholar]
- Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci U S A. 1996;93:8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. Folate and thiamine transporters mediated by facilitative carriers (SLC19A1–3 and SLC46A1) and folate receptors. Molecular aspects of medicine. 2013;34:373–385. doi: 10.1016/j.mam.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinck JW, de Groh M, MacFarlane AJ. Genetic modifiers of folate, vitamin B-12, and homocysteine status in a cross-sectional study of the Canadian population. The American journal of clinical nutrition. 2015;101:1295–1304. doi: 10.3945/ajcn.115.107219. [DOI] [PubMed] [Google Scholar]
- Zohn IE. Mouse as a model for multifactorial inheritance of neural tube defects. Birth Defects Res C Embryo Today. 2012;96:193–205. doi: 10.1002/bdrc.21011. [DOI] [PubMed] [Google Scholar]
- Zohn IE. Principles of Developmental Genetics. 2nd 2014. Neural Tube Defects. [Google Scholar]
- Zohn IE, De Domenico I, Pollock A, Ward DM, Goodman JF, Liang X, Sanchez AJ, Niswander L, Kaplan J. The flatiron mutation in mouse ferroportin acts as a dominant negative to cause ferroportin disease. Blood. 2007;109:4174–4180. doi: 10.1182/blood-2007-01-066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohn IE, Sarkar AA. Modeling neural tube defects in the mouse. Current topics in developmental biology. 2008;84:1–35. doi: 10.1016/S0070-2153(08)00601-7. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Sarkar AA. The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birth Defects Res A Clin Mol Teratol. 2010;88:593–600. doi: 10.1002/bdra.20705. [DOI] [PubMed] [Google Scholar]


