Abstract
We investigated associations of plasma lipoproteins with subclinical ILD by measuring high attenuation areas (HAA: lung voxels between −600 and −250 HU) in 6,700 adults and serum MMP-7 and SP-A in 1,216 adults age 45–84 without clinical cardiovascular disease in MESA. In cross-sectional analyses, each standard deviation (SD) decrement in HDL-C was associated with a 2.12% HAA increment (95% CI 1.44–2.79%), a 3.53% MMP-7 increment (95% CI 0.93–6.07) and a 6.37% SP-A increment (95% CI 1.35–11.13), independent of demographics, smoking, and inflammatory biomarkers. These findings support a novel hypothesis that HDL-C might influence subclinical lung injury and extracellular matrix remodeling.
Cardiovascular disease (CVD) is a prevalent comorbidity in adults with fibrotic ILD, yet the mechanisms underlying this association remain unclear.[1–3] Lipids and lipoproteins contribute to the pathogenesis of CVD and in recent years have been linked to a number of lung diseases, including asthma and COPD.[4 5] However, few studies have looked at the role of lipids and lipoproteins in interstitial lung injury, inflammation and fibrosis.[6 7]
Imaging-based identification of lung injury, matrix remodeling and fibrosis in asymptomatic individuals is a novel way of studying subclinical ILD and may lead to a better understanding of the early causes of fibrosis. Two methods have been developed and validated to identify subclinical ILD: automated detection using quantitative CT densitometry to measure increased lung attenuation (high attenuation areas, HAA) and visual inspection for the presence of interstitial lung abnormalities (ILA).[8–11] In the current study, we examined associations of plasma lipids and lipoproteins with HAA, ILA and serum biomarkers of lung inflammation and extracellular matrix remodeling (SP-A and MMP-7) in the Multi-Ethnic Study of Atherosclerosis (MESA).[12–15] We hypothesized that the presence of coronary artery calcium, high LDL-C, low HDL-C and their respective components would be associated with HAA, ILA, and higher MMP-7/SP-A levels independent of demographic characteristics, smoking and inflammatory biomarkers.
Full Methods are available in the Online Data Supplement.
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multi-center, prospective cohort study of 6,814 adults age 45–84 sponsored by the National Heart Lung and Blood Institute to investigate the progression of subclinical CVD. The participant selection criteria have previously been described.[16] Notably, there were no selection criteria based on lung disease, respiratory symptoms, or smoking history. MESA was approved by Institutional Review Boards at all collaborating centers, and all participants provided written informed consent for participation.
Lung attenuation was measured on cardiac CT scans performed at baseline. Quantitative image attenuation was measured using a modified version of the Pulmonary Analysis Software Suite at a single reading center by trained readers. HAA was defined as the volume of imaged lung having CT attenuation values between −600 and −250 Hounsfield Units (HU).[8 9] ILA was visually assessed on full lung CT scans, as previously described.[9–11]
Of the 6,814 MESA participants, there were 6,700 with available lipid measurements included in HAA analyses, 2,391 in ILA analyses, and 1,216 in MMP-7 and SP-A analyses, with sampling frame previously described.[9] The median (IQR) HAA was 4.2% (3.5–5.4%) of total imaged lung.
In multivariable-adjusted models, there was a significant association between the presence of coronary artery calcium, HAA and ILA. The presence of CAC was associated with 1.47% increment in HAA (95% CI 0.19 to 2.77) and with a 57% greater odds of ILA (OR 1.58, 95% CI 1.18 to 2.08). Greater total cholesterol, HDL-C, LDL-C, and triglyceride levels were each associated with lower HAA (Table 1). However, the associations of both LDL-C and triglycerides with HAA were greatly attenuated by further adjustment for left ventricular function. On the other hand, the association between greater HDL-C and lower HAA was only marginally changed and remained significant (Figure 1). This association also persisted across HDL particle size and for ApoA-1, the major protein component of HDL.
Table 1.
Associations of cholesterol and lipoproteins with HAA.
| % change in HAA (95% CI)* | P value | |
|---|---|---|
| Total cholesterol | −1.32 (−1.89 to −0.75) | <0.001 |
|
| ||
| HDL-C, mg/dL | −2.12 (−2.79 to −1.44) | <0.001 |
| LDL-C, mg/dL | −0.84 (−1.41 to −0.28) | 0.004 |
| Triglycerides | −0.82 (−1.45 to −0.18) | 0.01 |
|
| ||
| Large HDL, 9.4–14 nm | −1.10 (−1.93 to −0.26) | 0.01 |
| Medium HDL, 8.2–9.4 nm | −2.56 (−3.31 to −1.80) | <0.001 |
| Small HDL, 7.3–8.2 nm | −2.69 (−3.44 to −1.94) | <0.001 |
|
| ||
| Large LDL, 20.5–23 nm | −1.36 (−2.06 to −0.66) | <0.001 |
| Small LDL, 18–20.5 nm | −0.73 (−1.56 to 0.11) | 0.09 |
|
| ||
| Apolipoprotein A-1 | −1.66 (−2.36 to −0.97) | <0.001 |
| Apolipoprotein B | −0.69 (−1.37 to −0.01) | 0.048 |
Solid lines separate distinct models. Each model includes all exposure variables listed and is additionally adjusted for age, gender, race/ethnicity, educational attainment, height, body mass index, waist circumference, smoking status, cigarette pack-years, glomerular filtration rate, diuretic use, statin use, presence of hypertension, presence of diabetes mellitus, coronary artery calcium, study site, milliampere dose, total volume imaged lung, percent emphysema on CT, interleukin-6 and c-reactive protein.
Reported per standard deviation in each exposure variable.
Figure 1.
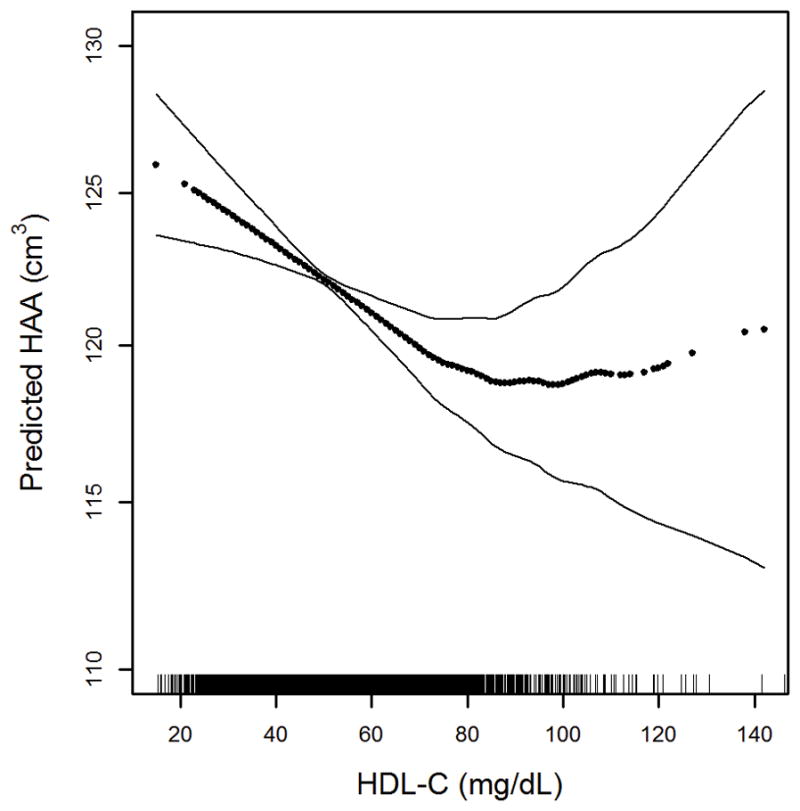
Continuous association between HDL-C and HAA adjusted for age, gender, race/ethnicity, educational attainment, height, body mass index, waist circumference, smoking status, cigarette pack-years, presence of hypertension, presence of diabetes, low density lipoprotein,, triglycerides, c-reactive protein, interleukin-6, glomerular filtration rate, statin use, diuretic use, coronary artery calcium, study site, milliAmpere dose, total volume imaged lung, percent emphysema on CT, left ventricular ejection fraction and left ventricular end-diastolic mass; p for nonlinearity 0.009, p for association <0.001. Dark dotted line is the continuous association. Thin solid lines are the 95% confidence bands. Each point in the graph and each vertical hashmark in the rug plot along the x-axis represent one study participant.
In a multivariable-adjusted model, there were no associations of baseline HDL-C, LDL-C, triglycerides, ApoA-1, ApoB, presence of DM, HTN, or statin use with the presence of ILA assessed at 10 year follow-up. In a multivariable-adjusted model (Table 2), each SD decrement in HDL-C was associated with a 3.53% increment in MMP-7 (95% CI 0.93 to 6.07%) and a 6.37% increment in SP-A (95% CI 1.35 to 11.13%).
Table 2.
Associations of cholesterol and lipoproteins with MMP-7 and SP-A.
| % change in MMP-7 (95% CI)* | P value | % change in SP-A (95% CI)* | P value | |
|---|---|---|---|---|
| Total cholesterol | −0.01 (−2.25 to 2.28) | 0.99 | 1.43 (−2.95 to 6.00) | 0.52 |
|
| ||||
| HDL-C, mg/dL | −3.53 (−6.07 to −0.93) | 0.008 | −6.37 (−11.13 to −1.35) | 0.01 |
| LDL-C, mg/dL | 0.35 (−2.55 to 1.89) | 0.76 | 3.44 (−0.98 to 8.05) | 0.13 |
| Triglycerides | 2.81 (0.30 to 5.37) | 0.03 | −3.11 (−7.69 to 1.70) | 0.20 |
|
| ||||
| Large HDL, 9.4–14 nm | −4.10 (−7.26 to −0.84) | 0.01 | −14.39 (−19.82 to −8.59) | <0.001 |
| Medium HDL, 8.2–9.4 nm | −4.92 (−7.61 to −2.14) | <0.001 | −1.67 (−7.06 to 4.02) | 0.56 |
| Small HDL, 7.3–8.2 nm | −5.32 (−8.03 to −2.53) | <0.001 | −8.23 (−13.31 to −2.86) | 0.003 |
|
| ||||
| Large LDL, 20.5–23 nm | −4.86 (−7.50 to −2.15) | <0.001 | −0.68 (−6.02 to 4.96) | 0.81 |
| Small LDL, 18–20.5 nm | −1.51 (−4.73 to 1.81) | 0.37 | −7.35 (−13.18 to −1.12) | 0.02 |
|
| ||||
| Apolipoprotein A-1 | −2.83 (−5.32 to −0.27) | 0.03 | −4.79 (−9.56 to 0.12) | 0.06 |
| Apolipoprotein B | 1.74 (−0.64 to 4.18) | 0.15 | 1.51 (−3.06 to 6.36) | 0.52 |
Solid lines separate distinct models. Each model includes all exposure variables listed and is additionally adjusted for age, gender, race/ethnicity, body mass index, smoking status, cigarette pack-years, presence of coronary artery calcium, statin use, interleukin-6 and c-reactive protein.
Reported per standard deviation in each exposure variable.
Additional analyses are presented in Supplementary Tables S1–S9 and Figures S1–S2 in the Online Data Supplement.
In this large cohort of community-dwelling adults, the presence of coronary artery calcium was associated with two measures of subclinical ILD (HAA and ILA), providing further support for a link between CVD and ILD.[1–3] We also found that lower plasma HDL-C and ApoA-1 were each associated with greater HAA and greater serum MMP-7 and SP-A levels, independent of smoking, demographic factors, inflammatory biomarkers and measures of left ventricular function, a finding consistent with a previous study that found lower HDL-C levels in 39 adults with pulmonary fibrosis compared to healthy controls.[6] Our findings suggest a novel hypothesis that HDL-C levels or its components might exert protective effects in subclinical ILD. Candidate mechanisms by which HDL-C might attenuate subclinical lung inflammation, extracellular matrix remodeling, and fibrosis include modulation of endothelial function, protection against inflammation and oxidative stress, and alterations in surfactant function.[17–19] ApoA-I has previously been shown to have beneficial effects in reducing lung inflammation and fibrosis in animal models,[7] and greater small and medium-size HDL particle concentrations are associated with increased noncardiovascular, noncancer chronic inflammation-related deaths in MESA,[20] supporting an anti-inflammatory role of small HDL particles in the lungs.
There are several limitations to our study, including the potential for residual confounding, the possibility that HAA likely includes pulmonary edema, the lack of pathological validation of HAA, and the use of cardiac rather than full lung CT scans to measure HAA. In addition, we found that HDL-C was not associated with ILA. This lack of consistency may reflect the ten year latency period between baseline measurements of lipids and ILA, or it may reflect different stages of disease identified by the two measures of subclinical ILD. Furthermore, the clinical significance of elevated HAA, MMP-7 and SP-A in asymptomatic individuals is uncertain, limiting inferences about the role of HDL-C in fibrotic lung disease.
In summary, we found novel associations of greater HDL-C and ApoA-1 levels with lower HAA, a quantitative CT-measure of subclinical lung inflammation and extracellular matrix remodeling, and with biomarkers of lung injury and extracellular matrix remodeling. This finding remains unexplained, but suggests a protective role of lipoproteins in ILD pathogenesis. More work is needed now to elucidate the mechanisms linking these two conditions, and the role that low lipoprotein levels might play in the pathogenesis of ILD.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
FUNDING
The work is funded by the National Institutes of Health contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 and grants UL1-TR-000040, UL1-TR-001079, R01-HL-103676, RC1-HL100543, R01-HL-093081, R01-HL-077612, T32-HL-105323, and K24-HL-131937; by the Pulmonary Fibrosis Foundation; and by the Rocco Guinta Research Fund.
Footnotes
CONTRIBUTORS
AJP, DJL, RGB, SJS, KW and SMK conceived and designed the study.
AJP, GR, MYT, RGB, EAH, FSA, SJS and DJL acquired the data.
AJP, SMK, PLE, EP, RS, DR, CJ, RGB, KHS, JJC, DRJ, KW, SJS and DJL analyzed the data.
AJP drafted the initial manuscript.
All authors contributed to the conception and design of the study and to the acquisition, analysis, or interpretation of data.
All authors revised the manuscript for important intellectual content.
All authors approved the final version of the manuscript.
All authors agree to be accountable for all aspects of the work.
COMPETING INTERESTS
Dr. Lederer has received modest consulting fees from Genentech/Roche, Boehringer-Ingelheim, Gilead, Pharmakea, Veracyte, Patara Pharmaceuticals, Degge Group, and the France Foundation related to IPF. Columbia University has received funding for clinical trials in IPF from Boehringer-Ingelheim, Gilead, Bayer, Global Blood Therapeutics. Columbia University has received funding from the Pulmonary Fibrosis Foundation for Dr. Lederer’s consulting services. Dr. Lederer has received modest fees for serving as a Deputy Editor for the Annals of the American Thoracic Society and as a Statistical Editor for Thorax.
Dr. Kawut reports grants from NIH during the conduct of the study and non-financial support from the ATS. He has received personal fees from the European Respiratory Journal for serving on an editorial board. The University of Pennsylvania has received grants from Actelion, grants from United Therapeutics, grants from Gilead, grants from Lung Biotech, grants from Bayer for CME courses.
Dr. Hoffman is a founder and shareholder in VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa.
Drs. Ahmed, Barr, Carr, Enright, Hinckley Stukovsky, Jacobs, Podolanczuk, Rabinowitz, Raghu, Shea, Sonti, Tsai, Watson, and Mr. Johnson and Peterson have no relevant disclosures.
References
- 1.Hubbard RB, Smith C, Le Jeune I, Gribbin J, Fogarty AW. The association between idiopathic pulmonary fibrosis and vascular disease: a population-based study. Am J Respir Crit Care Med. 2008;178(12):1257–61. doi: 10.1164/rccm.200805-725OC. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 2.Izbicki G, Ben-Dor I, Shitrit D, et al. The prevalence of coronary artery disease in end-stage pulmonary disease: is pulmonary fibrosis a risk factor? Respir Med. 2009;103(9):1346–9. doi: 10.1016/j.rmed.2009.03.012. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 3.Nathan SD, Basavaraj A, Reichner C, et al. Prevalence and impact of coronary artery disease in idiopathic pulmonary fibrosis. Respir Med. 2010;104(7):1035–41. doi: 10.1016/j.rmed.2010.02.008. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 4.Burkart KM, Manichaikul A, Wilk JB, et al. APOM and high-density lipoprotein cholesterol are associated with lung function and per cent emphysema. The European respiratory journal. 2014;43(4):1003–17. doi: 10.1183/09031936.00147612. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barochia AV, Kaler M, Cuento RA, et al. Serum Apolipoprotein A-I and Large HDL Particles are Positively Correlated with FEV in Atopic Asthma. Am J Respir Crit Care Med. 2015 doi: 10.1164/rccm.201411-1990OC. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aihara K, Handa T, Nagai S, et al. Impaired endothelium-dependent vasodilator response in patients with pulmonary fibrosis. Respir Med. 2013;107(2):269–75. doi: 10.1016/j.rmed.2012.10.005. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Kim TH, Lee YH, Kim KH, et al. Role of lung apolipoprotein A-I in idiopathic pulmonary fibrosis: antiinflammatory and antifibrotic effect on experimental lung injury and fibrosis. Am J Respir Crit Care Med. 2010;182(5):633–42. doi: 10.1164/rccm.200905-0659OC. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 8.Lederer DJ, Enright PL, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180(5):407–14. doi: 10.1164/rccm.200812-1966OC. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podolanczuk AJ, Oelsner EC, Barr RG, et al. High attenuation areas on chest CT in community-dwelling adults: The MESA Study. European Respiratory Journal. 2016 doi: 10.1183/13993003.00129-2016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putman RK, Hatabu H, Araki T, et al. Association Between Interstitial Lung Abnormalities and All-Cause Mortality. JAMA. 2016;315(7):672–81. doi: 10.1001/jama.2016.0518. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897–906. doi: 10.1056/NEJMoa1007285. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene KE, King TE, Jr, Kuroki Y, et al. Serum surfactant proteins-A and -D as biomarkers in idiopathic pulmonary fibrosis. The European respiratory journal. 2002;19(3):439–46. doi: 10.1183/09031936.02.00081102. [DOI] [PubMed] [Google Scholar]
- 13.Rosas IO, Richards TJ, Konishi K, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5(4):e93. doi: 10.1371/journal.pmed.0050093. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinder BW, Brown KK, McCormack FX, et al. Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest. 2009;135(6):1557–63. doi: 10.1378/chest.08-2209. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards TJ, Kaminski N, Baribaud F, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185(1):67–76. doi: 10.1164/rccm.201101-0058OC. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Pian MS, Dobbs LG. Lipoprotein-stimulated surfactant secretion in alveolar type II cells: mediation by heterotrimeric G proteins. The American journal of physiology. 1997;273(3 Pt 1):L634–9. doi: 10.1152/ajplung.1997.273.3.L634. [DOI] [PubMed] [Google Scholar]
- 18.Voyno-Yasenetskaya TA, Dobbs LG, Erickson SK, Hamilton RL. Low density lipoprotein- and high density lipoprotein-mediated signal transduction and exocytosis in alveolar type II cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(9):4256–60. doi: 10.1073/pnas.90.9.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabet F, Vickers KC, Cuesta Torres LF, et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat Commun. 2014;5:3292. doi: 10.1038/ncomms4292. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duprez DA, Otvos J, Tracy RP, et al. High-Density Lipoprotein Subclasses and Noncardiovascular, Noncancer Chronic Inflammatory-Related Events Versus Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4(9):e002295. doi: 10.1161/JAHA.115.002295. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


