Abstract
A carbapenem-resistant Acinetobacter baumannii strain was isolated in Toulouse, France, in 2003. Cloning and expression in Escherichia coli identified the carbapenem-hydrolyzing β-lactamase OXA-58, which is weakly related (less than 50% amino acid identity) to other oxacillinases. It hydrolyzed penicillins, oxacillin, and imipenem but not expanded-spectrum cephalosporins. The blaOXA-58 gene was located on a ca. 30-kb non-self-transferable plasmid. After electrotransformation in the A. baumannii CIP7010T reference strain, it conferred reduced susceptibility to carbapenems. The blaOXA-58 gene was bracketed by two novel ISAba3-like insertion elements. This study describes a newly characterized β-lactamase that may contribute to carbapenem resistance in A. baumannii.
Carbapenem resistance in Acinetobacter baumannii is observed increasingly in nosocomial isolates, especially in isolates recovered from intensive care units (4). This resistance phenotype is often associated with multidrug resistance, leading to limited choices for treating A. baumannii infections. This bacterial species naturally produces a chromosomally encoded cephalosporinase (6) that may be overexpressed due to insertion of ISAba1, which brings promoter sequences necessary for high-level expression of this β-lactamase (12, 36). Carbapenem resistance may be due to a reduced permeability related to porin deficiency and to modification of penicillin-binding protein affinity (10, 17, 39), but recent reports showed that β-lactamase-mediated carbapenem resistance is the most common mechanism (29). Only a few instances of metallo-β-lactamase have been described for A. baumannii (9, 15, 33, 37, 40), but identification of several Ambler class D β-lactamases (oxacillinases) has been reported recently (23).
Six oxacillinases with carbapenem-hydrolyzing activity have been sequenced from A. baumannii, and these isolates were responsible for nosocomial outbreaks in several cases (5, 14). OXA-23 (also named ARI-1) (16, 25) and OXA-27 (2) have 99% amino acid identity, whereas they share 60% identity with a second group of oxacillinases consisting of OXA-24, -25, -26, and -40, which differ by a few amino acid substitutions (2, 7, 18). In addition, OXA-48 was characterized recently from a Klebsiella pneumoniae clinical isolate that hydrolyzed imipenem to a significant extent (32). This plasmid-mediated Ambler class D β-lactamase likely originated from Shewanella spp., since it shared 92% amino acid identity with the naturally occurring β-lactamase OXA-54 from Shewanella oneidensis (31).
In this study, we have characterized a novel imipenem-hydrolyzing oxacillinase identified in A. baumannii.
MATERIALS AND METHODS
Bacterial strains and plasmids.
A. baumannii clinical isolate MAD was isolated in 2003. It was identified with the API20NE system (bioMérieux, Marcy-l'Etoile, France). Escherichia coli reference strain DH10B and plasmid pBK-CMV (Stratagene, Amsterdam, The Netherlands) were used for cloning experiments. A. baumannii CIP7010T (Pasteur Institute, Paris, France) and E. coli DH10B were used in transformation and conjugation experiments.
Antimicrobial agents and MIC determinations.
The antimicrobial agents and their sources have been referenced elsewhere (26). Antibiotic-containing disks were used for detection of antibiotic susceptibility with Mueller-Hinton agar plates and a disk diffusion assay (Sanofi Diagnostics Pasteur, Marnes-La-Coquette, France). MICs were determined by an agar dilution technique as previously reported (28), and results were interpreted according to the guidelines of the National Committee for Clinical Laboratory Standards (24).
Cloning experiments.
Whole-cell DNA of A. baumannii MAD was extracted as previously described (26). Cloning experiments were performed with partially Sau3AI-digested DNA of A. baumannii MAD and BamHI-restricted plasmid pBK-CMV, followed by expression of recombinant plasmids in E. coli DH10B, as described previously (28). Antibiograms were obtained with E. coli DH10B harboring recombinant plasmids, and sizes of the plasmid inserts were determined by restriction analysis (34). Recombinant plasmid pOXA-58 was retained for further analysis.
DNA sequencing and protein analysis.
Both strands of the cloned DNA fragments were sequenced with an Applied Biosystems sequencer (ABI 3100). The nucleotide and deduced protein sequences were analyzed with software available over the internet at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov).
Plasmid analysis and hybridizations.
Extraction of plasmid DNA from A. baumannii MAD and from electroporants was attempted by using the Kieser method (20). A Southern transfer was performed on a nylon membrane (Hybond N+; Amersham Pharmacia Biotech, Orsay, France), as described previously (27). The plasmid extract of A. baumannii MAD was also used for transformation experiments with a Gene Pulser II electroporator (Bio-Rad, Ivry-sur-Seine, France) and the A. baumannii CIP7010T and E. coli DH10B reference strains. Electroporation products were selected on ticarcillin (50 μg/ml)-containing plates.
Mating-out assays were attempted in liquid and solid media with A. baumannii MAD as the donor and A. baumannii CIP7010T as the recipient strain. Transconjugants were selected on MH plates containing ticarcillin (100 μg/ml).
The membrane was hybridized with a probe specific for the blaOXA-58 gene made of a 528-bp PCR-generated fragment (primers OXA-58A [5′-CGATCAGAATGTTCAAGCGC-3′] and OXA-58B [5′-ACGATTCTCCCCTCTGCGC-3′]). Southern hybridization was performed with the ECL nonradioactive labeling and detection kit as described by the manufacturer (Amersham Pharmacia Biotech).
IEF analysis.
Isoelectric focusing (IEF) analysis was performed with an ampholine polyacrylamide gel (pH 3.5 to 9.5) as described previously (28) with culture extracts of A. baumannii MAD and E. coli DH10B harboring recombinant plasmid pOXA-58.
β-Lactamase purification.
A culture of E. coli DH10B harboring recombinant plasmid pOXA-58 that produced OXA-58 was grown overnight at 37°C in 4 liters of Trypticase soy broth containing 100 μg of amoxicillin per ml and 30 μg of kanamycin per ml. The protein extracts obtained were purified as described previously (30). After sonication, the crude extract was treated with DNase and ultracentrifuged at 100,000 × g, and then the extracts were filtered through a 0.45-μm-pore-size filter and subjected to further purification steps, including ion-exchange chromatography with Q-Sepharose and 20 mM Tris-HCl buffer (pH 8). The β-lactamase was recovered in the flowthrough, and this partially purified extract was ultrafiltered with a Sartorius (Göttingen, Germany) instrument. The extract was subsequently dialyzed in 20 mM diethanolamine (pH 9.3) and loaded again on the Q-Sepharose column equilibrated with the same buffer. The β-lactamase was retained on the column, and elution was performed with a K2SO4 gradient to prevent any inhibition by NaCl. Finally, the fractions containing the highest β-lactamase activity were dialyzed against 100 mM phosphate buffer (pH 7.0). Their purity was estimated by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Kinetic studies.
Purified β-lactamase was used for kinetic measurements performed at 30°C in 100 mM sodium phosphate (pH 7.0) (28). The kcat and Km values were determined by analyzing β-lactam hydrolysis under initial-rate conditions with a UV spectrophotometer, as previously described (28). When biphasic hydrolysis was observed, reaction rates were measured at the steady state. The 50% inhibitory concentrations (IC50) of clavulanic acid, tazobactam, sulbactam, and NaCl were determined as described previously (28). Specific activities of protein extracts and purified β-lactamase from cultures of E. coli DH10B(pOXA-58) were determined as described previously (3). The protein content was determined by using the Bio-Rad DC protein assay. β-Lactamase specific activity for imipenem was also determined with culture extracts of 10 ml of TS broth of A. baumannii MAD by using UV spectrophotometry and imipenem as described previously (3). One unit of enzyme activity was defined as the activity that hydrolyzed one micromole of imipenem per minute per milligram of protein.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the EMBL/GenBank nucleotide sequence database under accession number AY570763.
RESULTS
Origin of the A. baumannii MAD isolate and preliminary antibiotic susceptibility testing.
A. baumannii MAD was isolated in August 2003 at the Rangueil University hospital (Toulouse, France) from a skin burn infection of a 24-year-old female. This patient had been treated with imipenem and amikacin. Antibiotic susceptibility testing revealed that this isolate was resistant to most tested β-lactams and was of intermediate susceptibility to aztreonam and imipenem (Table 1). Addition of clavulanic acid and tazobactam did not reduce the MICs of ticarcillin and piperacillin (Table 1). A. baumannii MAD was also resistant to fluoroquinolones, chloramphenicol, trimethoprim, and aminoglycosides and was susceptible to sulfonamides, tetracycline, and rifampin (data not shown). Culture extracts of A. baumannii MAD analyzed by IEF gave two β-lactamases with pIs of 7.2 and 9.4, the latter corresponding to the naturally produced AmpC of A. baumannii (data not shown). To determine whether resistance to carbapenems was at least partially β-lactamase mediated, a preliminary hydrolysis experiment was performed with culture extract of A. baumannii MAD and revealed a significant imipenem hydrolysis activity equal to that of OXA-40-producing A. baumannii CLA-1 (4.6 mU/mg of protein) (18).
TABLE 1.
MICs of β-lactams for the A. baumannii MAD clinical isolate, E. coli DH10B harboring recombinant plasmid pOXA-58, A. baumannii CIP7010T harboring natural plasmid pMAD, and the A. baumannii CIP7010T and E. coli DH10B reference strains
| β-Lactam(s)a | MIC (μg/ml) for:
|
||||
|---|---|---|---|---|---|
| A. baumannii MAD | A. baumannii CIP7010T (pMAD) | A. baumannii CIP7010T | E. coli DH10B (pOXA-58) | E. coli DH10B | |
| Amoxicillin | >512 | >512 | >512 | >512 | 4 |
| Amoxicillin + CLA | >512 | >512 | 512 | 128 | 4 |
| Ticarcillin | >512 | >512 | 4 | >512 | 4 |
| Ticarcillin + CLA | >512 | >512 | 4 | 256 | 4 |
| Piperacillin | 256 | 256 | 4 | 8 | 1 |
| Piperacillin + TZB | 256 | 128 | 4 | 8 | 1 |
| Cephalothin | >512 | >512 | >512 | 8 | 2 |
| Cefuroxime | >512 | 256 | 256 | 4 | 2 |
| Ceftazidime | 128 | 2 | 2 | 0.12 | 0.06 |
| Cefotaxime | 32 | 8 | 8 | 0.12 | 0.12 |
| Cefepime | 256 | 1 | 1 | 0.12 | 0.06 |
| Cefpirome | 256 | 8 | 2 | 0.25 | 0.06 |
| Moxalactam | 512 | 256 | 32 | 0.12 | 0.06 |
| Aztreonam | 32 | 64 | 64 | 0.12 | 0.12 |
| Imipenem | 32 | 2 | 0.25 | 0.5 | 0.06 |
| Meropenem | >64 | 2 | 0.25 | 0.5 | 0.06 |
CLA, clavulanic acid at a fixed concentration of 2 μg/ml; TZB, tazobactam at a fixed concentration of 4 μg/ml.
Cloning and sequencing of the β-lactamase.
Cloning of Sau3AI-restricted whole-cell DNA of A. baumannii MAD into pBK-CMV, followed by expression in E. coli DH10B gave E. coli DH10B(pOXA-58), expressing an oxacillinase resistance phenotype (pI, 7.2) with a carbapenem-hydrolyzing activity; the phenotype consisted of resistance to most penicillins that was not antagonized by clavulanic acid and a reduced susceptibility to imipenem (Table 1).
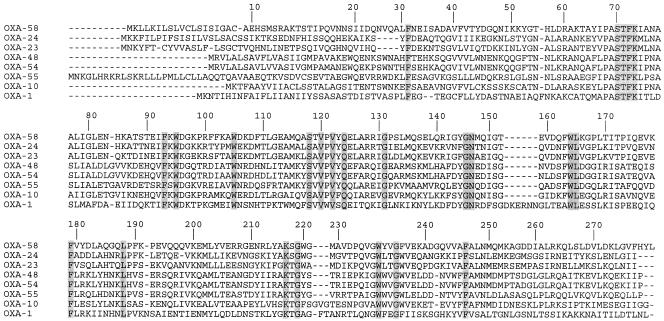
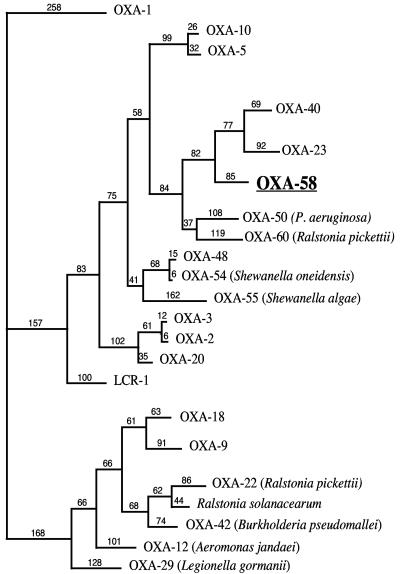
DNA sequence analysis of plasmid pOXA-58 identified a 843-bp open reading frame (ORF) for blaOXA-58, encoding a 280-amino-acid protein (Fig. 1). Within the deduced protein encoded by this ORF, a serine-threonine-phenylalanine-lysine tetrad (STFK) was found at positions DBL (Ambler class D β-lactamase numbering [13]) 70 to 73. A KSG element (positions 216 to 218) was found, as observed in the carbapenem-hydrolyzing β-lactamases OXA-24, -25, -26, and -40, whereas a KTG motif is present in the carbapenem-hydrolyzing oxacillinases OXA-23, -27, -48, -54, and -55 and in most of the class D β-lactamases without carbapenemase activity (13, 21, 23) (Fig. 2). The oxacillinase structural element YGN at positions DBL 144 to 146 was not replaced in OXA-58 by an FGN motif, in contrast to what is found in sequences of the carbapenem-hydrolyzing oxacillinases identified in A. baumannii (18). OXA-58 was weakly related to other oxacillinases sharing, 48 and 47% amino acid identity with OXA-23 and OXA-24, respectively, which were taken as representatives of the two carbapenem-hydrolyzing oxacillinase subgroups of A. baumannii (7, 16). In addition, OXA-58 shared 35, 33, and 18% amino acid identity with OXA-5, OXA-10, and OXA-1, respectively, and 32% amino acid identity with the naturally occurring carbapenem-hydrolyzing oxacillinases OXA-54 and OXA-55 from S. oneidensis and S., respectively (19, 31) (Fig. 3).
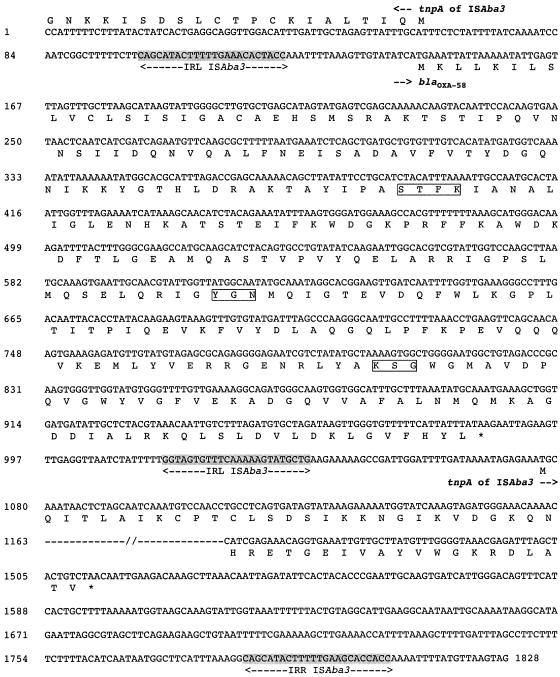
FIG. 1.
Nucleotide sequence of a 1,828-bp fragment of recombinant plasmid pOXA-58 containing the blaOXA-58 gene. The deduced amino acid sequence is designated in single-letter code below the nucleotide sequence. The start codons of the ORFs are indicated by horizontal arrows. The left inverted repeats (IRL) of ISAba3 are shaded in grey. The tnpA transposase gene of ISAba3 is indicated. Asterisks indicates stop codons.
FIG. 2.
Comparison of the amino acid sequence of OXA-58 with those of OXA-23, -24, -54, and -55, which possess carbapenem-hydrolyzing activity, and with those of reference oxacillinases OXA-1 and OXA-10. The conserved residues for oxacillinases are shaded. Numbering of β-lactamases is according to DBL numbering (13).
FIG. 3.
Dendrogram obtained for representative natural and acquired Ambler class D β-lactamases by parsimony analysis. The origins of the naturally occurring oxacillinases are indicated. The alignment used for tree calculation was performed with ClustalW followed by minor adjustments. Branch lengths are drawn to scale and are proportional to the number of amino acid changes. The distance along the vertical axis has no significance.
Genetic location of blaOXA-58.
Plasmid extraction of A. baumannii MAD revealed a ca. 30-kb plasmid that was successfully electroporated in A. baumannii CIP7010T and not in the E. coli DH10B reference strains. Thus, plasmid pMAD was likely not able to replicate in E. coli. The A. baumannii CIP7010T(pMAD) transformant had a β-lactam resistance pattern consistent with the expression of OXA-58, conferring resistance to ticarcillin and a reduced susceptibility to imipenem and meropenem (Table 1). No other additional antibiotic resistance markers were observed in A. baumannii CIP7010T(pMAD) transformants. Mating-out assays using A. baumannii MAD or A. baumannii CIP7010T (pMAD) as the donor and rifampin-resistant A. baumannii CIP7010T failed.
Downstream of the plasmid-carried blaOXA-58 gene, a novel insertion sequence (IS), ISAba3, was identified. It was 800 bp long and possessed a 145-amino-acid putative transposase weakly related to other transposase sequences, sharing 44% amino acid identity (56 amino acids) to part of the orfB transposase of IS1. In contrast to IS1, only one open reading frame is present in ISAba3. The perfect inverted repeats were 21 bp in length (Fig. 1). Immediately upstream of the blaOXA-58 gene, a similar ISAba3 element was present. The left inverted repeat of ISAba3 was identified 17 bp upstream of the start codon of the blaOXA-58 gene, and the 125-bp-long sequence of this IS revealed 100% nucleotide identity with ISAba3. Thus, blaOXA-58 likely is bracketed by two very similar IS elements. This structure may correspond to a composite transposon including the two ISAba3 elements.
Biochemical properties of β-lactamase OXA-58.
After purification from extracts of E. coli DH10B(pOXA-58), the specific activity of OXA-58 against benzylpenicillin was 2.3 U per mg of protein and its purification factor was 70-fold. This weak β-lactamase activity is similar to those of many purified oxacillinases (3, 26, 28). Protein purity was estimated to be >95% by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (data not shown). OXA-58 has a narrow-spectrum hydrolysis profile, including mostly penicillins (Table 2). Biphasic hydrolyses were obtained only for ampicillin, ticarcillin, and imipenem. Hydrolysis of imipenem was low, whereas hydrolysis of meropenem was not detected, although the MICs of both carbapenems were increased eightfold. However, determination of the Ki for meropenem revealed a strong affinity (0.075 μM) of OXA-58 for this substrate. In general, catalytic activities of OXA-58 were similar to those of OXA-40, which was taken as reference for an oxacillinase with carbapenem-hydrolyzing activity (Table 2). Nevertheless, the relative kcat/Km compared to that of benzylpenicillin showed that OXA-58 hydrolyzed imipenem twice as much as OXA-40 did. Additionally, OXA-58 had some hydrolytic activity against cefpirome, whereas activity against ceftazidime, cefotaxime, and cefepime remained undetectable (Table 2).
TABLE 2.
Kinetic parameters of purified β-lactamase OXA-58 from A. baumannii MAD compared to those of OXA-40 from A. baumannii CLA-1a
| Substrate | OXA-58
|
OXA-40b
|
||||||
|---|---|---|---|---|---|---|---|---|
| kcat (s−1) | Km (μM) | kcat/Km (mM−1 · s−1) | Relative kcat/Km | kcat (s−1) | Km (μM) | kcat/Km (mM−1 · s−1) | Relative kcat/Km | |
| Benzylpenicillin | 5.5 | 50 | 110 | 100 | 5 | 23 | 220 | 100 |
| Ampicillin | 1 | 130 | 8 | 7 | 5 | 220 | 20 | 9 |
| Ticarcillin | 1 | 240 | 4 | 8 | 1 | 60 | 20 | 9 |
| Piperacillin | 2.5 | 50 | 50 | 48 | 1 | 23 | 50 | 23 |
| Cephalothin | 0.1 | 150 | 1 | 1 | 3 | 72 | 50 | 23 |
| Cefotaxime | NDc | —d | — | — | ND | — | — | — |
| Ceftazidime | ND | — | — | — | 20 | 2,500 | 10 | 4.5 |
| Cefepime | ND | — | — | — | ND | — | — | — |
| Cefpirome | 0.1 | 200 | 0.5 | 0.5 | ND | — | — | — |
| Oxacillin | 1.5 | 70 | 2 | 2 | 2 | 876 | 3 | 0.1 |
| Aztreonam | ND | — | — | — | ND | — | — | — |
| Imipenem | 0.1 | 7.5 | 13.5 | 13 | 0.1 | 6.5 | 15 | 7 |
| Meropenem | <0.01 | 0.075e | <0.15 | <0.7 | ND | — | — | — |
Data are means from three independent experiments. Standard deviations were within 10% of the geometric means.
Data are from reference 18.
ND, no detectable hydrolysis (<0.01 s−1).
—, not determinable
The Ki was determined with benzylpenicillin as the substrate.
Studies of activity inhibition, as measured by determination of IC50, showed that OXA-58 was weakly inhibited by clavulanic acid (310 μM), tazobactam (60 μM), and sulbactam (2.5 mM), as found for most of the oxacillinases (21, 23). These IC50s were similar to those found for OXA-40 (18), except for sulbactam, which inhibits OXA-58 more than 10 times less. OXA-58 activity was well inhibited by NaCl (IC50, 12 mM).
DISCUSSION
In our preliminary experiments we were not able to identify known oxacillinase or metallo-β-lactamase genes in a carbapenem-resistant A. baumannii strain. Thus, we have cloned and characterized a novel carbapenem-hydrolyzing oxacillinase, OXA-58, that constitutes a novel group of oxacillinases. The β-lactamase OXA-58 hydrolyzed penicillins and imipenem significantly, sparing most expanded-spectrum cephalosporins. The significant kcat/Km values obtained for imipenem and meropenem are related more to a good affinity for these substrates than to a strong hydrolysis. Interestingly, the decrease of susceptibility to carbapenems conferred by OXA-58 in A. baumannii is significant, and in view of the kinetic data, it is likely the result of a competitive inhibition toward these molecules. It is likely, from comparison of the β-lactam resistance phenotypes of A. baumannii MAD and its transformant, that additional mechanisms are responsible for the high level of resistance to imipenem.
OXA-58 possesses the classical YGN motif in positions DBL 144 to 146 of oxacillinases instead of an FGN motif as found in all of the carbapenem-hydrolyzing oxacillinases identified in A. baumannii (18, 23). This confirms that a Phe residue in position DBL144 is not required by itself for providing carbapenem hydrolytic activity (18). Nevertheless, we have not identified the critical residue(s) involved in the carbapenem hydrolysis property of OXA-58. The inhibition of OXA-58 by NaCl confirmed that this property is related to a Tyr residue in position DBL 144, whereas the other carbapenem-hydrolyzing oxacillinases that have a Phe residue at this position are resistant to inhibition by NaCl (18). This discrepancy can be a useful tool for screening of OXA-58 from a crude extract of any carbapenem-resistant A. baumannii strain, since this is currently the only β-lactamase inhibited by NaCl that is able to hydrolyze imipenem in that species.
This is the second report of a tight association between IS elements and an oxacillinase gene, after that of IS1999 and blaOXA-48 in K. pneumoniae (32), which raises again the question of the origin of these oxacillinase genes and their mobilization process. As observed for the other carbapenem-hydrolyzing oxacillinase genes, blaOXA-58 was not present in the form of a gene cassette in a class 1 integron, a situation that contrasts to that found for most of the oxacillinase genes. The IS element located upstream of blaOXA-58 likely provided promoter sequences responsible for its expression. Further experiments are in progress to determine the putative role of this IS element in blaOXA-58 expression.
This study constitutes the second description of a plasmid-encoded carbapenem-hydrolyzing oxacillinase in A. baumannii, after that of OXA-23 and ARI-2 (8, 35). A plasmid location of that gene may enhance the spread of the carbapenem resistance marker in A. baumannii. A prevalence study of spread of similar oxacillinase genes among European isolates would be interesting, especially in southern Europe, where imipenem-resistant A. baumannii strains seem to be increasingly identified (1, 11, 22, 38).
Acknowledgments
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA3539), Université Paris XI, France, and by the European Community (6th PCRD, LSHM-CT-2003-503-335). L.P. is a researcher from the INSERM, France.
REFERENCES
- 1.Afzal-Shah, M., and D. M. Livermore. 1998. Worldwide emergence of carbapenem-resistant Acinetobacter spp. J. Antimicrob. Chemother. 41:576-577. [DOI] [PubMed] [Google Scholar]
- 2.Afzal-Shah, M., N. Woodford, and D. M. Livermore. 2001. Characterization of OXA-25, OXA-26, and OXA-27, molecular class D β-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 45:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert, D., L. Poirel, J. Chevalier, S. Léotard, J.-M. Pagès, and P. Nordmann. 2001. Oxacillinase-mediated resistance to cefepime and susceptibility to ceftazidime in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 45:1615-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergogne-Bérézin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bou, G., G. Cervero, M. A. Dominguez, C. Quereda, and J. Martinez-Beltran. 2000. Characterization of a nosocomial outbreak caused by a multiresistant Acinetobacter baumannii strain with a carbapenem-hydrolyzing enzyme: high-level carbapenem resistance in A. baumannii is not due solely to the presence of β-lactamases. J. Clin. Microbiol. 38:3299-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bou, G., and J. Martinez-Beltran. 2000. Cloning, nucleotide sequencing, and analysis of the gene encoding an AmpC β-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 44:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bou, G., A. Oliver, and J. Martinez-Beltran. 2000. OXA-24, a novel class D β-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob. Agents Chemother. 44:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, S., C. Bantar, H. K. Young, and S. G. Amyes. 1998. Limitation of Acinetobacter baumannii treatment by plasmid-mediated carbapenemase ARI-2. Lancet 351:186-187. [DOI] [PubMed] [Google Scholar]
- 9.Chu, Y.-W., M. Afzal-Shah, E. T. Houang, M. I. Palepou, D. J. Lyon, N. Woodford, and D. M. Livermore. 2001. IMP-4, a novel metallo-β-lactamase from nosocomial Acinetobacter spp. collected in Hong Kong between 1994 and 1998. Antimicrob. Agents Chemother. 45:710-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark, R. B. 1996. Imipenem resistance among Acinetobacter baumannii: association with reduced expression of a 33-36 kDa outer membrane protein. J. Antimicrob. Chemother. 38:245-251. [DOI] [PubMed] [Google Scholar]
- 11.Corbella, X., A. Montero, M. Pujol, M. A. Dominguez, J. Ayats, M. J. Argerich, F. Garrigosa, J. Ariza, and F. Gudiol. 2000. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J. Clin. Microbiol. 38:4086-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corvec, S., N. Caroff, E. Espaze, C. Giraudeau, H. Drugeon, and A. Reynaud. 2003. AmpC cephalosporinase hyperproduction in Acinetobacter baumannii clinical strains. J. Antimicrob. Chemother. 52:629-635. [DOI] [PubMed] [Google Scholar]
- 13.Couture, F., J. Lachapelle, and R. C. Lévesque. 1992. Phylogeny of LCR-1 and OXA-5 with class A and class D β-lactamases. Mol. Microbiol. 6:1693-1705. [DOI] [PubMed] [Google Scholar]
- 14.Dalla-Costa, L. M., J. M. Coelho, H. A. Souza, M. E. Castro, C. J. Stier, K. L. Bragagnolo, A. Rea-Neto, S. R. Penteado-Filho, D. M. Livermore, and N. Woodford. 2003. Outbreak of carbapenem-resistant Acinetobacter baumannii producing the OXA-23 enzyme in Curitiba, Brazil. J. Clin. Microbiol. 41:3403-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Da Silva, G. J., M. Correia, C. Vital, G. Ribeiro, J. C. Sousa, R. Leitao, L. Peixe, and A Duarte. 2002. Molecular characterization of blaIMP-5, a new integron-borne metallo-β-lactamase gene from an Acinetobacter baumannii nosocomial isolate in Portugal. FEMS Microbiol. Lett. 215:33-39. [DOI] [PubMed] [Google Scholar]
- 16.Donald, H. M., W. Scaife, S. G. B. Amyes, and H.-K. Young. 2000. Sequence analysis of ARI-1, a novel OXA β-lactamase responsible for imipenem resistance in Acinetobacter baumannii 6B92. Antimicrob. Agents Chemother. 44:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gehrlein, M., H. Leying, W. Cullmann, S. Wendt, and W. Opferkuch. 1991. Imipenem resistance in Acinetobacter baumannii is due to altered penicillin-binding protein. Chemotherarpy 37:405-412. [DOI] [PubMed] [Google Scholar]
- 18.Héritier, C., L. Poirel, D. Aubert, and P. Nordmann. 2003. Genetic and functional analysis of the chromosome-encoded carbapenem-hydrolyzing oxacillinase OXA-40 of Acinetobacter baumannii. Antimicrob. Agents Chemother. 47:268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Héritier, C., L. Poirel, and P. Nordmann. 2004. Genetic and biochemical characterization of a chromosome-encoded carbapenem-hydrolyzing Ambler class D β-lactamase from Shewanella algae. Antimicrob. Agents Chemother. 48:1670-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kieser, T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19-36. [DOI] [PubMed] [Google Scholar]
- 21.Ledent, P., X. Raquet, B. Joris, J. Van Beeumen, and J.-M. Frère. 1993. A comparative study of class-D β-lactamases. Biochem. J. 292:555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manikal, V. M., D. Landman, G. Saurina, E. Oydna, H. Lal, and J. Quale. 2000. Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin. Infect. Dis. 31:101-106. [DOI] [PubMed] [Google Scholar]
- 23.Naas, T., and P. Nordmann. 1999. OXA-type β-lactamases. Curr. Pharm. Design 5:865-879. [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing. Eleventh informational supplement. M100-S11. NCCLS, Wayne, Pa.
- 25.Paton, R., R. S. Miles, J. Hood, and S. G. B. Amyes. 1993. ARI-1: β-lactamase mediated imipenem resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents 2:81-88. [DOI] [PubMed] [Google Scholar]
- 26.Philippon, L. N., T. Naas, A.-T. Bouthors, V. Barakett, and P. Nordmann. 1997. OXA-18, a class D clavulanic-acid inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41:2188-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poirel, L., T. Naas, M. Guibert, E. B. Chaibi, R. Labia, and P. Nordmann. 1999. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob. Agents Chemother. 43:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirel, L., D. Girlich, T. Naas, and P. Nordmann. 2001. OXA-28, an extended-spectrum variant of OXA-10 β-lactamase from Pseudomonas aeruginosa and its plasmid- and integron-located gene. Antimicrob. Agents Chemother. 45:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poirel, L., and P. Nordmann. 2002. Acquired carbapenem-hydrolyzing β-lactamases and their genetic support. Curr. Pharm. Biotechnol. 3:117-127. [DOI] [PubMed] [Google Scholar]
- 30.Poirel, L., C. Héritier, I. Podglajen, W., Sougakoff, L. Gutmann, and P. Nordmann. 2003. Emergence in Klebsiella pneumoniae of a chromosome-encoded SHV β-lactamase that compromises the efficacy of imipenem. Antimicrob. Agents Chemother. 47:755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poirel, L., C. Héritier, and P. Nordmann. 2004. Chromosome-encoded Ambler class D β-lactamase of Shewanella oneidensis as a progenitor of carbapenem-hydrolyzing oxacillinase. Antimicrob. Agents Chemother. 48:348-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poirel, L., C. Héritier, V. Tolün, and P. Nordmann. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riccio, M. L., N. Franceschini, L. Boschi, B. Caravelli, G. Cornaglia, R. Fontana, G. Amicosante, and G. M. Rossolini. 2000. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of blaIMP allelic variants carried by gene cassettes of different phylogeny. Antimicrob. Agents Chemother. 44:1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Scaife, W., H. K. Young, R. H. Paton, and S. G. B. Amyes. 1995. Transferable imipenem resistance in Acinetobacter species form a clinical isolate source. J. Antimicrob. Chemother. 36:585-587. [DOI] [PubMed] [Google Scholar]
- 36.Segal, H., E. C. Nelson, and B. Gay Elisha. 2004. Genetic environment of ampC in an Acinetobacter baumannii clinical isolate. Antimicrob. Agents Chemother. 48:612-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata, N., Y. Doi, K. Yamane, T. Yagi, H. Kurokawa, K. Shibayama, H. Kato, K. Kai, and Y. Arakawa. 2003. PCR typing of genetic determinants for metallo-β-lactamases and integrases carried by gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J. Clin. Microbiol. 41:5407-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi, A., S. Yomoda, I. Kobayashi, T. Okubo, M. Tsunoda, and S. Iyobe. 2000. Detection of carbapenemase-producing Acinetobacter baumannii in a hospital. J. Clin. Microbiol. 38:526-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urban, C., E. S. Go, K. S. Meyer, N. Mariano, and J. J. Rahal. 1995. Interactions of sulbactam, clavulanic acid and tazobactam with penicillin-binding proteins of imipenem-resistant and susceptible Acinetobacter baumannii. FEMS Microbiol. Lett. 125:193-197. [Google Scholar]
- 40.Yum, J. H., K. Yi, H. Lee, D. Yong, K. Lee, J. M. Kim, G. M. Rossolini, and Y. Chong. 2002. Molecular characterization of metallo-β-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the blaVIM-2 gene cassettes. J. Antimicrob. Chemother. 49:837-840. [DOI] [PubMed] [Google Scholar]