Abstract
Background
Cardiomyocytes are resistant to radiation. However, cardiac radiation exposure causes coronary microvascular endothelial inflammation, a perturbation implicated in the pathogenesis of heart failure (HF) and particularly, HF with preserved ejection fraction (HFpEF). Radiotherapy for breast cancer results in variable cardiac radiation exposure and may increase the risk of HF.
Methods
We conducted a population-based case-control study of incident HF in 170 female residents of Olmsted County, Minnesota (59 cases and 111 controls) who underwent contemporary (1998–2013) radiotherapy for breast cancer utilizing computed tomography-assisted radiotherapy planning. Controls were matched to cases for age, tumor side, chemotherapy use, diabetes and hypertension. Mean cardiac radiation dose (MCRD) in each patient was calculated from their computed tomography images and radiotherapy plan.
Results
Mean age at radiotherapy was 69±9 years. Of HF cases, 38 (64%) had EF≥ 50% (HFpEF), 18 (31%) had EF<50% (HFrEF) and 3 (5%) did not have EF measured. The EF was ≥ 40% in 50 (89%) of the 56 HF cases with an EF measurement. The mean interval from radiotherapy to HF was 5.8±3.4 years. The odds of HF was higher in patients with a prior history of ischemic heart disease or atrial fibrillation. The MCRD was 2.5 Gy (range 0.2 to 13.1 Gy) and higher in cases (3.3±2.7 Gy) than controls (2.1±2.0 Gy, p=0.004). The odds ratio (95% confidence interval) for HF per log MCRD was 9.1 (3.4, 24.4) for any HF, 16.9 (3.9,73.7) for HFpEF and 3.17 (0.8,13.0) for HFrEF. The increased odds of any HF or HFpEF with increasing MCRD remained significant after adjustment for HF risk factors and in sensitivity analyses matching by cancer stage rather than tumor side. Only 18.6% of patients experienced new or recurrent ischemic events between radiotherapy and onset of HF.
Conclusion
The relative risk of HFpEF increases with increasing cardiac radiation exposure during contemporary conformal breast cancer radiotherapy. These data emphasize the importance of radiotherapy techniques which limit MCRD during breast cancer treatment. Moreover, these data provide further support for the importance of coronary microvascular compromise in the pathophysiology of HFpEF.
Keywords: Heart Failure, Heart Failure with Preserved Ejection Fraction, HFpEF, Diastolic Heart Failure, Radiotherapy, Cardio-oncology, Radiation Heart Disease, Ionizing Radiation
Journal Subject Terms: Heart Failure
Introduction
Breast conserving surgery plus radiotherapy has emerged as the standard approach for localized breast cancer and in more advanced disease, radiotherapy improves local control and survival.1–4 The high doses of thoracic radiation used with thoracic tumors and older breast cancer radiotherapy techniques increase the risk of cardiac disease.5–8 Advances in radiotherapy planning, including the use of computed tomography (CT) assisted radiotherapy planning, can substantially reduce cardiac radiation exposure during contemporary breast cancer radiotherapy.5 However, even low levels of cardiac radiation during breast cancer radiotherapy increase the risk of coronary events.6
Cardiomyocytes are resistant to radiation. However, radiation induces coronary microvascular endothelial damage and inflammation leading to microvascular rarefaction and myocardial inflammation, oxidative stress and fibrosis.7–10 Comorbidity-driven coronary microvascular endothelial inflammation with similar subsequent myocardial effects has been implicated as a key factor in the pathophysiology of heart failure (HF) with preserved ejection fraction (HFpEF).11, 12 While major cardiomyocyte loss due to infarction or other factors is the primary etiologic insult in reduced ejection fraction HF (HFrEF), comorbidity-driven coronary microvascular endothelial inflammation can contribute to global myocardial dysfunction and HF progression.11, 12 Accordingly, we hypothesized that cardiac radiation exposure during contemporary breast cancer radiotherapy may increase the risk of HF and particularly, HFpEF. We performed a population-based case-control study of breast cancer patients treated with CT-guided radiotherapy, relating the odds of incident HF after radiotherapy to mean cardiac radiation dose (MCRD) and HF risk factors.
METHODS
Study Population
This study was restricted to appropriately consented residents of Olmsted County, Minnesota. The study was approved by the Institutional Review Boards of the Mayo Clinic and the Olmsted Medical Center.
For Olmsted County residents, radiotherapy is provided solely by the Mayo Clinic. Using the resources of the Rochester Epidemiology Project and the Mayo Clinic Cancer Registry (Supplemental Methods), all female patients over 18 years of age who had undergone radiotherapy for a histologically proven diagnosis of breast cancer in the era where CT- guided radiotherapy planning was beginning to be integrated into clinical practice (January, 1998 through December, 2013) and who resided in Olmsted County, Minnesota at the time of and subsequent to radiotherapy were identified (Supplemental Figure 1). The date of first appearance of diagnostic codes for HF and relevant comorbidities (Supplemental Table 1) was extracted for all patients. Patients with a HF diagnosis, thoracic radiation, or chemotherapy prior to the breast cancer diagnosis date were excluded from consideration as cases or controls.
We manually reviewed the medical records of patients with a HF diagnostic code to further confirm the absence of pre-existing HF or cardiomyopathy and to determine if patients met the modified Framingham criteria for HF13 (Supplemental Table 2) or if a physician had indicated a diagnosis of HF in the medical record with supportive clinical symptoms, signs and chest radiograph or echocardiographic evidence of HF (Supplemental Table 3). Patients with other explanations (ie lung metastasis) for HF symptoms were excluded. The medical records of potential controls were also reviewed using free text data searches of the electronic medical record for terms consistent with HF (Supplemental Methods). If such terms were present, charts were manually reviewed to confirm HF as above. Assessment for incident HF included the interval from breast cancer diagnosis through December 31, 2014.
Cases and controls with bilateral tumors, distant metastases at initial diagnosis, additional radiotherapy or chemotherapy after their initial breast cancer treatment or who did not have CT-based radiotherapy planning were excluded. At least one and up to two radiated breast cancer controls corresponding to each HF case were matched for factors known to increase HF risk including age at the breast cancer diagnosis (within 10 years), use of anthracycline, use of trastuzumab and prior history of hypertension or diabetes. As the cardiac chambers exposed to radiation may vary by tumor side, we also matched by tumor side.14, 15 Controls were required to have follow-up (index interval) equivalent to or greater than the time from radiotherapy to HF diagnosis of the corresponding case.
Comorbid conditions, cardiovascular medications, lifestyle information and cardiac imaging data were extracted from the medical record. The presence of ischemic heart disease was defined as a history of myocardial infarction, coronary bypass grafting or percutaneous coronary intervention and ascertained as previously described.16 Availability of ejection fraction measurement at the time (30 days before or after) of HF diagnosis was assessed and used to characterize HF as preserved (equal to or greater than 50%), reduced (less than 50%) or indeterminate (unavailable) ejection fraction HF.
Higher cancer stage often mandates more extensive radiotherapy and increases MCRD but also may result in heightened surveillance and bias HF ascertainment. Thus, we performed a sensitivity analysis re-matching controls to cases using the same criteria as above except matching for cancer stage rather than tumor side (Supplemental Methods).
Breast Cancer Treatment and Dosimetry
Breast cancer characteristics and treatment including use of systemic therapy and details of radiation therapy were extracted from Mayo Cancer Registry database, radiation oncology record and manual record review. In each patient, MCRD was calculated using simulation software (Eclipse, Varian Medical System, Inc. Palo Alto, CA) integrating the patient’s complete chest CT image set and their radiotherapy plan (Supplemental Methods). Dose sparing techniques were integrated over the study period (Supplemental Methods).
Statistical Analyses
Conditional logistic regression, conditioning on the matching factors (age, tumor side, chemotherapy use, diabetes and hypertension), was used to estimate incident HF odds ratios associated with clinical characteristics not used to match cases and controls. Similar models were used to calculate incident HF (overall and by HF type) odds ratios per (natural) log MCRD. Odds ratios were estimated without or with adjustment for clinical characteristics not used as matching factors but associated with HF incidence by including these adjustment factors as covariates in the conditional logistic regression models. The natural logarithm of MCRD was applied prior to analyses due to the skewed distribution of MCRD; this transformation improved model fit and also lowered the potential of influential observations with very large values. As the age matching criteria was fairly broad, we also performed analysis adjusting for age as a continuous variable. Interaction terms were added to the models to test for differences in dose effects and time to HF onset.
Comparison of crude HF frequency (HF or No HF) with increasing MCRD category were analyzed using the Cochran Armitage test for trend. Significance tests were two-sided. A p value less than 0.05 was considered statistically significant. Calculations were performed with the use of SAS version 9.4 (SAS Institute Inc).
RESULTS
Characteristics of the study patients
Among 945 female Olmsted County residents with breast cancer who underwent radiotherapy during 1998–2013 (median age 59), we identified 77 patients that developed new onset validated HF after radiotherapy. Of these 77 potential cases, 60 met final entry criteria (Supplemental Figure 1). No matching control was found for one case, only one matching control was found for seven cases, and two matching controls were found for 52 cases. Thus, the study included 59 HF cases and 111 non-HF controls. Of the HF cases, 43 fulfilled Framingham criteria and the remaining 16 had a physician’s diagnosis of HF recorded in the medical record with objective evidence of HF (Supplemental Table 3). Of HF cases, 38 (64%) had HFpEF, 18 (31%) had HFrEF and 3 (5%) did not have ejection fraction measured coincident with HF diagnosis. Of note, the EF was ≥ 40% in 50 (89%) of the 56 HF cases with an EF measurement. The majority of cases (57, 97%) and controls (105, 97%) were white. The mean interval from radiotherapy to HF diagnosis and corresponding index interval in controls was 5.8 ± 3.4 years. Matched characteristics were similar in cases and controls (Table 1). The relative risk of HF was higher in patients with more advanced cancer stage and in those with a prior history of ischemic heart disease or atrial fibrillation (Table 1).
Table 1.
Clinical characteristics at breast cancer diagnosis and relative risk of heart failure
| Cases (n=59) | Controls (n=111) | Odds Ratio | p-value | |
|---|---|---|---|---|
| Matched characteristics | ||||
| Age at breast cancer diagnosis, year | 69.8±9.6 | 68.3±8.8 | NA | NA |
| Left sided breast cancer, n (%) | 24 (41%) | 43 (39%) | NA | NA |
| Anthracycline therapy, n(%) | 7 (12%) | 13 (12%) | NA | NA |
| Trastuzumab therapy, n(%) | 0 (0%) | 0 (0%) | NA | NA |
| Hypertension, n(%) | 36 (61%) | 68 (61%) | NA | NA |
| Diabetes, n(%) | 13 (22%) | 22 (20%) | NA | NA |
| Cancer stage, n(%) | 0.03 | |||
| Stage 0 | 6 (10) | 24 (21) | 1.0 | |
| Stage 1 | 31 (53) | 62 (56) | 2.14 (0.79, 5.77) | |
| Stage 2 (A and B, n=40 ) or 3 (A–C, n=7) | 22 (37) | 25 (23) | 4.63 (1.45, 14.78) | |
| Surgical therapy | 0.67 | |||
| Mastectomy | 6 (10) | 10 (9) | 1.0 | |
| Breast-conserving surgery | 52 (88) | 101 (91) | 0.77 (0.22, 2.65) | |
| None | 1 (2) | 0 | N/A | |
| Adjuvant Paclitaxel therapy, n(%) | 0.25 | |||
| No | 53 (90) | 104 (94) | 1.0 | |
| Yes | 6 (10) | 7 (6) | 2.73 (0.50, 15.04) | |
| Adjuvant hormonal therapy, n(%) | 0.87 | |||
| No | 25 (42) | 48 (43) | 1.0 | |
| Yes | 34 (58) | 63 (57) | 1.05 (0.56, 1.96) | |
| Obesity (Body Mass Index ≥ 30 kg/m2) | 29.4±6.1 | 29.5±6.0 | 0.55 | |
| No | 37 (63) | 65 (59) | 1.0 | |
| Yes | 22 (37) | 46 (41) | 0.82 (0.43, 1.57) | |
| History of ischemic heart disease, n(%) | 0.02 | |||
| No | 51 (86) | 108 (97) | 1.0 | |
| Yes | 8 (14) | 3 (3) | 5.06 (1.34, 19.13) | |
| History of atrial fibrillation or flutter, n(%) | 0.009 | |||
| No | 45 (76) | 102 (92) | 1.0 | |
| Yes | 14 (24) | 9 (8) | 3.41 (1.36, 8.55) | |
| History of chronic lung disease, n(%) | 0.11 | |||
| No | 53 (90) | 107 (96) | 1.0 | |
| Yes | 6 (10) | 4 (4) | 2.82 (0.79, 10.03) | |
| Smoking, n(%) | 0.11 | |||
| Current | 10 (17) | 9 (8) | 2.56 (0.89, 7.37) | |
| Ever | 12 (20) | 31 (28) | 0.68 (0.29, 1.59) | |
| Never | 37 (63) | 71 (64) | 1.0 | |
| Medication use | ||||
| ACE or ARB, n(%) | 0.32 | |||
| No | 38 (64) | 78 (70) | 1.0 | |
| Yes | 21 (36) | 33 (30) | 1.56 (0.65, 3.72) | |
| Beta blocker, n(%) | 0.11 | |||
| No | 32 (54) | 73 (66) | 1.0 | |
| Yes | 27 (46) | 38 (34) | 1.76 (0.88, 3.50) | |
Abbreviations: ACE, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker Ischemic Heart Disease was defined as a history of myocardial infarction, coronary bypass grafting or percutaneous coronary intervention prior to breast cancer diagnosis
Impact of mean cardiac radiation dose on the relative risk of incident heart failure
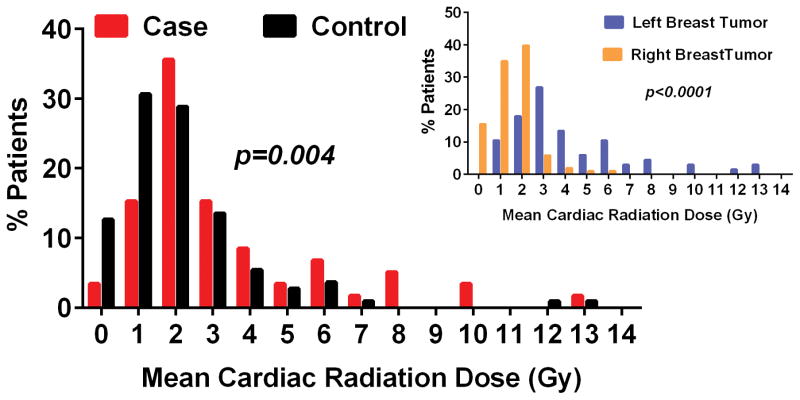
The overall MCRD was 2.5 (range 0.2 to 13.1) Gy and higher in cases (3.3±2.7 Gy) than controls (2.1±2.0 Gy, p=0.004) (Figure 1). The average MCRD was higher in women with left (4.1, range 0.6 to 13.1 Gy) versus right (1.5, range 0.2 to 5.6 Gy, p<0.001) sided tumors (Figure 1, insert). The MCRD was higher in patients with higher cancer stage (Supplemental Figure 2), likely owing to internal mammary node treatment. In the entire study population, tumor side explained 37% (p<0.001) of the variation and tumor side and cancer stage together explained 44% (p<0.001 for both) of the variation in MCRD. MCRD decreased over the study era (Supplemental Figure S3).
Figure 1. Distribution of mean cardiac radiation dose in study patients.
The mean cardiac radiation dose in cases and controls and in patients with right or left sided tumors (insert) are shown.
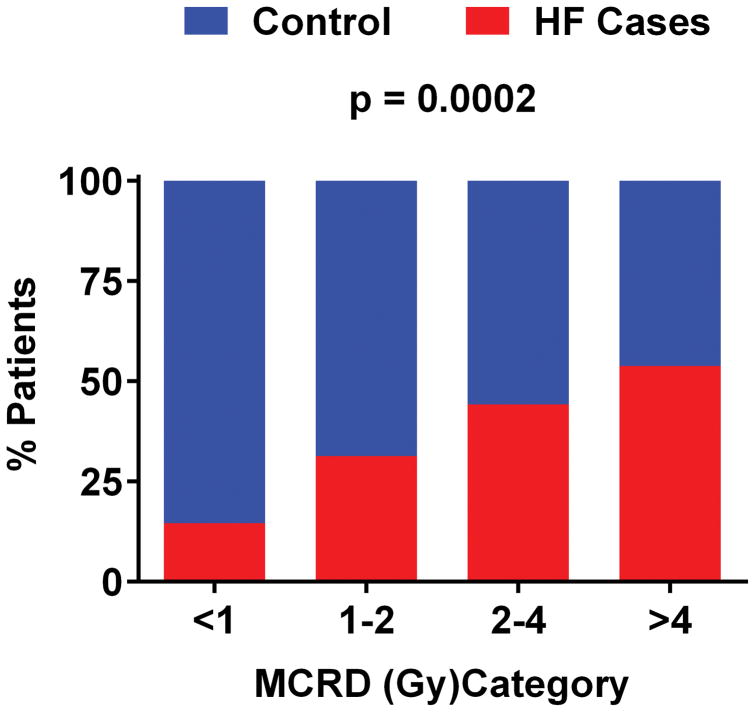
The crude frequency of HF cases versus controls increased with higher MCRD (Figure 2). The odds of incident HF (any) and of HFpEF increased with higher MCRD (Table 2), even after adjustment for age, cancer stage and prior history of ischemic heart disease or atrial fibrillation. The crude frequency of HF at any MCRD was numerically higher in those with versus those without a prior history of ischemic heart disease or atrial fibrillation but the crude HF frequency increased with increasing MCRD in both groups (Figure 3) as in the overall conditional regression analysis (Table 2). The effect of MCRD on the odds of incident HF was apparent and statistically significant in patients with left or right sided tumors (Supplemental Table 4). Further, consistent with our findings matched by use of chemotherapy (Table 2), the crude frequency of HF increased with MCRD when analysis was restricted to patients not receiving chemotherapy (Supplemental Figure 4).
Figure 2. Crude frequency of heart failure cases versus controls according to category of mean cardiac radiation dose.
Heart failure (red) relative to controls (blue) increased with increasing mean cardiac radiation dose (MCRD). Abbreviations: HF, heart failure
Table 2.
Association between mean cardiac radiation dose and relative risk of incident heart failure
| Odds Ratio Per Log MCRD |
p value | |
|---|---|---|
| All Heart Failure | ||
| Unadjusted | 9.14 (3.43, 24.37) | <0.001 |
| Adjusted for Age | 8.57 (3.22, 22.85) | <0.001 |
| Adjusted for History of Ischemic Heart Disease (IHD) | 8.16 (3.05, 21.83) | <0.001 |
| Adjusted for History of Atrial Fibrillation/Flutter (AF) | 8.65 (3.23, 23.16) | <0.001 |
| Adjusted for Cancer Stage | 8.66 (3.23, 23.23) | <0.001 |
| Adjusted for Age/IHD/AF/cancer stage | 7.40 (2.77, 19.81) | <0.001 |
| Heart Failure with Preserved Ejection Fraction | ||
| Unadjusted | 16.88 (3.86, 73.74) | <0.001 |
| Adjusted for Age | 16.31 (3.67, 72.48) | <0.001 |
| Adjusted for History of Ischemic Heart Disease (IHD) | 16.45 (3.72, 72.75) | <0.001 |
| Adjusted for History of Atrial Fibrillation/Flutter (AF) | 20.83 (4.24, 102.30) | <0.001 |
| Adjusted for cancer stage | 18.93 (4.12, 87.02) | <0.001 |
| Adjusted for Age/IHD/AF/cancer stage | 22.70 (4.48, 115.10) | <0.001 |
| Heart Failure with Reduced Ejection Fraction | ||
| Unadjusted | 3.17 (0.77, 12.96) | 0.11 |
| Adjusted for Age | 3.22 (0.79, 13.10) | 0.10 |
| Adjusted for History of Ischemic Heart Disease (IHD) | 1.96 (0.48, 7.95) | 0.35 |
| Adjusted for History of Atrial Fibrillation/Flutter (AF) | 3.33 (0.76, 14.69) | 0.11 |
| Adjusted for cancer stage | 2.24 (0.48, 10.52) | 0.31 |
| Adjusted for Age/IHD/AF/cancer stage | 2.33 (0.53, 10.17) | 0.26 |
Abbreviations: MCRD, mean cardiac radiation dose; vs, versus
Figure 3. Crude frequency of heart failure cases versus controls according to category of mean cardiac radiation dose and stratified by history of heart failure risk factors prior to breast cancer diagnosis.
Heart failure (red) relative to controls (blue) increased with increasing mean cardiac radiation dose (MCRD) irrespective of the presence or absence of atrial fibrillation (AF) or ischemic heart disease (IHD) prior to breast cancer diagnosis. Abbreviations: HF, heart failure
The odds of incident HFrEF increased with higher MCRD but this association was not significant (Table 2). Adjusting for age, HF risk factors and cancer stage, there was no difference in the association between MCRD effect and odds of HF by time from radiotherapy (interaction radiation dose*time p = 0.61, Table 3).
Table 3.
Association between mean cardiac radiation dose and relative risk of incident heart failure by time from breast cancer diagnosis
| Time period after breast cancer diagnosis (above or below the median index interval) | Odds Ratio Per Log MCRD* |
p value |
|---|---|---|
| < 5.9 years | 8.47(2.03, 36.42) | 0.003 |
| ≥ 5.9 years | 5.65 (1.50, 21.30) | 0.01 |
Adjusted for age, history of ischemic heart disease, history of atrial fibrillation or flutter and cancer stage. The p value for the interaction between radiation dose effect and time to heart failure was 0.61.
Abbreviations: MCRD, mean cardiac radiation dose.
Sensitivity analyses
In a cohort matched by the same factors except cancer stage rather than tumor side, clinical characteristics associated with HF incidence (Supplemental Table 5) were similar to the primary analysis. The MCRD was associated with HF and HFpEF incidence (Supplemental Table 6), even after adjustment for pertinent covariates. The magnitude of odds per log MCRD was lower than in the primary analysis but remained substantial, particularly if the analysis was adjusted for tumor side. The effect of MCRD on the odds of incident HF was apparent and statistically significant when patients who did not fulfill Framingham criteria for HF diagnosis were excluded both in the primary analysis cohort matched by tumor side (Supplemental Table 7) and in the sensitivity analysis cohort matched by cancer stage (Supplemental Table 8).
Factors associated with development of heart failure after radiotherapy
Of patients who developed HF after radiotherapy, 11 (18.6%) had new or recurrent ischemic heart disease events, 15 (25.4%) had new or recurrent atrial fibrillation, and 22 (37.3%) had either of these conditions after radiotherapy but prior to or coincident with the HF diagnosis.
Discussion
In this population-based case-control study of older women with breast cancer treated with contemporary conformal radiotherapy, the odds of incident HF after radiotherapy increased with higher MCRD. The predominant form of HF was HFpEF or HF with “mid-range” (40–49%) EF17 and the odds for any HF and for HFpEF increased with MCRD, even after adjustment for other known risk factors and cancer stage. The mean time from radiotherapy to HF was 5.8 years. A minority of women developed ischemic events between radiotherapy and HF diagnosis, suggesting that myocardial infarction due to epicardial coronary disease was not the predominant mediator of incident HF. The effect of MCRD on HF incidence was still apparent in sensitivity analyses addressing the potential for surveillance bias associated with higher cancer stage.
In 40 year old women, the lifetime risks of both breast cancer (12%) and HF (20%) are significant.18, 19 Adjuvant radiotherapy reduces breast cancer loco-regional recurrence and mortality in some breast cancer subgroups.2–4 The excellent survival following treatment for localized breast cancer mandates attention to survivorship issues, including cardiovascular complications of radiotherapy.20 The risk of cardiac toxicity with high dose thoracic radiotherapy is well documented.2, 7, 8, 10, 20 While MCRD varies with tumor side and treatment of nodal beds, individual variation in thoracic and cardiac anatomy contributes significantly to cardiac exposure, as seen here. Thus, while on average, MCRD is quite low with contemporary conformal breast cancer radiotherapy, significant individual variation exists.5, 14, 15 A growing number of radiotherapy techniques can reduce cardiac exposure,5 but these are inconsistently utilized. Indeed, average cardiac doses and importantly, maximal cardiac doses in a meta-analysis of contemporary breast cancer RT studies substantially exceed those observed here.5 Further, even as MCRD falls with improved techniques, our data emphasize that women treated before such advances remain at increased risk of HF. The current data also underscore the need to reduce MCRD, particularly in older women with HF risk factors.
Consistent with our findings, the ongoing study of atomic bomb survivors in Japan has demonstrated that total body radiation exposures of less than 2.5 Gy leads to significant increases in the incidence of HF (excess risk 22% per Gy) but not myocardial infarction.7 Meta-analyses have suggested that cardiovascular mortality and some assessed cardiovascular morbid events are not increased in women treated with more contemporary breast cancer radiotherapy techniques20, 21. However, these studies acknowledge the limited follow-up duration, the lack of individual cardiac dose data and importantly, the potential for interaction between pre-existing clinical or subclinical cardiovascular abnormalities and the impact of cardiac radiation dose.20, 21
Beyond differences in therapeutic era, the design of studies assessing radiotherapy cardiac toxicity have varied, and comparisons between breast cancer patients with or without radiotherapy or between left or right sided tumor radiotherapy patients have significant limitations due to confounders6 and the inability of tumor laterality to precisely reflect individual cardiac dose, as also demonstrated here. To address these limitations, Darby et al used a case control design with estimations of individual patient MCRD derived from the radiotherapy treatment plan and a single “representative” CT scan. Even after adjustment for coronary risk factors, the risk of major coronary events increased in proportion to the MCRD (7% per Gy) and over a fairly short interval following radiotherapy.6 The absolute risk was highest in older women with coronary risk factors.
The current study used a case-control design rather than a cohort study design. Results from previous cohort studies of the effect of breast cancer radiotherapy on HF incidence have been mixed.22–25 No study has specifically examined the effect of individually calculated MCRD on the incidence of preserved and reduced ejection fraction HF and study designs were subject to the limitations noted above as well as complexities of HF (and particularly HFpEF) case ascertainment.26 The incidence of HF increases dramatically with age, and in the community, the mean age at HF diagnosis is 78 years for HFpEF and 72 years for HFrEF.27 Given the average age (61 years) and low MCRD in the majority of contemporary breast cancer patients,5, 15 the likely critical interaction between the impact of MCRD and pre-existing age and comorbidity related myocardial abnormalities, underutilization of radiotherapy in older patients with HF risk factors28, 29 and the challenges in HF case ascertainment, a general breast cancer cohort study may fail to detect the impact of radiation dose on HF incidence without accurate cardiac dose assessment and sensitive case ascertainment methodology.
Several studies have documented new cardiac perfusion defects (without interim myocardial infarction) after breast cancer radiotherapy consistent with microvascular rarefaction.30 Comorbidity driven coronary microvascular endothelial inflammation is believed to play a key role in the pathophysiology of HFpEF. Microvascular endothelial inflammation leads to microvascular dysfunction and rarefaction with reduction in coronary flow reserve and myocardial inflammation and fibrosis as well as oxidative stress, which may impair nitric oxide – cyclic guanosine monophosphate signaling and potentiate cardiomyocyte hypertrophy and myofiber diastolic stiffness.11, 12, 31 The mechanism of radiation induced myocardial disease is well described with microvascular damage leading to inflammatory and thrombotic changes, microvascular rarefaction, myocardial inflammation, oxidative stress and fibrosis as well as focal ischemia.4, 6, 7, 20 Thus, the current findings are consistent with, and lend further support to, the pivotal role of the microvasculature in the pathophysiology of HFpEF,31, 32 where microvascular inflammation has been histologically demonstrated.33 Studies of heart disease after higher doses of cardiac radiation in younger persons7 suggest that HF is a late occurrence. However, older women receiving breast cancer radiotherapy have comorbidities and may already have significant but subclinical coronary microvascular and myocardial disease. Thus, even low doses of cardiac radiation may have impact, providing the further disruption in microvascular structure and function required to precipitate overt HF.
Potential Limitations and Strengths
The study size was small but the design was strengthened by the use of precise MCRD calculation using the complete set of CT images, matching or adjusting for HF risk factors, complete patient level data, rigorous case ascertainment techniques, the community based setting and our sensitivity analyses. Few women developed HFrEF and even among the HFrEF group, most had “midrange EF” (40–49%)17 often considered as HFpEF and thus the impact of MCRD on HFrEF incidence is uncertain. Restriction to the contemporary therapeutic era limits the ability to detect longer term risks in younger women. Specific cardiac chamber doses were not assessed and may be important14 as impairment in atrial and right ventricular function both contribute to the pathophysiology of HFpEF.34, 35 While the analysis adjusted for non-matched variables associated with HF (ischemic heart disease and atrial fibrillation), we cannot exclude residual confounding but the effect of dose on crude HF odds ratios in patients with or without these risk factors was still apparent. While we confined analysis to the era where CT- guided radiotherapy planning was beginning to be integrated into clinical practice, this was an incremental practice change and not all radiotherapy patients had CT scans for MCRD calculations.
Conclusion
In older women, undergoing contemporary breast cancer radiotherapy, the relative risk of HFpEF increases in proportion to calculated MCRD, begins within a few years after radiotherapy and is not mediated solely by coronary events. These data suggest that cardiac dose and HF risk factors should be considered in decisions regarding breast cancer radiotherapy and underscore the importance of techniques to reduce cardiac dose. Moreover, these data provide further support for the importance of coronary microvascular compromise in the pathophysiology of HFpEF.
Supplementary Material
Clinical Perspective.
What is new?
In this population-based case-control study of older women with breast cancer treated with contemporary conformal radiotherapy, the odds of incident heart failure (HF) after radiotherapy increased with higher mean cardiac radiation dose (MCRD).
The predominant form of HF was HF with preserved (≥ 50%; HFpEF) or HF with “mid-range” (40–49%) ejection fraction.
The relative risk for any HF and for HFpEF increased with MCRD, even after adjustment for other known risk factors and cancer stage.
Myocardial infarction due to epicardial coronary disease was not the predominant mediator of incident HF.
What are the clinical implications?
These data emphasize the importance of radiotherapy techniques which limit MCRD during breast cancer treatment.
Moreover, these data provide further support for the importance of coronary microvascular compromise in the pathophysiology of HFpEF.
Acknowledgments
Funding Sources:
Resources utilized in this study were provided by the Mayo Foundation and the National Institutes of Health (CTSA, UL1 TR000135; Rochester Epidemiology Project, RO1 AG034676). Dr. Saiki’s time was funded by the Mayo Foundation (2014–2015), Saitama medical university (2013–2014), the International exchange aid, Fukuda Foundation for Medical Technology, Japan (Dr. Saiki, 2013), and Miyata Research Promotion Foundation, Japan (Dr. Saiki, 2014). Dr Redfield’s time was funded by the Mayo Foundation and the National Institutes of Health (P01 HL 76611, U10 HL 110262 and RO1 HL 105418). Dr. Ruddy’s time was funded by the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (UL1 TR000135 and KL2TR000136-09). This paper’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Disclosures:
none
References
- 1.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A, Giordano SH, Goetz M, Goldstein LJ, Hudis CA, Isakoff SJ, Marcom PK, Mayer IA, McCormick B, Moran M, Patel SA, Pierce LJ, Reed EC, Salerno KE, Schwartzberg LS, Smith KL, Smith ML, Soliman H, Somlo G, Telli M, Ward JH, Shead DA, Kumar R. Breast Cancer Version 2.2015. J Natl Compr Canc Netw. 2015;13:448–475. doi: 10.6004/jnccn.2015.0060. [DOI] [PubMed] [Google Scholar]
- 2.Brown LC, Mutter RW, Halyard MY. Benefits, risks, and safety of external beam radiation therapy for breast cancer. Int J Womens Health. 2015;7:449–458. doi: 10.2147/IJWH.S55552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, Gray R, Mannu G, Peto R, Whelan T, Wang Y, Wang Z, Darby S. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor CW, Wang Z, Macaulay E, Jagsi R, Duane F, Darby SC. Exposure of the Heart in Breast Cancer Radiation Therapy: A Systematic Review of Heart Doses Published During 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93:845–853. doi: 10.1016/j.ijrobp.2015.07.2292. [DOI] [PubMed] [Google Scholar]
- 6.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 7.Stewart FA, Seemann I, Hoving S, Russell NS. Understanding radiation-induced cardiovascular damage and strategies for intervention. Clin Oncol (R Coll Radiol) 2013;25:617–624. doi: 10.1016/j.clon.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Stewart FA. Mechanisms and dose-response relationships for radiation-induced cardiovascular disease. Ann ICRP. 2012;41:72–79. doi: 10.1016/j.icrp.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 10.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, Mabuchi K, Marks LB, Mettler FA, Pierce LJ, Trott KR, Yeh ET, Shore RE. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 14.Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, Hayman JA, Jagsi R, Jolly S, Larouere J, Soriano J, Marsh R, Pierce LJ. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansen S, Tjessem KH, Fossa K, Bosse G, Danielsen T, Malinen E, Fossa SD. Dose Distribution in the Heart and Cardiac Chambers Following 4-field Radiation Therapy of Breast Cancer: a Retrospective Study. Breast Cancer (Auckl) 2013;7:41–49. doi: 10.4137/BCBCR.S11118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P Authors/Task Force M. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 19.Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst. 2011;103:1397–1402. doi: 10.1093/jnci/djr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeboa DN, Evans SB. Contemporary Breast Radiotherapy and Cardiac Toxicity. Semin Radiat Oncol. 2016;26:71–78. doi: 10.1016/j.semradonc.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Demirci S, Nam J, Hubbs JL, Nguyen T, Marks LB. Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys. 2009;73:980–987. doi: 10.1016/j.ijrobp.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, Taylor CW, van Leeuwen FE. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 23.Patt DA, Goodwin JS, Kuo YF, Freeman JL, Zhang DD, Buchholz TA, Hortobagyi GN, Giordano SH. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol. 2005;23:7475–7482. doi: 10.1200/JCO.2005.13.755. [DOI] [PubMed] [Google Scholar]
- 24.McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, Bennet AM, Fornander T, Gigante B, Jensen MB, Peto R, Rahimi K, Taylor CW, Ewertz M. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol. 2011;100:167–175. doi: 10.1016/j.radonc.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, Solin LJ. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 26.Nolan MT, Russell DJ, Marwick TH. Long-term Risk of Heart Failure and Myocardial Dysfunction After Thoracic Radiotherapy: A Systematic Review. Can J Cardiol. 2016;32:908–920. doi: 10.1016/j.cjca.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballard-Barbash R, Potosky AL, Harlan LC, Nayfield SG, Kessler LG. Factors associated with surgical and radiation therapy for early stage breast cancer in older women. J Natl Cancer Inst. 1996;88:716–726. doi: 10.1093/jnci/88.11.716. [DOI] [PubMed] [Google Scholar]
- 29.Louwman WJ, Janssen-Heijnen ML, Houterman S, Voogd AC, van der Sangen MJ, Nieuwenhuijzen GA, Coebergh JW. Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: a population-based study. Eur J Cancer. 2005;41:779–785. doi: 10.1016/j.ejca.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 30.Marks LB, Yu X, Prosnitz RG, Zhou SM, Hardenbergh PH, Blazing M, Hollis D, Lind P, Tisch A, Wong TZ, Borges-Neto S. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 31.Mohammed SF, Majure DT, Redfield MM. Zooming in on the Microvasculature in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.1116.003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Kimura K, Umemura S. Impairment of Coronary Flow Reserve Evaluated by Phase Contrast Cine-Magnetic Resonance Imaging in Patients With Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.1115.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, Paulus WJ, Hamdani N. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:312–324. doi: 10.1016/j.jchf.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee NA, Steiner J, Lewis GD. It is time to look at heart failure with preserved ejection fraction from the right side. Circulation. 2014;130:2272–2277. doi: 10.1161/CIRCULATIONAHA.114.013536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. doi: 10.1161/CIRCHEARTFAILURE.114.001667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.