Abstract
Failure to maintain a healthy body weight may reflect a long-term imbalance between the executive control and reward systems of the brain. The current study examined whether the anatomical connectivity between these two systems predicted individual variability in achieving a healthy body weight, particularly in chronic dieters. Thirty-six female chronic dieters completed a food-cue reactivity task in the scanner. Two region-of-interest (ROIs) were defined from the reactivity task: the inferior frontal gyrus (IFG), which engages cognitive control, and the orbitofrontal cortex (OFC), which represents reward value. A white matter tract connecting these two ROIs was identified across participants using diffusion tensor imaging and probabilistic tractography. Results showed a negative relationship between body fat percentage and white matter integrity within the identified tract. This suggests that reduced structural integrity between the OFC and IFG may be related to self-regulatory problems for those who chronically diet to control body weight.
Introduction
Obesity and dieting have increased substantially in recent decades, with many dieters failing to lose weight over long periods of time and becoming chronic dieters (Andreyeva, Long, Henderson, & Grode, 2010). Among these individuals, some go on to gain even more weight after years of dieting (van Strien, Herman, & Verheijden, 2014), thus engaging in behaviors that undermine their goals. A comprehensive review of obesity suggests that not only the heightened reward sensitivity to food cues, but also failures in self-regulation play a role in obesity (Volkow, Wang, Tomasi, & Baler, 2013). Similarly, individual differences in achieving or maintaining long-term dieting success may reflect differences in self-regulatory ability (Heatherton, 2011). According to the balance model, such self-regulatory outcomes reflect individual differences in the balance between brain regions involved in executive control, e.g., the inferior frontal gyrus (IFG), and regions representing reward value, e.g., the orbitofrontal cortex (OFC) (Heatherton & Wagner, 2011). A balance between reward and control is dependent on the communication between the IFG and the OFC (Wagner, Altman, Boswell, Kelley, & Heatherton, 2013), which is reflected in the connectivity between them. Individual differences in the structural integrity of these anatomical pathways may predict the long-term outcome of chronic dieting, such as body fat percentage.
In order to isolate regions of the brain known to be involved in processing the appetitive value of food, we used a food cue reactivity task to localize the IFG and OFC ROIs using functional magnetic resonance imaging (fMRI). Reviews of this research indicate that chronic dieters tend to show exaggerated activity to appetitive food cues in both reward regions and regions involved in executive control after being exposed to conditions associated with diet failure in the real world, such as consuming high-calorie food or being depleted in their cognitive control (Kelley, Wagner, & Heatherton, 2015; Volkow et al., 2013). Thus, we used a food cue reactivity task to localize the extent of the IFG and OFC regions involved in food-cue processing, and we then used these two regions as seed masks for probabilistic tractography in a diffusion tensor imaging (DTI) analysis to calculate white matter integrity between these two regions. The aim of this study was to use a multimodal approach to isolate white matter tracts between functionally defined regions of the IFG and OFC. This approach offers a targeted way to examine whether structural integrity of this tract predicts chronic dieters’ variability in body fat percentage. We hypothesized that chronic dieters who had the highest body fat percentage would show lowest structural integrity in the tract connecting the IFG and OFC.
Methods
Forty right-handed female chronic dieters who score higher than 15 on the Restraint Scale, a measure of chronic dieting (Heatherton, Herman, Polivy, King, & McGree, 1988), were recruited in this study from a large sample of college students. Participants were screen to have no history of metabolic, psychological, or neurological abnormalities. Four participants were excluded due to excessive head movements (more than 3 mms in either x-, y- or z-direction) during scanning, resulting in a sample size of 36 chronic dieters. Dieters were weighted using a Tanita scale (model TBF-300A Arlington Heights), which has been proven to reliably measure body fat percentage. Among these chronic dieters, mean body fat percentage was 29.6% (s.d. = 5.5%; range = 16.6% – 38.2%) and mean body mass index (BMI) was 23.9 (s.d. = 3.1%, range = 17.2 – 33.7, Table. 1).
Table 1.
Demographic and dieting characteristics of the population.
| Mean (S.D.) | Range | |
|---|---|---|
| Age (years) | 19.639 (1.312) | 18 to 23 |
| BMI (kg/m2) | 23.872 (3.092) | 17.2 to 33.7 |
| Body fat percentage (%) | 29.6 (5.5) | 16.6 to 38.2 |
| Restraint Scale scores | 19.361 (3.673) | 15 to 29 |
In order to localize the IFG and the OFC ROIs involved in food-cue processing, we used a food-cue reactivity paradigm as a localizer task. Prior to this localizer task, we used a depleting inhibitory control task, as this task helps elicit robust reward activity (Wagner et al., 2013). In this depleting inhibitory control task, participants were asked to view a 7-minute video about Canadian bighorn mountain sheep with a series of distractor words showing from the bottom to the center of the screen in 3 seconds (40 words in total). Participants were then instructed to avoid reading the distractor words and only focus on watching the video. Immediately following the depleting inhibitory control task, participants received the food-cue reactivity task. This task consisted of 90 appetizing food images, and another 180 images involving people or natural scenes as control images. Each image was presented for 2000 ms, and followed by a fixation for another 500 ms in an event-related design. Participants were instructed to make an indoor/outdoor judgment for each image by pressing buttons. This judgment task made participants keep their focus on this task without being aware of our study purpose, which was to localize brain regions responding to food cues.
The fMRI data from the food-cue reactivity task were preprocessed and analyzed with SPM8 (Wellcome Department of Cognitive Neurology, London, England). The preprocessing procedures included motion-correction, realignment, unwarping, normalization to standard space, and 6-mm FWHM Gaussian kernel smoothing (see the Supplementary Materials for a detailed description of the fMRI scanning parameters). For each participant, a general linear model incorporating task effects and covariates of no interest was computed and convolved with a canonical hemodynamic response function (HRF). Food-vs.-control contrast images were created for each participant and then submitted to a random-effect whole-brain analysis, corrected for p < 0.05 with Monte Carlo simulations using AFNI’s AlphaSim (Fig. 1(a), Table. S1). The IFG and OFC ROIs were defined based on this whole-brain analysis and used as masks (Fig. 1(b)) in a two-mask seeding method for DTI probabilistic tractography. The DTI data was preprocessed and analyzed with Diffusion Toolbox in FSL (T. Behrens et al., 2003). We used a two-mask seeding method to ensure that the tractography maps only included streamlines passing through both seed masks. Five thousand probabilistic tract streamlines were sent from each voxel within both seed masks, and these results were normalized to MNI standard space. In order to determine a common tractography map among individuals, each individual’s tractography results were binarized and overlaid with all others to create a group-level tract probability map. To make the tractography map robust, it was then thresholded at a 50% tract probability across all participants in the standard space (Fig. 1(c)). This common tractography map was then overlaid on each participant’s fractional anisotropy (FA) images (a general measure of white matter integrity or coherence), and FA values were extracted from all voxels within this tractography map. For each participant, an averaged FA score was calculated to represent their white matter integrity. The participants’ age, BMI, and FA scores were then taken as independent variables in the multiple regression analysis with body fat percentage as the dependent variable, examining whether individual differences in white matter integrity reflected long-term self-regulatory success in dieting.
Figure 1.
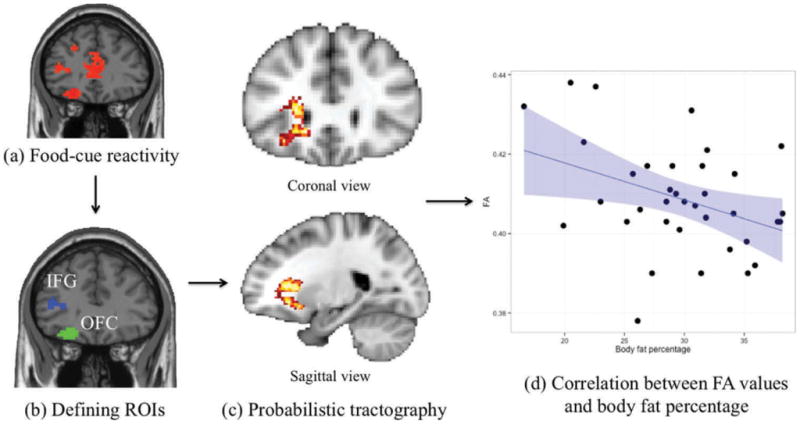
(a) The food-cue reactivity fMRI paradigm elicited activation in regions of OFC and IFG, consistent with previous findings in the literature. (b) OFC and IFG regions from the fMRI analysis were then used as seed masks for the DTI probabilistic tractography analysis. (c) From the DTI analysis, we then delineated a white matter pathway between the IFG and OFC which was consistent across subjects. White matter integrity within this pathway was then correlated with each subject’s body fat. (d) FA value extracted from the IFG-OFC tract showed a negative correlation with body fat percentage (shaded area represents 95% confidence interval).
Results
FMRI results from the food-cue reactivity task revealed that both the IFG and OFC showed greater activity for food than control images (Fig. 1(a), Table. S1), consistent with findings from previous studies (Lopez, Hofmann, Wagner, Kelley, & Heatherton, 2014; Wagner et al., 2013). The resulting IFG and OFC clusters from the fMRI analysis were used as seed regions for the DTI probabilistic tractography (Fig. 1(b)). Cross-subject DTI probabilistic tractography analysis revealed a robust white matter tract linking the left IFG to the left OFC (Fig. 1(c)). Average FA values within this tract showed a significantly negative correlation with body fat percentage (R36 = −0.379, p = 0.023), indicating that dieters with lower body fat percentage showed greater white matter integrity (Fig. 1(d)) 1. After controlling for age and BMI, average FA values within the tract continued to show a significantly negative correlation with body fat percentage (beta = −0.247, t(35) = −2.862, p = 0.007).
General Discussion
Our findings support the hypothesis that the structural integrity between the IFG, a key region in response inhibition (Aron, 2011) and the OFC, a region representing subjective reward value of food (van der Laan, de Ridder, Viergever, & Smeets, 2011), is related to individual differences in body fat percentage, a putative index of long-term success in dieting. Based on the balance model of self-regulation (Heatherton & Wagner, 2011), failures in self-regulation results from the imbalance between the executive control and reward regions. Individuals with reduced white matter integrity within the tract connecting these regions may have lower efficiency in the communication between these regions than those with high integrity (Madden et al., 2012). With an inefficient communication between the executive control and reward regions, individuals with reduced integrity may have difficulty in overriding rewarding temptations, leading to a greater chance of becoming obese than those with higher structural integrity.
Although prior studies have used DTI to examine the relationship between obesity and white matter integrity (Kullmann, Schweizer, Veit, Fritsche, & Preissl, 2015), this study is novel in employing a multimodal approach to systematically target a specific tract implicated in regulating food desires. A recent study has shown that both the IFG and the OFC engage in regulating food consumption in daily life (Lopez et al., 2014). Using this fMRI paradigm, we replicated the previous finding and even found that both the IFG and the OFC showed robust activity when chronic dieters were exposed to appetitive food cues, suggesting that the reward and executive control processing may both spontaneously engage during the cue exposure period (Wagner et al., 2013). The food-cue reactivity localizer task offers a more targeted approach to defining IFG and OFC regions than atlas-based masking and ensures that the tractography results were based on functionally relevant regions related to food-cue processing within the same sample. This fMRI localizer task may help researchers identify key white matter tracts relevant to the control of eating behavior.
It remains unclear whether individual differences in the white matter integrity result from repeated dieting. Although a previous study found that repeatedly practicing a task can lead to increased FA in particular fiber tracts (Scholz, Klein, Behrens, & Johansen-Berg, 2009), it is also possible that failures in dieting lead to obesity and obesity-related factors, such as inflammation and dyslipidaemia, which may cause alterations in white matter integrity (Shimoji et al., 2013). Future longitudinal studies are needed to determine whether alteration in white matter integrity is caused by repeated practice in dieting or obesity-related factors. Longitudinal studies are also needed to examine the relationship between white matter integrity and body fat percentage in chronic dieters. Although the exclusive recruitment of female dieters may prevent the confounding effect from gender difference, future studies are still needed to compare whether white matter integrity differentially influences the dieting outcomes between male and female dieters.
In conclusion, the current study provides evidence that white matter integrity is related to individual differences in body fat percentage in tracts that connect regions involved in food-cue reactivity. These results are consistent with balance model of self-regulation and suggest that structural integrity of pathways connecting executive control and reward regions may play a critical role in achieving long-term self-regulatory success in dieting.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institute of Health (R01DA022582) to T.F. Heatherton.
Footnotes
Additionally, this finding also replicated in an independent sample of thirty-two chronic dieters taken from a separate study. For details, see the Supplementary Materials.
References
- Andreyeva T, Long MW, Henderson KE, Grode GM. Trying to Lose Weight: Diet Strategies among Americans with Overweight or Obesity in 1996 and 2003. Journal of the American Dietetic Association. 2010;110(4):535–542. doi: 10.1016/j.jada.2009.12.029. http://doi.org/10.1016/j.jada.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological Psychiatry. 2011;69(12):e55–68. doi: 10.1016/j.biopsych.2010.07.024. http://doi.org/10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. http://doi.org/10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Heatherton TF. Neuroscience of Self and Self-Regulation. Annual Review of Psychology. 2011;62(1):363–390. doi: 10.1146/annurev.psych.121208.131616. http://doi.org/10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15(3):132–139. doi: 10.1016/j.tics.2010.12.005. http://doi.org/10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Herman CP, Polivy J, King GA, McGree ST. The (mis)measurement of restraint: An analysis of conceptual and psychometric issues. Journal of Abnormal Psychology. 1988;97(1):19–28. doi: 10.1037//0021-843x.97.1.19. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Wagner DD, Heatherton TF. In Search of a Human Self-Regulation System. Annual Review of Neuroscience. 2015;38(1):389–411. doi: 10.1146/annurev-neuro-071013-014243. http://doi.org/10.1146/annurev-neuro-071013-014243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Schweizer F, Veit R, Fritsche A, Preissl H. Compromised white matter integrity in obesity. Obesity Reviews. 2015;16(4):273–281. doi: 10.1111/obr.12248. http://doi.org/10.1111/obr.12248. [DOI] [PubMed] [Google Scholar]
- Lopez RB, Hofmann W, Wagner DD, Kelley WM, Heatherton TF. Neural Predictors of Giving in to Temptation in Daily Life. Psychological Science. 2014;25(7):1337–1344. doi: 10.1177/0956797614531492. http://doi.org/10.1177/0956797614531492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2012;1822(3):386–400. doi: 10.1016/j.bbadis.2011.08.003. http://doi.org/10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. http://doi.org/10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji K, Abe O, Uka T, Yasmin H, Kamagata K, Asahi K, et al. White matter alteration in metabolic syndrome: diffusion tensor analysis. Diabetes Care. 2013;36(3):696–700. doi: 10.2337/dc12-0666. http://doi.org/10.2337/dc12-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Laan LN, de Ridder DTD, Viergever MA, Smeets PAM. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. Neuro Image. 2011;55(1):296–303. doi: 10.1016/j.neuroimage.2010.11.055. http://doi.org/10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- van Strien T, Herman CP, Verheijden MW. Dietary restraint and body mass change. A 3-year follow up study in a representative Dutch sample. Appetite. 2014;76:44–49. doi: 10.1016/j.appet.2014.01.015. http://doi.org/10.1016/j.appet.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obesity Reviews. 2013;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. http://doi.org/10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Altman M, Boswell RG, Kelley WM, Heatherton TF. Self-Regulatory Depletion Enhances Neural Responses to Rewards and Impairs Top-Down Control. Psychological Science. 2013;24(11):2262–2271. doi: 10.1177/0956797613492985. http://doi.org/10.1177/0956797613492985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


