Abstract
Background
Central nervous system (CNS) infiltration by CD8+ T cells is associated with neuroinflammation in many neurodegenerative diseases, including HIV-associated dementia. However, the role of CD8+ T cells in the CNS during acute HIV infection is unknown.
Methods
We analyzed the phenotype, gene expression, TCR repertoire and HIV-specificity of CD8+ T cells in cerebrospinal fluid (CSF) of a unique cohort captured during the earliest stages of acute HIV infection (AHI) (n=26), chronic (n=23), and uninfected (n=8).
Results
CSF CD8+ T cells were elevated in AHI compared to uninfected controls. The frequency of activated CSF CD8+ T cells positively correlated to CSF HIV RNA and to markers of CNS inflammation. In contrast, activated CSF CD8+ T cells during chronic infection (CHI) were associated with markers of neurological injury and microglial activation. CSF CD8+ T cells in AHI exhibited increased functional gene expression profiles associated with CD8+ T cells effector function, proliferation and TCR signaling, a unique restricted TCR Vbeta repertoire and contained HIV-specific CD8+ T cells directed to unique HIV epitopes compared to the periphery.
Conclusions
These results suggest that CSF CD8+ T cells in AHI expanding in the CNS are functional and directed against HIV antigens. These cells could thus play a beneficial role protective of injury seen in CHI if cART is initiated early.
Keywords: HIV, cytotoxic T lymphocytes, HIV-specific CD8+ T cells, neuroinflammation, HIV-associated neurocognitive disorders
BACKGROUND
HIV infects the central nervous system (CNS) within days of initial exposure and induces neuroinflammation that includes invasion of infected mononuclear cells and subsequent activation of localized inflammatory cells. These activated CNS resident cells release an array of neurotoxins that can be measured in the cerebral spinal fluid (CSF),1 and additional neuronal injury can occur directly from HIV proteins, such as tat and gp120.2 Tissue damage persists despite combination antiretroviral therapy (cART) due to incomplete eradication of HIV reservoirs and sustained CNS inflammation, in part as a result of limitations in CNS drug penetration.3–5 This ongoing injury likely plays a role in a pervasive low-level encephalopathy presenting as continued mild cognitive impairment.6–8 These inflammatory mechanisms need to be elucidated to develop therapeutic strategies to limit CNS damage and preserve or restore cognitive function in HIV-infected individuals.
Infiltration of CD8+ T cells into the CNS is a recognized feature of many neurodegenerative diseases associated with neuroinflammation, including multiple sclerosis, Alzheimer’s disease, and various encephalitides.9 The frequency of CD8+ T cells among all lymphocytes in CSF is substantially elevated during HIV infection compared to other CNS diseases.10 Recent studies have reported that cognitive decline was associated with ongoing CSF CD8+ T cell activation among HIV-infected individuals, linking CSF CD8+ T cells to HIV neuropathogenesis in individuals.11,12 HIV-specific CD8+ T cells have been detected in CSF of ART-naïve individuals and their presence has been associated in the past with HIV dementia in chronically infected subjects.13–15 A recent study found HIV-specific CD8+ T cells were most frequent in the CSF of individuals with a CD4 count below 500 cells/μL, suggesting a possible association between HIV-specific CD8+ T cells in the CSF and disease progression.16 High frequencies of SIV-specific CD8+ T cells have also been detected in CSF of macaques chronically infected with SIV.17,18 Recent studies suggested that early ART initiation would preserve these effective responses in the periphery.19–22 CD8+ T cells constitute the majority of white blood cells in the CSF of acute HIV-infection (AHI) compared to uninfected individuals associated with a decreased CD4:CD8 ratio.23 However, data on CNS trafficking of CD8+ T cells during AHI are few, due to the difficulty to recruit participants within days of acquiring HIV. The RV254/SEARCH010 cohort provides a unique opportunity to analyze CD8+ T cells in the CSF and periphery within the first 20 days of estimated exposure. In this cohort, we recently reported that HIV-specific CD8+ T cell responses present in the periphery at peak plasma viral load, have an enhanced capacity to kill HIV-infected cells, and are associated with viral load decline and reduced seeding of the HIV reservoir after cART initiation (pending). Preserving potent HIV-specific CD8+ T cells in the CNS could be useful in reducing and possibly eliminating the persistent HIV replication in cART treated individuals as it has been proposed in the SIV model.24 The CNS remains a potential site for persistant HIV replication, despite the level at which HIV replicates during ART being controversial.25 However, the presence of these cells in acute infection in the CNS and the extent to which they have the ability to limit HIV replication in the CNS is unknown.
METHODS
Study participants
All clinical work was completed at the Thai Red Cross AIDS Research Center in Bangkok, Thailand. Blood and CSF samples were collected from untreated AHI (RV254/SEARCH010), CHI (SEARCH011) and uninfected participants (RV304/SEARCH013). Subjects from RV254/SEARCH010 were classified based on the 4th generation (4G) immunoassay (IA): stage 1 (4G IA−, 3G IA−), stage 2 (4G IA+, 3G IA−), stage 3 (4G IA+, 3G IA+, Western blot-/indeterminate).26 All participants signed the study consent forms approved by ethics committees at Chulalongkorn University, the Walter Reed Army Medical Center, Yale University and UCSF.
CSF biomarker analyses
CSF concentration of the neuroaxonal injury marker neurofilament light chain (NFL) was measured using the NF-light enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (UmanDiagnostics, Umeå, Sweden). CSF concentration of the astrocyte and macrophage activation marker YKL-40 was measured using an ELISA from R&D systems (Minneapolis, MN). All measurements were performed by board-certified laboratory technicians in one round of experiments using one batch of reagents. Intra-assay coefficients of variation were below 10%. Monocyte chemotactic protein 1 (MCP-1) in the CSF were quantified by customized multiplex ELISA (Quansys Biosciences, UT) captured on the Odyssey infrared imaging system (Li-Cor Biosciences, NE) and analyzed using Quansys Q-view Plus software (Quansys Biosciences). Traditional single-analyte ELISAs were used to measure other CNS injury markers IP-10 (Life Technologies, NY) and neopterin (GenWay Biotech, CA) and analyzed with SoftMax Pro (Molecular Devices, CA).
Cell sorting and phenotypic analysis
Thawed PBMCs or CSF were stained for surface markers at 4°C for 20 minutes with the following monoclonal antibodies: αCD27-FITC, αCD8-PE, αPD1-PECy7, αCD14-BV650 (BioLegend), αHLA-DR-PerCP, αCD38-APC, αCD3-A700, αCD45RA-APCH7 (BD Biosciences), αCD4-BV605 (Life Technologies), and αCD127-V450 (Affymetrix eBioscience). Live/dead stain with Vivid-amcyan was used to exclude dead cells from the sort. Cells were sorted on a BD Facs Aria II (BD Biosciences) and analyzed with FlowJo software (Treestar).
Primary CD8+ T cell expansion for TCR repertoire and HIV-specificity analyses
Primary sorted CD8+ T cells were expanded in RPMI supplemented as previously described.27 Briefly, CD8+ T cells were expanded with PHA in 8% human serum culture medium supplemented with both natural and recombinant IL-2 in the presence of feeder cells (irradiated fresh PBMCs from 3 different donors and irradiated B-EBV cells in a 10/1 ratio).
Intracellular staining (ICS) and TCR Vbeta repertoire analysis
B-EBV lines were generated for each donor by culturing PBMCs in RPMI, 20% fetal bovine serum (FBS), 20nM FK506 (AG Scientific), and Ebstein-barr virus (EBV)-containing supernatant from the virus-producing B95.8 marmoset cell line (ATCC) at an MOI of 100. B-EBV cell lines were then loaded with 0.5–5 mg/mL HIV Clade AE peptide pools overnight. Peptide pools were made with 20 15-mer peptides per pool of HIV PTE and HIV Consensus A peptides (gag, pol, nef, env) obtained through the AIDS Reagent Program, Division of AIDS, NIAID. Expanded primary CD8+ T cells were added to the loaded B-EBV (2:1), co-stimulated with 1 μg/μL αCD28/CD49d (BD Biosciences) and incubated for 12 hours with GolgiPlug protein transport inhibitor (BD Biosciences). After incubation, cells were stained with αCD8-FITC, αCD3-PacificBlue (BD Biosciences), and αCD20-PECy7 (BioLegend) prior to fixation/permeabilization and intracellular staining with αIFNγ-APC (BD Biosciences). Live/dead stain with Vivid-amcyan was used to exclude dead cells from the analysis. The T-cell receptor Vbeta repertoire was analyzed by flow cytometry using the IOTest® Beta mark (Beckman Coulter) in conjunction with αCD8-Pacific Blue (BD Biosciences). Stained CD8+ T cells were run on an LSRII flow cytometer using DiVA software (BD Biosciences) and analyzed with FlowJo (Treestar).
Gene expression analysis
CSF cells were pelleted from 7 mL of CSF and frozen in 3 aliquots. CD8+ T cells were sorted from a single CSF aliquot into 96 well plates at 100 cells/well to perform the Fluidigm’s Biomark Assay at one gene/well. Assays (primers and probes) were designed using the Roche Universal Probe Library Assay Design Center (www.universalprobelibrary.com) and were designed to detect 96 gene transcripts, without respect to isoform prevalence. RNA was reverse transcribed and amplified in a single-step RT-STA (Specific Template Amplification) using a pool of all the primer sets and with the Superscript III Platinum One-Step qRT-PCR Kit (Life Technologies, Grand Island, NY) for 18 cycles. Unincorporated primers and any generated non-specific single-stranded products were then removed by an Exonuclease I step. High-throughput qPCR on the pre-amplified samples was performed on a 96.96 BioMark™ Dynamic Array (Fluidigm, South San Francisco, CA)28 for 40 cycles. Threshold cycle (CT) values were calculated by the Real-Time PCR Analysis Software (Fluidigm) and failed reactions were discarded from the analysis. qPCR amplification curves were validated using gene expression Fluidigm BioMark real-time PCR analysis software on a Biomark (Fluidigm). Statistical analysis was performed using GenEx software (MultidAnalyses) and one-way ANOVA with Geisser-Greenhouse correction and Tukey-Kramer’s post-hoc test used to assess significance. RPL13A and IPO8 were used as housekeeping genes and fold change calculated with infected compared to uninfected samples. Only those genes that were significant (p<0.05) were used in the heatmap.
Statistical analyses
P values were calculated using One-Way ANOVA and Kruskal-Wallis with post-hoc Dunn multiple comparison analysis for group comparisons. Statistical analysis for correlations was performed using nonparametric Spearman correlation 95% confidence intervals using the Prism 7 for Macintosh (GraphPad software).
RESULTS
Clinical Composition
We evaluated 57 individuals from uninfected (n=8), acute HIV-infection (AHI) stage 1/2 (n=9), AHI stage 3 (n=17) and chronic HIV-infection (CHI) (n=23) groups that were similar in demographic variables (Supplemental Table 1). The acutely HIV-infected subjects were recruited during the earliest stages of acute infection within the first 20 days of acquiring HIV corresponding to pre-peak to peak viremia, as previously described.29 Acute HIV-infected subjects were classified into different AHI stages using the 4th generation immunoassay comparable to the Fiebig staging.26 AHI stages 1 and 2 were grouped together since both stages are typically before peak viremia and no significant differences in plasma or CSF viral load were found between the groups (data not shown) whereas AHI stage 3 corresponds to peak viremia. Untreated CHI participants were unaware of duration of infection and referred from clinics in Bangkok because they met Thai Ministry of Health criteria for initiating cART (symptomatic disease or CD4 count <350 cells/mm3).
Activated CD8+ T cells are elevated in the CSF during AHI
The AHI stage 3 group had an elevated mean plasma HIV RNA compared to the AHI stage 1/2 group (6.9log10 vs. 4.9log10 copies/mL; p=0.026) and tended to also have elevated plasma HIV RNA compared to the CHI group (5.3log10; p=0.164, Fig. 1A). The mean CSF HIV RNA level was higher in CHI participants compared to AHI stage 1/2 participants (5.0log10 vs. 2.2log10 copies/mL; p=0.046), but not different than the AHI stage 3 group (4.5log10 copies/mL; p=0.303). The average number CSF CD8+ T cells per milliliter of CSF was highest in the CHI (3899 cells/mL compared to both the AHI stage 1/2 (140 cells/mL; p<0.0001), AHI stage 3 (1498 cells/mL; p=0.011) and uninfected groups (308 cells/mL; p=0.002); but a slight increase in the number of CD8+ T cells was already detected in AHI stage 3 compared to stage 1/2 (Fig. 1B). Furthermore, the average frequency of activated CD8+ T cells in the CSF characterized by CD38+ CD127− was elevated in CHI participants (67%) compared to controls (4.6%, p<0.0001) and AHI stage 1/2 (6.7%, p<0.0001), but similar to that of the AHI stage 3 group (43%, p=0.255). The AHI stage 3 group already showed increased activated CD8+ T cell frequencies compared to the AHI stage 1/2 group (p=0.011) and uninfected controls (p=0.010, Fig. 1C, D).
Figure 1. Activated CD8+ T cells are elevated in the CSF during acute HIV infection.
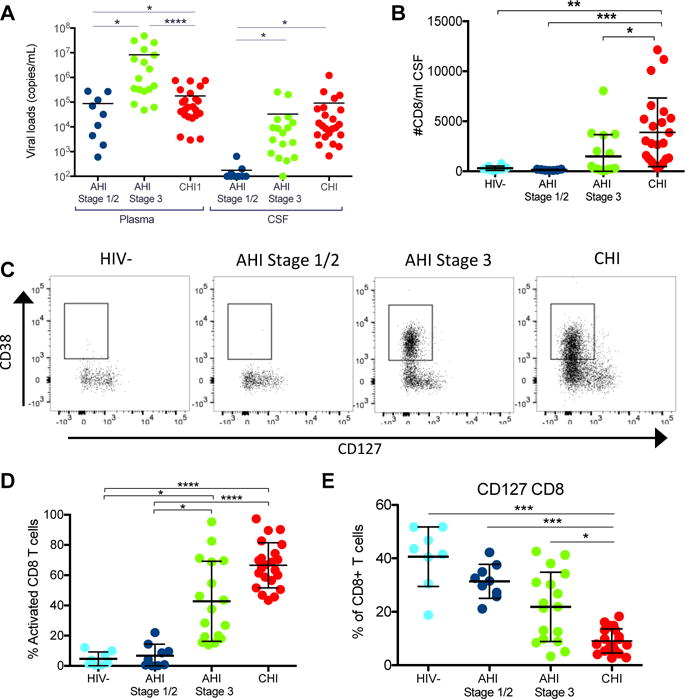
A. CSF viral load in acute HIV infection Stage1/2 (dark blue) (n=9), acute HIV infection Stage 3 (green) (n=17) and chronic HIV infection (CHI) (red) (n=23) samples. Limit of detection 100 viral copies/ml. B. Number of CD8+ T cells per mL of CSF in the different groups of donors including HIV-1 (n=8). C. Representative dot plots of activated CD8+ T cells defined by CD38+ CD127− in the CSF of a representative subject in the different groups. D. Frequency of activated CD8+ T cells (CD38+, CD127−) within the CD8+ T cells in CSF in the different groups. E. Frequency of CD127+ CD8+ T cells within CD8+ T cells in CSF in the different groups. Uninfected participants (light blue), AHI Stage 1/2 participants (dark blue), AHI Stage 3 participants (green) and CHI subjects (red). Asterisks denote different P values: *P< 0.05; **P < 0.005; *** < 0.0001.
There was no significant difference in number of CSF CD4+ T cells between the groups (Supp. Fig. 1A). However, the frequency of activated CD4+ T cells was significantly higher in CHI participants (HIV-: p<0.0001, AHI 1/2: p<0.0001, AHI 3: p=0.025) compared to other groups. The frequency of activated CD4+ T cells already started to increase in the AHI stage 3 compared to stage 1/2 groups (3.6% vs. 14%, p=0.089, Supp. Fig. 1B). Further phenotypic analyses revealed that CD127 expression, the alpha chain of IL-7 receptor as a marker of survival potential, was slightly lower on CD8+ during AHI stage 3 compared to uninfected (22% vs. 41%; p=0.056) and was lowest in the chronic stage compared to uninfected (9%; p<0.0001, Fig. 1E). A similar decrease in frequency of CD127 expression was observed on CD4+ T cells in CHI (15%) vs. uninfected (59%; p<0.0001), AHI stage 1 (58%, p<0.0001) and AHI stage 3 (47%, p=0.007) (Supp. Fig. 1C). Full characterization of the CD8+ T cell phenotype revealed no significant difference between the memory CD8+ T cell subsets naïve (CD45RA+CD27+), TEMRA (terminal differentiated effector memory) (CD45RA+CD27-), TTM (transitional memory) (CD45RA-CD27+), or TEM (effector memory) (CD45RA-CD27-) in the CSF of the different groups (Supp. Fig. 2).
CSF activated CD8+ T cells are associated with CSF HIV RNA and neuroinflammatory markers during AHI
To investigate whether the activation states of CD8+ T cells in AHI stage 3 and CHI were associated with a pathogenic or beneficial effect on viral replication in the CNS, we analyzed the association between the frequency of activated CD8+ T cells in the CSF with concurrent CSF HIV RNA and levels of common neuroinflammatory markers measured in the CSF. In AHI stage 3, the frequency of activated CD8+ T cells in the CSF was associated with CSF HIV RNA (r=0.66, p=0.004; Fig. 2A), but not with plasma HIV RNA (r=0.382, p=0.131, Fig. 2A). Regarding neuroinflammatory markers, we found that the frequency of activated CD8+ T cells in CSF during AHI stage 3 was positively correlated with CSF levels of neopterin (r=0.53, p=0.005), interferon-gamma induced protein 10 (IP-10) (r=0.47, p=0.016), and soluble CD163 (r=0.75, p<0.0001), all synthesized by activated myeloid cells (Fig. 2B). CSF levels of neuroinflammatory markers common in later stages of neuroinflammation and neuronal damage, neurofilament light chain (NFL) (CSF marker of inflammation neuronal damage) and YKL-40 (marker of astrocyte and macrophage activation) did not correlate with frequency of activated CD8+ T in the CSF of AHI stage 1/2 or 3 (Fig. 2C). In contrast to AHI, the frequency of activated CD8+ T cells in CHI did not correlate with HIV RNA in the CSF (r=0.399, p=0.066), but did correlate with plasma HIV RNA (r=0.45, p=0.035; Supp. Fig. 3A). No correlations were seen in CHI with neopterin (r=0.317, p=0.173) or IP-10 (r=0.179, p=0.451); Supp. Fig. 3B) as was seen in AHI. Importantly, the late stage neuroinflammatory markers positively correlated with frequency of activated CD8+ T cells in CSF of CHI participants (r=0.58, p=0.007 and r=0.45, p=0.049 respectively; Supp. Fig. 3C) as expected, but not with activated CD4+ T cells (Supp. Fig. 4A). The frequency of activated CD4+ T cells in the AHI CSF did not correlate with the neuroinflammatory markers neopterin (r=0.36, p=0.069) and IP-10 (r=0.34, p=0.089), but did correlate with sCD163 (r=0.55, p<0.007), Supp. Fig. 4B).
Fig 2. Activated CD8+ T cells in CSF from acute subjects positively correlates with CSF viral load and CSF markers of neuroinflammation.
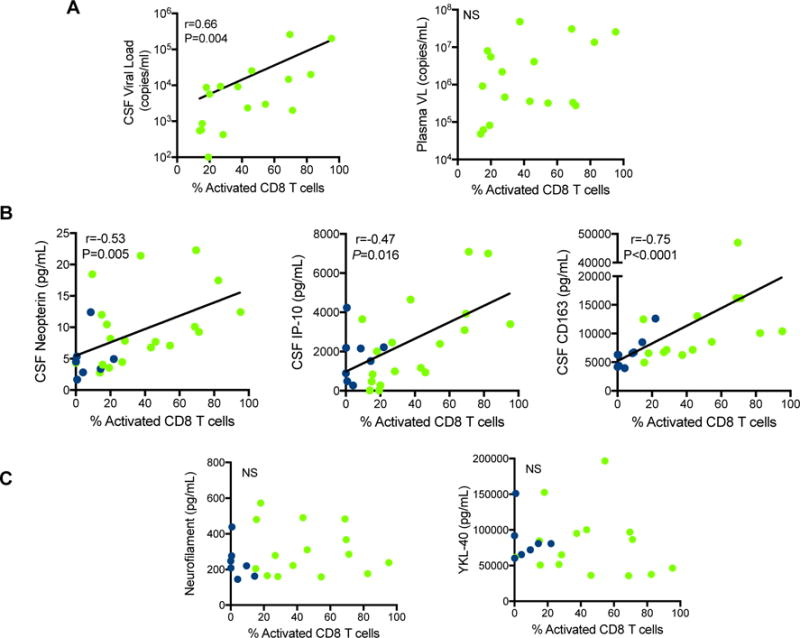
A. Correlation of activated CD8+ T cells with viral load in the CSF in AHI Stage 3 participants (n=17). Frequency of activated CD8+ T cells in the CSF does not correlate with plasma viral load (VL) in AHI. B. Frequency of CSF activated CD8+ T cells in AHI Stage 1/2 (dark blue) (n=9) and 3 (green) (n=17) correlates with CSF neuroinflammatory markers neopterin (p=0.005), IP-10 (p=0.016), and CD163 (p<0.0001). C. Frequency of activated CD8+ T cells in the CSF does not correlate with late neuroinflammatory markers (NFL and YKL-40) in acute HIV infection (AHI Stage 1/2 (dark blue) (n=7) and 3 (green) (n=17)).
CSF CD8+ T cell associated genes are upregulated in AHI
To assess the potential beneficial role of CD8+ T cells in CSF during AHI compared to CHI, we measured the gene expression profile (96 genes) of CD8+ T cells in CSF using a high-throughput nanofluidic qPCR system. Genes associated with CD8+ T cytolytic effector function, cell cycle, T cell receptor (TCR) signaling, and transcription factors were elevated in CSF CD8+ T cells during AHI stage 3 compared to stage 1/2 and to CHI (Fig. 3A, B). Increased expression of these genes could be seen as early as AHI stage 1/2, however, by chronic stage the expression of these pathways was downregulated compared to uninfected controls suggesting CD8+ T cell exhaustion (p-values and list of genes in Supp. Table 2).
Fig 3. Gene expression of CD8+ T cells in the CSF of acute and chronic HIV infected participants.
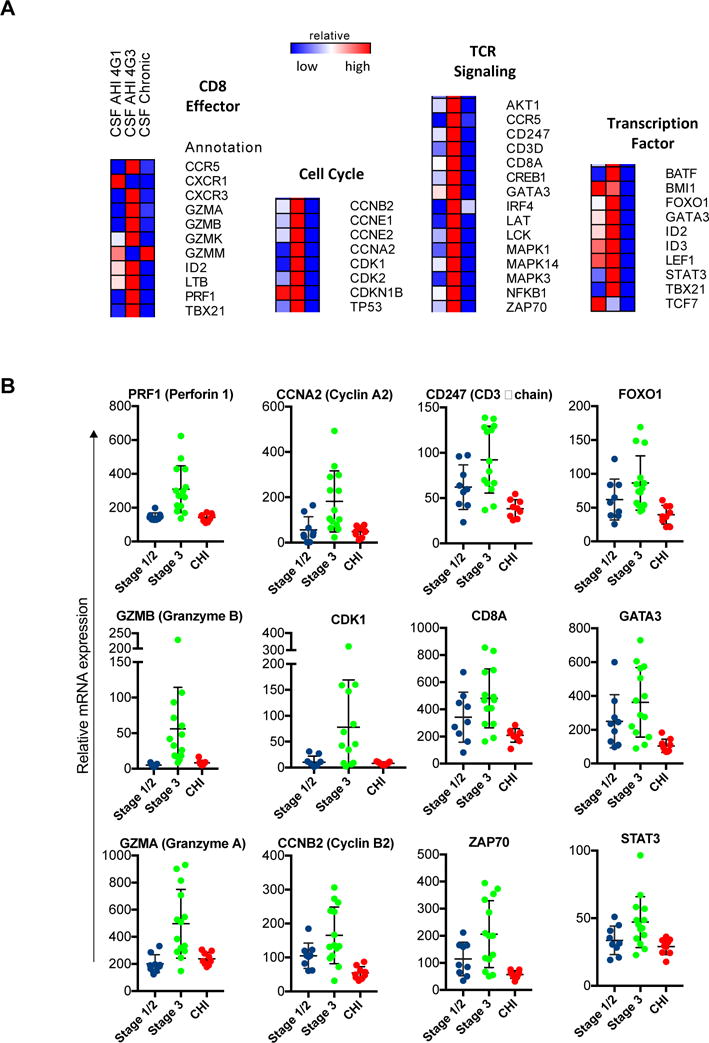
A. Targeted gene expression profile of 96 genes by multiplex qPCR involved in cell cycle, transcription factors, CD8 effector functions, and TCR signaling pathways for the CD8+ T cells in the CSF of AHI stage ½ (n=9), AHI stage 3 (n=14), and CHI (n=9) participants compared to uninfected participants (n=6). Scale represents fold change in gene expression of 100 total CD8+ T cells in the analyzed group compared to uninfected participants. Forty-two genes were significantly different (p<0.05) are displayed in heatmap based on one-way ANOVA with Geisser-Greenhouse correction and Tukey-Kramer’s post-hoc test. B. Graphs represent relative mRNA expression for individual subjects for select genes from each pathway. AHI: acute HIV-infection, CHI: chronic HIV-infection.
CSF CD8+ T cells express a unique TCR Vbeta repertoire compared to activated CD8+ T cells in the periphery
To determine whether the presence of activated CD8+ T cells in the CSF is unique to the CNS compartment and is not only a passive migration of peripheral activated CD8+ T cells, we analyzed the TCR Vbeta repertoire of CD8+ T cells from AHI stage 3 participants in CSF, and activated and non-activated CD8+ T cells in the periphery. The results from the 8 AHI stage 3 participants analyzed showed that CD8+ T cells in CSF expressed a unique TCR repertoire profile compared to the periphery (Fig. 4A). The TCR Vbeta repertoire diversity was significantly lower in the CD8+ T cells in CSF compared to activated and non-activated CD8+ T cells in peripheral blood mononuclear cells (PBMCs) (Fig. 4B). Importantly, CSF CD8+ T cells showed up to 40% unique Vbeta usage compared to their matched activated CD8+ T cells from the periphery (Fig. 4C). Despite CSF CD8+ T cells expressing lower TCR repertoire diversity compared to the periphery, they displayed a unique TCR Vbeta repertoire in the CSF during acute infection reflecting differences in compartmentalization and suggesting specific expansion of certain T cell clonotypes in the CNS.
Fig 4. TCR Vbeta repertoire of CD8+ T cells in CSF and PBMCs from 8 AHI stage 3 participants.
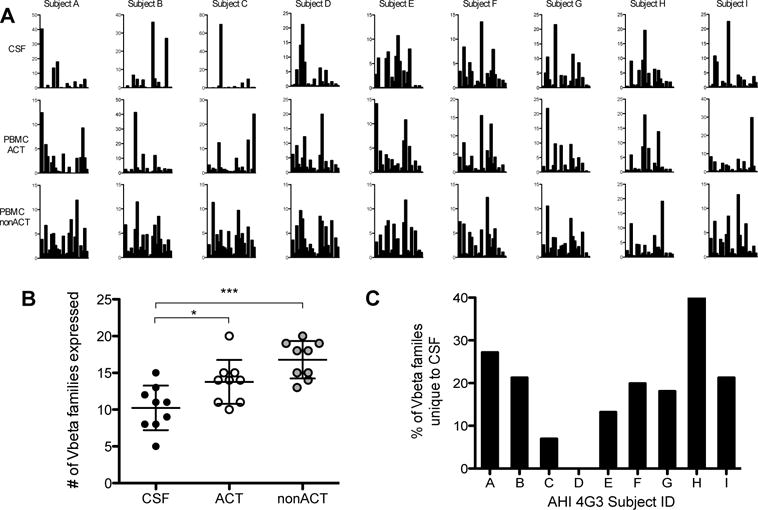
A. Frequency of Vbeta family usage of CD8+ T cells per AHI stage 3 participant (n=8) from the CSF, activated CD8+ T cells in PBMCs (PBMC ACT), and non-activated CD8+ T cells in PBMCs (PBMC nonACT). B. Number of Vbeta families out of the 24 Vbeta families tested that were expressed on CD8+ T cells from CSF, activated and non-activated CD8+ T cells in PBMCs per participant in each compartment, *p<0.05, ***p<0.0005. C. Frequency of Vbeta families unique to the CSF compared to the PBMCs for each AHI stage 3 participant analyzed.
HIV-specific CD8+ T cells are present in the CSF in acute infection
The presence of HIV-specific CD8+ T cells in CNS is expected to have a beneficial effect on neuronal damage by killing HIV infected cells and decreasing HIV replication in the brain. In order to determine the presence and specificity of CD8+ T cells in CSF, we generated CD8+ T cell lines and autologous B-EBV cell lines as previously described27 from either CSF cells pellets or peripheral PBMCs from 10 AHI stage 3 participants. B-EBV cells are used as antigen presenting cells and loaded with peptide pools for gag, pol, env, and nef. These pools were selected based on potential T cell epitopes common in HIV clade AE, which is dominant in Thailand. HIV peptide loaded B-EBV cells were then co-cultured with donor matched CD8+ T cells from either the CSF or periphery (activated and non-activated CD8+ T cells) and IFN-gamma production was measured. HIV-specific CD8+ T cells were present in high frequency in the activated CD8+ T cell population in the CSF and PBMCs, but not in the non-activated CD8+ T cell population in the PBMCs (Fig. 5A). When assessing the specific peptides recognized by CD8+ T cells from CSF and PBMCs in the same participants, we found that CD8+ T cells were directed against shared, but also unique HIV epitopes in CSF compared to periphery (Fig. 5B).
Figure 5. Frequency of HIV-specific CD8+ T cells in the CSF and PBMCs in acute HIV infection.
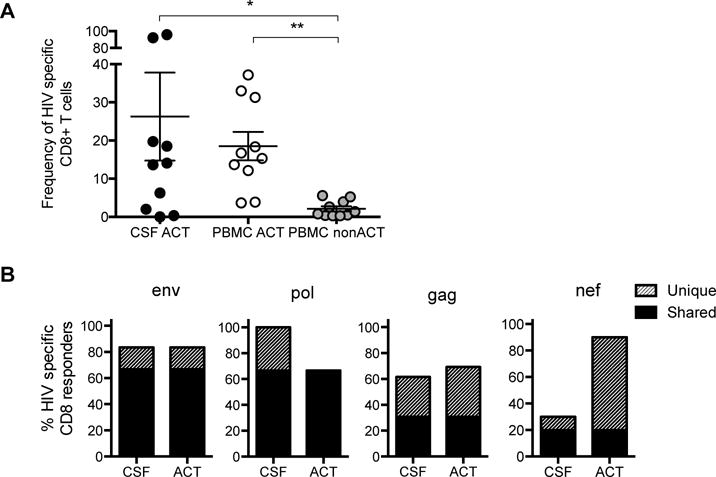
A. Frequency of activated CD8+ T cells from AHI stage 3 participants (n=10) expressing IFNγ in response to stimulation with Gag, Pol, Env, and Nef in activated CD8+ T cells in the CSF (CSF ACT), activated CD8+ T cells in the periphery (PBMC ACT), and non-activated CD8+ T cells in the periphery (PBMC non-ACT), *p<0.05, **p<0.005. B. Frequency of HIV-specific CD8+ T cells that responded to peptide pool stimulation (4-5 pools per HIV protein) in the CSF and activated PBMCs (ACT) by measuring IFNγ production. Grey bar color represents HIV-specific CD8+ T cell responses that were unique to either the CSF or PBMCs and black represents responses that were shared by both compartments.
DISCUSSION
In this study, we found that activated CD8+ T cells are elevated in the CSF very early after HIV infection, are associated with viremia and neuroinflammation and are functionaly active compared to activated CD8+ in the CSF of chronically infected individuals. Importantly, these activated CD8+ T cells recognize HIV antigen and might play a beneficial role early in infection to mitigate neuropathogenesis in people treated in acute HIV infection. Evaluating T cells in CSF is typically limited by the small amount of cells that can be extracted from the CSF and is not equivalent to analyzing brain tissue, but it is the best available cells that we have access to to assess the immunologic status of the brain in these acutely-infected individuals. We developed novel techniques that allowed for the comprehensive characterization of the phenotype, gene expression profile, TCR repertoire and HIV specificity of CD8+ T cells on a very small number of cells detected in the CSF. However, with some experiments, such as the TCR Vbeta analysis, we were limited to looking at individuals with enough cells available and matched donor compartments of cells (e.g. CSF with matching peripheral PBMCs samples). A strength of this work is the access to a population of individuals exposed to HIV within days and weeks, however, this limited our sample size initially. A longitudinal follow-up will be conducted on this group to look at longitudinal early effects of CD8+ T cell function on neurocognitive impairment. Despite these limitations, we were still able to extract significant data and the sample size was similar to those done in past studies by our group.30
Previously, Valcour et. al. detected HIV RNA in the CSF of participants from this acute infection cohort as early as 8 days after estimated HIV infection and identified elevated neuroinflammatory markers in the CSF in these acutely-infected participants.31 We found positive correlations with the frequency of activated CD8+ T cells in the CSF to early biomarkers of immune activation in the CNS (CSF neopterin, CSF IP-10, and CSF CD163) as well as CSF viral load at peak viremia in acute infection. These early markers of CNS inflammation did not correlate with activated CD8+ T cells in chronically-infected participants, where levels of NFL (biomarker of neuroaxonal injury)32 and YKL-40 (CSF inflammatory marker)33,34 correlated with the frequency of activated CD8+ T cells in the CSF. These results depict a very distinct immune response and inflammatory environment between acute and chronic infection and suggest that activated CD8+ T cells are recruited early in acute HIV infection responding to early neuroinflammatory markers in the CNS and might play a beneficial role in preventing neuronal damage if viral replication is halted at that stage by cART by killing HIV infected cells.
Our group previously demonstrated phenotypic, gene expression and functional differences in CD8+ T cell profiles in the periphery of acutely and chronically HIV-infected participants.35 In the current study, we found that activated CD8+ T cells exhibited distinct gene expression pathways associated with CD8+ T cells activation such as cell cycle, TCR signaling, effector function in the CSF in AHI compared to CHI. We have previously shown that chronic inflammation results in a dramatic clonal focusing of HCMV-specific CD8+ T cells in the synovial fluid compared to periphery in rheumatoid arthritis individuals suggesting that inflammation in tissues is reflected by T cell selection more significantly than in the PBMCs.27 Here we show that in AHI, T cell clonotypes are expanded as CD8+ T cells in CSF exhibited unique Vbeta usage compared to their matched activated CD8+ T cells from the periphery. These data suggest that CD8+ T cells in CSF exhibit a restricted unique TCR repertoire that allows for the longitudinal follow up of these clones over time and provide the rationale to assess whether persisting clonotypes in CSF under cART associate with residual neuroinflammation. Future studies will determine if CD8+ T cell clonotypes that persist after cART in CSF are associated with persistent CNS neuroinflammation injury.
The presence of HIV-specific CD8+ T cells in CNS is expected to have a beneficial effect on neuronal damage by killing HIV infected cells and decreasing HIV replication in the brain. However, there is still a possibility that CD8+ T cells may contribute to inflammation and damage in the CNS, as a recent study found an association with the presence of HIV-specific CD8+T cells in the CSF to disease progression.16 Little is known about the frequency of CD8+ T cells recognizing HIV antigens over the course of HIV infection especially in the CNS. A recent study suggested that inflammatory cytokines present during untreated chronic HIV infection triggers proliferation and expression of activation markers in CD8+ T cells in the periphery independent of antigen specificity.36 These bystander cells might be unable to eliminate HIV-infected cells. However, we demonstrated here that HIV-specific CD8+ T cells are present in AHI already at peak viremia in the CSF and cART initiation in AHI could lead to the preservation of these effector HIV-specific CD8+ T cells and could be critical for reducing the residual HIV replication in the CNS. Here, we demonstrated not only that HIV-specific CD8+ T cells are present in the CSF, but that they respond to unique HIV epitopes in the CSF compared to the CD8+ T cells in the periphery. This data along with the specific TCR Vbeta repertoire suggest very early infiltration and local expansion of HIV-specific CD8+ T cells in the CNS that may be interacting with unique HIV epitopes that are present in the CNS and not in the periphery.
Our data demonstrate the presence of highly activated and HIV-specific CD8+ T cells in the CNS within the first weeks after HIV exposure and provide the rationale to analyze CSF cell populations rather than PBMCs to understand immune responses in the CNS. Determining the role of CD8+ T cells, beneficial or pathogenic, in the CNS of HIV-infected participants will be determined by future longitudinal studies in this unique acute infection cohort initiating ARVs in the earliest stage of infection infection as it will determine if early cART initiation will preserve beneficial HIV-specific CD8+ T cells in the brain, that have the potential to control viral replication. These findings will be critical in targeting the HIV reservoirs that persists in the CNS despite cART.
Supplementary Material
Acknowledgments
The authors would like to thank the research participants in the SEARCH cohorts. We would also like to thank Collin Adams, Derek Ochi and Leah Le for administrative support and Zhong He for the flow cytometry sorting. The study team is grateful to the contribution of the staff at the Thai Red Cross AIDS Research Centre and the Department of Retrovirology, U.S. Army Medical Component, Armed Forces Research Institute of Medical Sciences (AFRIMS).
The RV254/SEARCH 010 Study Group includes from SEARCH/TRCARC/HIV-NAT:
Eugene Kroon, Donn Colby, Nitiya Chomchey, Peeriya Prueksakaew, Sasiwimol Ubolyam, Naphassanant Laopraynak, Suwanna Puttamaswin, and Putthachard Karnsomlap; from Chulalongkorn University: Mantana Mothisri; from AFRIMS: Robert O’ Connell, Rapee Trichavaroj, Siriwat Akapirat, Bessara Nuntapinit, Nantana Tantibul, Hathairat Savadsuk and Vatcharain Assawadarachai; from the US Military HIV Research Program: Jerome Kim, Silvia Kim and Sodsai Tovanabutra.
Victor Valcour has served as a consultant for ViiV Healthcare and Merck, each of whom provide support for medications to these participants. S. Spudich has received travel support and an honorarium from AbbVie. J. Ananworanich has received honorarium from ViiV Healthcare, Merck and Tetralogic.
Financial Support
This work was supported by the National Institutes of Health grants [R01AI10843] (LT), [R01MH106466] (LT), [R21MH086341] (VV), [R01MH095613] (VV & SS), and a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DoD). Thai Pharmaceutical Organization, Merck, ViiV Healthcare and Gilead.
Footnotes
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense.
Potential Conflicts of Interest
Other authors declare no conflicts of interest.
Previous Presentations
The results of this study were presented as a poster at the 2015 Keystone Symposium in Boston, Massachusetts and as an oral report at the International NeuroHIV Cure Consortium in Silver Springs, Maryland in 2015.
References
- 1.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 2.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. The Journal of infectious diseases. 2002;186(Suppl 2):S193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 3.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. The Journal of infectious diseases. 2007;196(12):1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. Journal of acquired immune deficiency syndromes. 2008;47(2):168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. Aids. 2011;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langford TD, Letendre SL, Larrea GJ, Masliah E. Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain pathology. 2003;13(2):195–210. doi: 10.1111/j.1750-3639.2003.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letendre SL, Ellis RJ, Everall I, Ances B, Bharti A, McCutchan JA. Neurologic complications of HIV disease and their treatment. Topics in HIV medicine: a publication of the International AIDS Society, USA. 2009;17(2):46–56. [PMC free article] [PubMed] [Google Scholar]
- 8.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Annals of neurology. 2010;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 9.Neumann H, Medana IM, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends in neurosciences. 2002;25(6):313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- 10.Kowarik MC, Grummel V, Wemlinger S, et al. Immune cell subtyping in the cerebrospinal fluid of patients with neurological diseases. Journal of neurology. 2014;261(1):130–143. doi: 10.1007/s00415-013-7145-2. [DOI] [PubMed] [Google Scholar]
- 11.Grauer OM, Reichelt D, Gruneberg U, et al. Neurocognitive decline in HIV patients is associated with ongoing T-cell activation in the cerebrospinal fluid. Ann Clin Transl Neurol. 2015;2(9):906–919. doi: 10.1002/acn3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrier RD, Hong S, Crescini M, et al. Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND) PLoS One. 2015;10(2):e0116526. doi: 10.1371/journal.pone.0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jassoy C, Johnson RP, Navia BA, Worth J, Walker BD. Detection of a vigorous HIV-1-specific cytotoxic T lymphocyte response in cerebrospinal fluid from infected persons with AIDS dementia complex. J Immunol. 1992;149(9):3113–3119. [PubMed] [Google Scholar]
- 14.Petito C, Torres-Muñoz J, Zielger F, McCarthy M. Brain CD8+ and cytotoxic T lymphocytes are associated with, and may be specific for, human immunodeficiency virus type 1 encephalitis in patients with acquired immunodeficiency syndrome. J Neurovirol. 2006;12(4):272–283. doi: 10.1080/13550280600879204. [DOI] [PubMed] [Google Scholar]
- 15.Sadagopal S, Lorey SL, Barnett L, et al. Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. J Virol. 2008;82(21):10418–10428. doi: 10.1128/JVI.01190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganesh A, Lemongello D, Lee E, et al. Immune Activation and HIV-Specific CD8(+) T Cells in Cerebrospinal Fluid of HIV Controllers and Noncontrollers. AIDS Res Hum Retroviruses. 2016;32(8):791–800. doi: 10.1089/aid.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Herrath M, Oldstone MB, Fox HS. Simian immunodeficiency virus (SIV)-specific CTL in cerebrospinal fluid and brains of SIV-infected rhesus macaques. J Immunol. 1995;154(10):5582–5589. [PubMed] [Google Scholar]
- 18.Moniuszko M, Brown C, Pal R, et al. High frequency of virus-specific CD8+ T cells in the central nervous system of macaques chronically infected with simian immunodeficiency virus SIVmac251. J Virol. 2003;77(22):12346–12351. doi: 10.1128/JVI.77.22.12346-12351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oxenius A, Price DA, Easterbrook PJ, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A. 2000;97(7):3382–3387. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streeck H, Jessen H, Alter G, et al. Immunological and virological impact of highly active antiretroviral therapy initiated during acute HIV-1 infection. The Journal of infectious diseases. 2006;194(6):734–739. doi: 10.1086/503811. [DOI] [PubMed] [Google Scholar]
- 21.Cellerai C, Harari A, Stauss H, et al. Early and prolonged antiretroviral therapy is associated with an HIV-1-specific T-cell profile comparable to that of long-term non-progressors. PloS one. 2011;6(4):e18164. doi: 10.1371/journal.pone.0018164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freel SA, Picking RA, Ferrari G, et al. Initial HIV-1 antigen-specific CD8+ T cells in acute HIV-1 infection inhibit transmitted/founder virus replication. J Virol. 2012;86(12):6835–6846. doi: 10.1128/JVI.00437-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho EL, Ronquillo R, Altmeppen H, Spudich SS, Price RW, Sinclair E. Cellular Composition of Cerebrospinal Fluid in HIV-1 Infected and Uninfected Subjects. PLoS ONE. 2013;8(6):e66188. doi: 10.1371/journal.pone.0066188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham DR, Gama L, Queen SE, et al. Initiation of HAART during acute simian immunodeficiency virus infection rapidly controls virus replication in the CNS by enhancing immune activity and preserving protective immune responses. Journal of neurovirology. 2011;17(1):120–130. doi: 10.1007/s13365-010-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS. 2016;11(4):417–423. doi: 10.1097/COH.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ananworanich J, Fletcher JL, Pinyakorn S, et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology. 2013;10:56. doi: 10.1186/1742-4690-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trautmann L, Rimbert M, Echasserieau K, et al. Selection of T Cell Clones Expressing High-Affinity Public TCRs within Human Cytomegalovirus-Specific CD8 T Cell Responses. The Journal of Immunology. 2005;175(9):6123–6132. doi: 10.4049/jimmunol.175.9.6123. [DOI] [PubMed] [Google Scholar]
- 28.Spurgeon SL, Jones RC, Ramakrishnan R. High throughput gene expression measurement with real time PCR in a microfluidic dynamic array. PLoS One. 2008;3(2):e1662. doi: 10.1371/journal.pone.0001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ananworanich J, Phanuphak N, de Souza M, et al. Incidence and characterization of acute HIV-1 infection in a high-risk Thai population. Journal of acquired immune deficiency syndromes. 2008;49(2):151–155. doi: 10.1097/QAI.0b013e318183a96d. [DOI] [PubMed] [Google Scholar]
- 30.Valcour VG, Ananworanich J, Agsalda M, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PloS one. 2013;8(7):e70164. doi: 10.1371/journal.pone.0070164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. The Journal of infectious diseases. 2012;206(2):275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuire JL, Gill AJ, Douglas SD, Kolson DL, group CHA-RTER Central and peripheral markers of neurodegeneration and monocyte activation in HIV-associated neurocognitive disorders. J Neurovirol. 2015;21(4):439–448. doi: 10.1007/s13365-015-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonneh-Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. J Neuroinflammation. 2010;7:34. doi: 10.1186/1742-2094-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peluso MJ, Valcour V, Phanuphak N, et al. Immediate initiation of cART is associated with lower levels of cerebrospinal fluid YKL-40, a marker of microglial activation, in HIV-1 infection. Aids. 2016 doi: 10.1097/QAD.0000000000001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trautmann L, Mbitikon-Kobo FM, Goulet JP, et al. Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood. 2012;120(17):3466–3477. doi: 10.1182/blood-2012-04-422550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bastidas S, Graw F, Smith MZ, Kuster H, Gunthard HF, Oxenius A. CD8+ T cells are activated in an antigen-independent manner in HIV-infected individuals. J Immunol. 2014;192(4):1732–1744. doi: 10.4049/jimmunol.1302027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


