Abstract
Background
Peripartum immunologic changes may affect latent TB infection (LTBI) diagnostic performance among HIV-infected women.
Methods
HIV-infected women were serially-tested with tuberculin skin test (TST) and interferon gamma release assay (IGRA) (QuantiFERON® TB Gold In-tube [QFT]) in pregnancy and 6 weeks postpartum in Kenya. Prevalence, sensitivity and agreement, and correlates of QFT/TST positivity were assessed. Quantitative QFT mitogen and M. tuberculosis antigen (Mtb-Ag) responses were compared by peripartum stage. Incidence of test conversion at 6 weeks postpartum was evaluated in baseline TST−/QFT− women.
Results
Among 100 HIV-infected women, median age was 26 years, median CD4 was 555 cells/mm3, and 88% were on antiretrovirals. More women were QFT+ than TST+ in pregnancy (35.4% vs. 13.5%, p=0.001), and postpartum (29.6% vs. 14.8%, p<0.001). Among 18 consistently QFT+ women, 8 (44%) converted from TST− to TST+, with improved test agreement postpartum (56.9%, κ=0.20 to 82.4%, κ=0.60). Three initially QFT−/TST− women had test conversion (TST+ and/or QFT+), suggesting new infection [incidence 13.4/100 person-years]. Mean QFT mitogen (4.46 vs. 7.64 IU/mL, p<0.001) and Mtb-Ag (1.03 vs. 1.54 IU/mL, p=0.03) responses were lower among all women retested in pregnancy vs. postpartum, and specifically among persistently QFT+ women (Mtb-Ag: 3.46 vs. 4.48 IU/mL, p=0.007). QFT indeterminate rate was higher in pregnancy (16%) compared to postpartum (0%) due to lower mitogen response.
Conclusion
QFT identified >2-fold more women with LTBI compared to TST in pregnancy and postpartum. Lower QFT Mtb-Ag and mitogen responses in pregnancy compared to postpartum suggest pregnancy-associated immunologic changes may influence LTBI test performance.
Keywords: latent tuberculosis infection (LTBI), HIV, pregnancy, tuberculin skin testing (TST), interferon gamma-release assay (IGRA), QuantiFERON® TB Gold In-tube (QFT), diagnostics
INTRODUCTION
Tuberculosis disease (TB) during pregnancy and the postpartum period is associated with poor maternal and infant outcomes.[1, 2] Pregnancy and HIV have both been associated with increased risk of progression to TB disease in women with M. tuberculosis (Mtb) infection.[3–5] Detecting latent TB infection (LTBI) is useful to identify individuals who may most benefit from TB prevention therapies.
LTBI diagnostics, including tuberculin skin tests (TST) and interferon gamma-release assays (IGRA), detect cell-mediated immune responses to Mtb infection.[6] IGRAs measure interferon gamma (IFN-γ) released from T cells stimulated by Mtb-specific antigens in vitro, whereas TSTs assess the delayed-type hypersensitivity response to purified protein derivative (PPD) which triggers skin induration in vivo.[7] In high-risk populations IGRAs have higher specificity compared to TSTs, due to less cross-reactivity with Bacillus Calmette-Guérin (BCG) vaccination and nontuberculous mycobacteria,[6] but are thought to generally have similar sensitivity.[8]
Cell-mediated immunity is altered in pregnancy[9] and HIV[5], and there is evidence that both pregnancy[10, 11] and HIV[8] effect LTBI diagnostic performance. Two studies in India have compared IGRAs to TSTs in peripartum women in a high TB burden setting,[10, 11] one of which focused on HIV-infected women.[11] In both studies, IGRAs identified a higher proportion of women with LTBI compared to TSTs during pregnancy and postpartum. This pattern of discordance (IGRA+/TST−) differs from the predominant discordance pattern (TST+/IGRA−) observed in studies of HIV-negative pregnant women in low TB incidence settings.[12–14] In settings with lower risk of TB transmission, a positive TST may reflect false positivity due to BCG vaccination rather than Mtb infection, leading to a higher proportion of TST+/IGRA−.[15] Additionally, most studies have been cross-sectional, and do not capture potential dynamic effects of peripartum stage on LTBI diagnostics.
There are no published prospective longitudinal studies evaluating LTBI diagnostics in HIV-infected peripartum women in Africa. In this study, we aimed to determine the effect of peripartum stage on the performance of TST and the QuantiFERON® TB Gold In-tube (QFT) IGRA, for LTBI detection among HIV-infected women in Kenya.
METHODS
Study setting and participants
This was a prospective cohort study of HIV-infected pregnant women at two antenatal care clinics in the Nyanza region of western Kenya. This region has the highest prevalence of HIV in Kenya (15%), and TB-HIV co-infection is common with ~68% of TB cases occurring among those with HIV.[16] Antenatal HIV prevalence estimates are 19–26%,[17] and the burden of culture-confirmed pulmonary TB among HIV-infected pregnant women in this setting is 2.4–5.9%.[18, 19] In Kenya, BCG administration is administered once at birth, and BCG coverage is high (79–99%).[20] At the time of this study, pregnant HIV-infected women were not routinely offered isoniazid prophylaxis. HIV-infected pregnant women ≥16 years were eligible for study enrollment. Women were ineligible if they received treatment for TB disease or LTBI within the past year.
Procedures
Enrollment in pregnancy
HIV-infected pregnant women accessing prevention of maternal-to-child transmission (PMTCT) services were consecutively recruited and screened. After informed consent, participants were interviewed regarding sociodemographic information, pregnancy, HIV, TB, and LTBI history. Participants were asked whether they or household members had TB symptoms using the WHO 4-part symptom screen (fever, any cough, weight loss, night sweats),[21] as well as prolonged cough (>2 weeks), hemoptysis, and lymphadenopathy, or if they had known exposure to a TB case. Data extracted from clinic charts included medication history, CD4 cell count, and gestational age (estimated by last menstrual period).
QFT
Blood was collected in heparinized tubes and transported daily to an ISO 15189-accredited KEMRI/CDC laboratory, where blood was transferred into QFT tubes per manufacturer recommendations.[22] An Mtb antigen (Mtb-Ag) response of ≥0.35 IU/ml (Mtb-Ag minus nil, with nil <8 IU/ml and positive mitogen control) was considered positive.
TST
After QFT blood collection, TSTs were placed using 5 tuberculin units (0.1 ml) of PPD (RT 23 solution, Sanofi Pasteur) and read by study nurses within 48–96 hours using the “ball-point” and ruler technique.[23, 24] A positive TST was defined as ≥5 mm of induration in this HIV-infected population.[25]
Sputum Collection and Testing
Women provided one expectorated sputum specimen at enrollment. Sputum samples were refrigerated immediately after collection and transported on ice the same day to the KEMRI/CDC laboratory for processing with N-acetyl-L-cysteine and sodium hydroxide. Sputa were examined by AFB-smear microscopy using Ziehl-Neelsen technique. Mycobacterial culture was performed with MGIT Manual Mycobacterial Growth System (Becton-Dickinson, Franklin Lakes, NJ), and speciation using Capilia TB Test Kit (TAUNS, Numazu, Japan).
Follow-up at 6 weeks postpartum
Participants were re-evaluated at 6 weeks postpartum. Women with either both QFT−/TST−, or discordant QFT/TST results on enrollment, had repeat QFT and TST performed at follow-up. Women with both TST+/QFT+ in pregnancy were seen in follow-up, but not retested with QFT/TST postpartum per pre-defined protocol.
Study Endpoints and Statistical Analysis
Participants with either positive TST or QFT, and negative sputum AFB smear and culture were considered to have LTBI. Baseline characteristics were evaluated as potential correlates of LTBI and further stratified by TST or QFT positivity using univariate logistic regression or Fisher’s exact test as appropriate. The proportion of positive QFT and TST results were compared using Chi-square tests. Given the lack of “gold standard,” we used a composite of either QFT+ or TST+ as a reference standard to indicate LTBI to calculate sensitivity. The effect of peripartum stage on sensitivity was assessed using McNemar's test. QFT/TST agreement (proportion of QFT/TST tests both either positive or negative) was assessed and concordance measured using kappa (κ) statistics by peripartum stage. Mean quantitative mitogen (QFT mitogen minus nil) and Mtb antigen (QFT Mtb-Ag minus nil) responses were calculated per manufacturer recommendations, and compared by peripartum stage and QFT/TST result by paired or unpaired t-tests as appropriate. Estimates were reported using 95% confidence intervals (CI); all statistical tests were two-sided with α=0.05. Analyses were performed using Stata 13 (StataCorp, College Station, Texas, USA) and/or GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
Ethics Approval
This study was approved by the Kenyatta National Hospital-University of Nairobi Ethics and Research Committee and University of Washington Institutional Review Board.
RESULTS
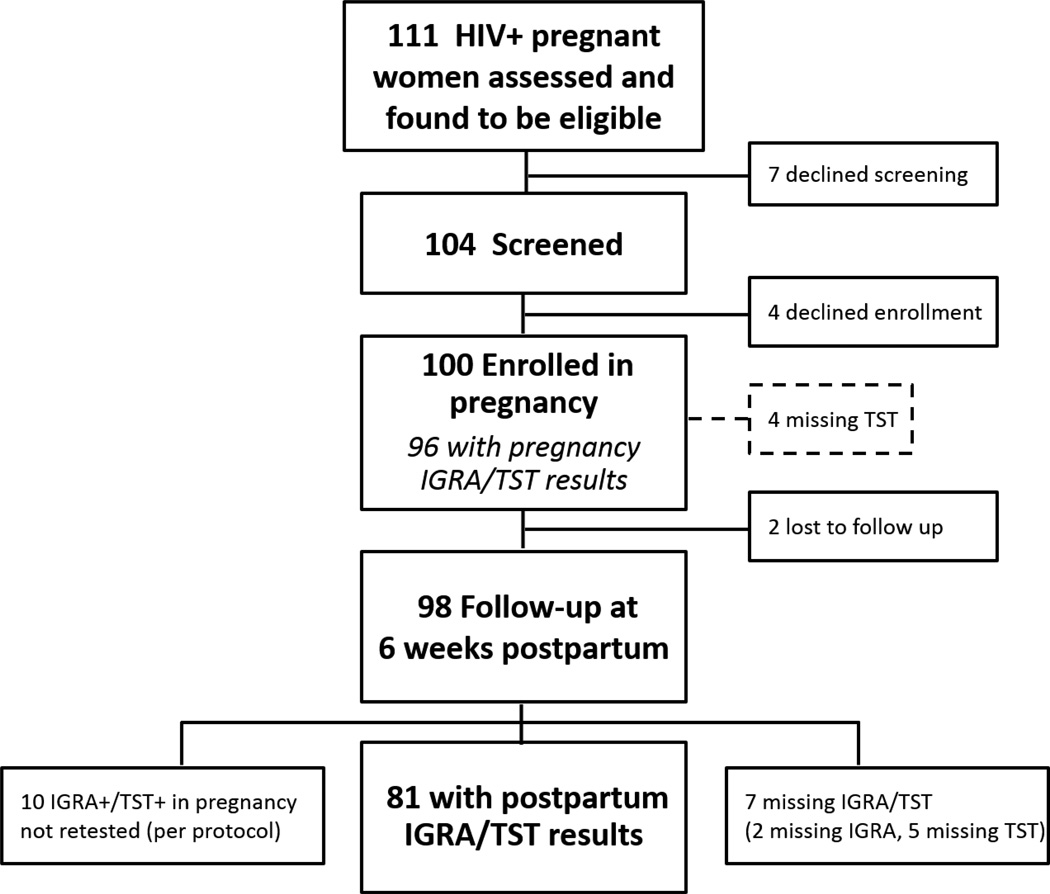
Between August 2014 and August 2015, 111 HIV-infected pregnant women were approached and all were eligible for study screening. Seven women declined screening, and 4 declined enrollment after screening. The remaining 100 women were enrolled (Figure 1).
Figure 1.
Study flow of HIV-infected peripartum women evaluated for LTBI by TST and IGRA in western Kenya.
Baseline characteristics
Median maternal age was 26 years (IQR 22–32), and median gestational age was 27 weeks (IQR 20–32) (Table 1). Median CD4 cell count was 555 cells/mm3 (IQR 340–730). Most women were on antiretroviral therapy at enrollment (92%), of which 88 were on combination antiretroviral treatment (ART), and 4 were on nevirapine or zidovudine alone for PMTCT. Although 74 (74%) knew their HIV sero-positive status prior to this pregnancy, only 37 women had initiated ART prior to pregnancy. Six had a history of TB, and 23% reported TB contact exposure. None reported previous LTBI diagnosis or treatment.
Table 1.
Baseline characteristics of HIV-infected pregnant women in western Kenya and correlates of LTBI (either TST+ or QFT+) in pregnancy
| All participants | LTBI (TST+ or QFT+)a |
No LTBI (TST− and QFT−) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Correlate | N= 100 | N=36 | N=60 | OR | 95% CI | p | |||
| n(%), or median (IQR) |
n(%), or median (IQR) |
n(%), or median (IQR) |
|||||||
|
Sociodemographic characteristics |
|||||||||
| Age (years) | 26 | 22–32 | 30 | 25–34 | 24 | 21–31 | 1.08 | 1.01–1.17 | 0.02 |
| Gestational age (weeks) | 27 | 20–32 | 24 | 20–28 | 28 | 20–32 | 0.96 | 0.90–1.02 | 0.19 |
| BMI (kg/m2) | 23 | 21–25 | 23 | 21–26 | 23 | 21–25 | 1.05 | 0.93–1.19 | 0.40 |
| Education (years) | 8 | 7–10 | 8 | 7–8 | 8 | 7–12 | 0.97 | 0.82–1.15 | 0.71 |
| Employed | |||||||||
| Yes | 61 | 61 | 25 | 26.0 | 35 | 36.5 | 1.62 | 0.68–3.90 | 0.28 |
| No | 39 | 39 | 11 | 11.5 | 25 | 26.0 | ref | ||
| Residential Conditions | |||||||||
| Persons in household | 4 | 3–5 | 4 | 3–5 | 4 | 3–5 | 1.05 | 0.85–1.31 | 0.63 |
| Single room household | |||||||||
| Yes | 28 | 28 | 7 | 7.3 | 19 | 19.8 | 0.52 | 0.19–1.40 | 0.20 |
| No | 72 | 72 | 29 | 30.2 | 41 | 42.7 | ref | ||
| HIV | |||||||||
| CD4 cell count (cells/mm3)(N=75) |
555 | 340–730 | 588 | 321–733 | 542 | 386–720 | 1.00 | 1.00–1.00 | 0.96 |
| ≤250 | 10 | 13.0 | 4 | 5.3 | 6 | 8.0 | 0.94 | 0.24–3.65 | 0.93 |
| >250 | 67 | 87.0 | 27 | 36.0 | 38 | 50.7 | ref | ||
| HIV status known prior to this pregnancy |
|||||||||
| Yes | 74 | 74 | 27 | 28.1 | 44 | 45.8 | 1.09 | 0.42–2.81 | 0.86 |
| No | 26 | 26 | 9 | 9.4 | 16 | 6.7 | ref | ||
| ART prior to this pregnancy (n=95) |
|||||||||
| Yes | 37 | 38.9 | 21 | 23.1 | 15 | 16.5 | 4.10 | 1.67–10.07 | 0.002 |
| No | 58 | 61.1 | 14 | 15.4 | 41 | 45.1 | ref | ||
| ART on enrollment | |||||||||
| PMTCTb | 4 | 4 | 1 | 1.0 | 3 | 3.1 | 2.33 | 0.11–50.98 | 0.59 |
| ART | 88 | 88 | 34 | 35.4 | 50 | 52.1 | 4.76 | 0.56–40.46 | 0.15 |
| None | 8 | 8 | 1 | 1.0 | 7 | 7.3 | ref | ||
| Partner’s HIV status | |||||||||
| Positive | 38 | 38 | 17 | 17.7 | 19 | 19.8 | 1.57 | 0.38–6.30 | 0.63 |
| Negative | 12 | 12 | 4 | 4.2 | 7 | 7.3 | ref | ||
| Unknown | 50 | 50 | 15 | 15.6 | 34 | 35.4 | 0.77 | 0.20–3.04 | 0.71 |
| TB history, symptoms, exposures | |||||||||
| History of TB (n=99) | |||||||||
| Yes | 6 | 6.1 | 4 | 4.2 | 2 | 2.1 | 3.56 | 0.62–20.5 | 0.16 |
| No | 93 | 93.9 | 32 | 33.7 | 57 | 60.0 | ref | ||
| Any WHO TB symptom positivec |
|||||||||
| Yesd | 17 | 17 | 5 | 5.2 | 10 | 10.4 | 0.81 | 0.25–2.58 | 0.72 |
| No | 83 | 83 | 31 | 32.3 | 50 | 52.1 | ref | ||
| Cough >2 weeks | |||||||||
| Yes | 4 | 4 | 3 | 3.1 | 1 | 1.0 | 5.36 | 0.54–53.65 | 0.15 |
| No | 96 | 96 | 33 | 34.4 | 59 | 61.5 | ref | ||
| Lymphadenopathy | |||||||||
| Yes | 2 | 2 | 0 | 0 | 2 | 2.1 | - | 0.52 | |
| No | 98 | 98 | 36 | 37.5 | 58 | 60.4 | |||
| Hemoptysis | |||||||||
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | - | - | |
| No | 100 | 100 | 37 | 37.7 | 63 | 62.5 | |||
| Household WHO TB symptom positive |
|||||||||
| Yes | 1 | 1 | 0 | 0.0 | 1 | 1.0 | - | 1.00 | |
| No | 99 | 99 | 36 | 37.5 | 59 | 61.5 | |||
| TB exposure | |||||||||
| Yes | 23 | 23 | 10 | 10.4 | 13 | 13.5 | 1.39 | 0.54–3.61 | 0.50 |
| No | 77 | 77 | 26 | 27.1 | 47 | 49.0 | ref | ||
| Household TB contact (n=94) |
|||||||||
| Yes | 16 | 16.3 | 9 | 9.6 | 7 | 7.4 | 2.43 | 0.81–7.24 | 0.11 |
| No | 82 | 83.7 | 27 | 28.7 | 51 | 54.3 | ref | ||
Excludes 4 participants with missing TST (1 QFT+, 1 QFT −, 2 QFT indeterminate)
PMTCT with either nevirapine or zidovudine alone
Cough (any duration), fever, weight loss, or night sweats
Of 17 women with a positive WHO TB symptom screen: 11 had cough only, 2 cough + night sweats, 1 cough + fever, 1 cough + weight loss, 1 night sweats only, and 1 fever only
Abbreviations: BMI, body mass index; ART, antiretroviral therapy; PMTCT, prevention of maternal to child transmission; ART, antiretroviral therapy; TST, tuberculin skin test; mm, millimeter.
Enrollment testing in pregnancy
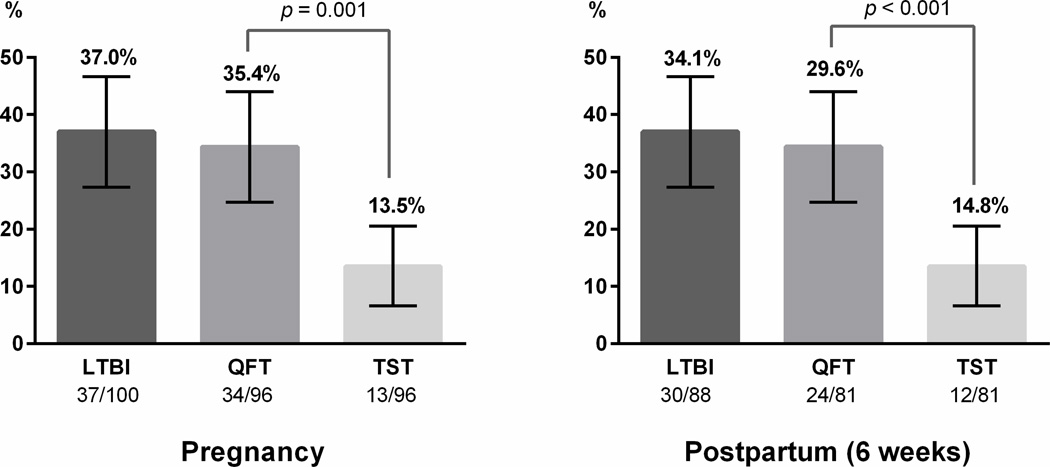
Five women were unable to produce sputum; the remaining 95 had sputum culture negative for M. tuberculosis. QFT was performed on all 100 participants, of which 34 were positive, 50 negative, and 16 indeterminate. Four participants did not have their TST read within 96 hours (Figure 1). Of 96 women with TST results, 13.5% were positive. The prevalence of LTBI in pregnancy, measured by either positive TST or QFT, was 37.0% (95% CI 27.4–46.6) (Figure 2). Of 96 women with both QFT and TST results in pregnancy, a significantly higher proportion were QFT+ compared to TST+ (35.4% vs. 13.5%, p=0.001).
Figure 2. Proportion of positive QFT and TST tests among HIV-infected women in western Kenya by peripartum stage.
LTBI was defined as either QFT or TST positive. Of the 100 women enrolled in pregnancy, 96 had QFT and TST results (4 missing TST). In postpartum, 10 women with QFT+/TST+ results in pregnancy were not tested postpartum per protocol and 2 women were lost-to follow-up. Of 88 women retested postpartum, 81 had both QFT and TST results (5 missing TST, 2 missing QFT). In both pregnancy and in postpartum, the proportion of QFT+ was significantly higher than TST+.
The sensitivity of QFT to identify LTBI was higher than TST, using a composite reference of either positive test (91.9% vs. 36.1%, p<0.0001) (Supplemental Table 1). Ten women (10.4%) were concordant positive (QFT+/TST+), 47 (49.0%) were concordant negative (QFT−/TST−), and 14 (14.6%) had indeterminate QFT results due to low mitogen response (13 TST−, 1 TST+) (Supplemental Table 2). The most common pattern of discordance in pregnancy was QFT+/TST− (23/96, 24%). QFT and TST agreement was 56.9% (κ= 0.20, 95% CI 0.16–0.24).
6 weeks postpartum follow-up
Of 100 women enrolled in pregnancy, 98 women were seen at 6 weeks postpartum follow-up, and 2 were lost to follow-up (Figure 1). Per protocol, 10 women with concordant positive QFT+/TST+ results in pregnancy were not retested. Of 88 women retested postpartum, the proportion of either positive QFT or TST was 34.1% (30/88, 95% CI 23.9–44.1) (Figure 2). Five had missing TST and 2 had missing QFT results. Among 81 with both postpartum QFT and TST results, a higher proportion of women were QFT+ compared to TST+ (29.6% vs. 14.8%, p<0.001) (Figure 2). There were no QFT indeterminates postpartum. Assuming 10 women initially QFT+/TST+ (not retested per protocol) remained positive, prevalence of LTBI at 6 weeks postpartum was estimated to be 39.6% (36/91, 95% CI 29.3–49.8) (Supplemental Table 1).
Postpartum, the sensitivity of QFT to identify LTBI remained higher than TST (94.4% vs. 61.1%, p=0.003) (Supplemental Table 1). TST sensitivity improved significantly from pregnancy to postpartum (36.1% to 61.1%, p=0.02), while QFT sensitivity remained similarly high (91.9% to 94.4%, p=0.26). Among 81 women with both QFT/TST postpartum results, 12.4% were concordant positive (QFT+/TST+), and 67.9% were concordant negative (QFT−/TST−) (Supplemental Table 2). Comparable to pregnancy, the most common pattern of discordance was QFT+/TST− (17.3%, 14/81). Including 10 women initially QFT+/TST+ in pregnancy (not retested per protocol), IGRA/TST agreement improved to 82.4% postpartum (κ=0.60, 95% CI 0.42–0.77) (Supplemental Table 2).
Test conversion/reversion
Seventy-eight women underwent both QFT and TST testing in pregnancy and postpartum (Supplemental Table 3). The most common test conversion occurred among women with consistently positive QFT, who converted from TST− to TST+ by 6 weeks postpartum (10.3%, 8/78). Of 12 women with initially indeterminate QFT, the majority were QFT negative postpartum (10/12). Three women with negative LTBI testing in pregnancy (QFT−/TST−) had at least one test conversion by 6 weeks postpartum (1 with both QFT+/TST+ conversion, and 2 with QFT+ conversion but remaining TST−) for an estimated LTBI infection incidence of 13.4/100 person-years. Three women had test reversion to negative: 2 women reverted from QFT+ to QFT− without baseline TST change (1 TST+, 1 TST−), and 1 woman reverted from TST+ to TST− without baseline QFT− result change (Supplemental Table 3).
Correlates of LTBI in pregnancy and postpartum
Both older age (OR 1.1 [95% CI 1.0–1.2]) per year, p=0.02) and ART prior to pregnancy (OR 4.1 [95% CI 1.7–10.1], p=0.002) were associated with LTBI (either positive TST or QFT) in pregnancy (Table 1). Women with earlier gestational age were less likely to be TST+ (OR 0.91 [95% CI 0.83–0.99] per week gestation, p=0.03) (Supplemental Table 4). Positive QFT or TST in the postpartum period were more strongly associated with reported household TB exposure than during pregnancy.
Quantitative mitogen and Mtb-Ag IFN-γ response
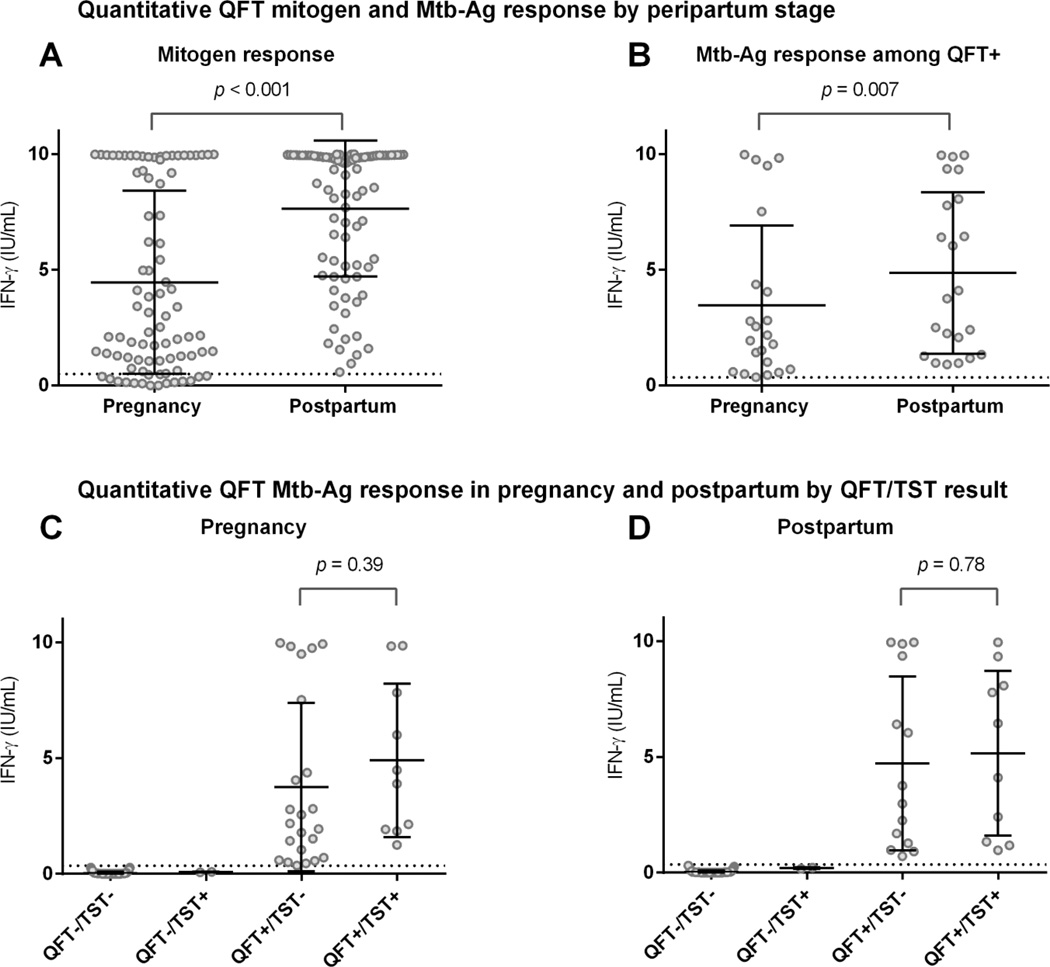
Of 86 women QFT-tested both in pregnancy and postpartum, mean mitogen (4.46 vs. 7.64 IU/mL, p<0.001) (Figure 3, Panel A) and Mtb-Ag (1.03 vs. 1.54 IU/mL, p=0.03) IFN-γ responses were lower in pregnancy compared to postpartum. This pattern of lower mean Mtb-Ag IFN-γ response in pregnancy was similar when restricted to women who remained QFT+ at both time points (3.46 vs. 4.48 IU/mL, p=0.007) (Figure 3, Panel B). Most QFT−/TST− pregnant women remained QFT−/TST− postpartum (41/44), without change in Mtb-Ag IFN-γ (0.04 vs. 0.04 IU/mL, p=0.93). Among women with initially negative testing (QFT−/TST−) in pregnancy, 1 converted to QFT+/TST+ (both test conversion) with corresponding increase in Mtb-Ag IFN-γ from 0.03 to 8.07 IU/ml, and 2 converted to QFT+/TST− (QFT+ conversion only) with mean Mtb-Ag IFN-γ increase from 0.0 to 2.33 IU/ml. Mean Mtb-Ag IFN-γ was higher (though not statistically significant) among QFT+/TST+ compared to those with discordant QFT+/TST− in pregnancy and postpartum (Figure 3 Panels C & D).
Figure 3. Quantitative QFT mitogen and Mtb-Ag response by peripartum stage and QFT/TST result.
Panel A: Comparison of mean quantitative QFT mitogen response between pregnancy and postpartum among all women tested at both time points (n=86). The dotted line represents the cut-off for mitogen responses (0.50 IU/ml), below which are considered to be indeterminate by QFT manufacturer recommendations. There were 14 women tested at both time points with indeterminates in pregnancy with no indeterminates postpartum. Mean mitogen response was significantly lower during pregnancy compared to postpartum (4.46 vs. 7.64 IU/ml, p<0.001). Panel B: Comparison of quantitative QFT Mtb-Ag response between pregnancy and postpartum among women who remained QFT+ at both time points (n=22). The dotted line represents the cut-off for QFT+ responses (≥ 0.35 IU/ml). Mean QFT Mtb-Ag response was significantly lower during pregnancy compared to postpartum (3.46 vs. 4.48 IU/ml, p=0.007). Panels C & D: Comparison of mean QFT Mtb-Ag response by combined QFT/TST results in pregnancy and postpartum. Mean QFT Mtb-Ag response was not statistically significantly higher among QFT+/TST+ compared to QFT+/TST− in both pregnancy and postpartum, but considerably above the ≥0.35 IU/mL positive threshold (represented by the dotted line). For all panels the solid lines indicate mean and 95% CI. Mtb-Ag indicates Mtb-Ag minus nil and mitogen indicates mitogen minus nil as calculated per manufacturer recommendations.
DISCUSSION
In this longitudinal study of HIV-infected women in western Kenya, a higher proportion were identified as having LTBI by QFT compared to TST in both pregnancy and early postpartum. Our findings of lower likelihood of TST+ at later gestational age (when immunosuppression peaks just prior to delivery) and lower proportion of positive TST in pregnancy and postpartum compared to QFT, suggest TST is more influenced by peripartum stage than QFT. TST conversion from negative in pregnancy to positive postpartum among women who remained QFT positive at both time points, supports this hypothesis. In addition, lower mean QFT Mtb-Ag and mitogen responses and higher indeterminate rates (due to low mitogen levels) in pregnancy compared to postpartum, suggest IGRAs are also effected by pregnancy-related immunologic changes. The relatively high incidence of postpartum test conversion, may indicate the peripartum period is a time of increased risk for Mtb infection.
In this study, QFT identified more than twice as many women with LTBI compared to TST in the peripartum period. These results are similar to recent studies of HIV-infected[11] and uninfected[10] peripartum women in India, and suggest that TST often fails to detect LTBI in peripartum women. Among previously TST positive women in the US, in-vitro lymphocyte responses to PPD decreased in late pregnancy and delivery, returning to higher early pregnancy levels within 24 hours postpartum.[26] We observed decreased likelihood of TST positivity later in pregnancy when the relative immune suppression of pregnancy may be greatest. The high proportion of postpartum TST− to TST+ conversion among consistently QFT+ women in our cohort may represent pregnancy associated-blunting of the PPD response in pregnancy with recovery early postpartum.
QFT responses were also affected by pregnancy. In the HIV-infected Indian cohort described by Mathad et al., median mitogen and Mtb-Ag IFN-γ responses decreased from pregnancy to delivery, with postpartum increases.[11] Similarly in our cohort, mean mitogen and Mtb-Ag IFN-γ responses were significantly lower in pregnancy compared to postpartum. We noted higher rates of indeterminates in pregnancy compared to postpartum, all due to low mitogen response. Both IGRA and TST measure Th-1 immune responses. Increasing levels of progesterone throughout pregnancy favor transition from Th-1 to Th-2 T-cell responses.[9] Th-1 responses reach a nadir late in the 3rd trimester, with decreased cellular expression of IFN-γ in later pregnancy with postpartum rebound.[27, 28]
Similar mechanisms responsible for the blunted response of LTBI diagnostics in pregnancy may contribute to the observed increase in active TB noted in the postpartum period. Decreased Th-1 responses in pregnancy have been speculated to increase risk of influenza,[9, 29] and potentially TB.[1] Early postpartum Th-1 response rebound has been described as analogous to immune reconstitution inflammatory syndrome,[30] and may account for the increased incidence of both IGRA conversion and active TB disease observed in the early postpartum period.[3, 4, 31] In a large cohort in the United Kingdom, there was a nearly 2-fold increased incidence of active TB in the early postpartum period.[3] Given difficulties with TB screening and diagnoses in pregnancy [18, 19], this early postpartum risk could reflect TB during pregnancy not detected until postpartum[3], or pregnancy-related risk of progression from infection to disease. In a cohort of HIV-infected women in Kenya, positive IGRA results in pregnancy were associated with increased postpartum active tuberculosis and mortality among mothers and their infants.[32] Mathad et al. found a similar increase in postpartum TB among IGRA positive HIV-infected pregnant women, and noted greater decreases in mitogen and Mtb-Ag IFN-γ and IL-2 responses from pregnancy to delivery compared to IGRA+ women who did not develop TB, suggesting a potential mechanism for this observation.[11]
Our study had several limitations. We did not retest women with QFT+/TST+ results in pregnancy, though the low level of reversions among women with either QFT+ or TST+ tests suggest that re-testing QFT+/TST+ women would not have resulted in substantial reversions. Because there is no gold standard diagnostic test for LTBI, we assumed that either positive QFT or TST represented “true” LTBI in estimating sensitivity and are therefore unable to estimate specificity. Latent class analysis models could be used to estimate LTBI prevalence in the absence of a gold standard, however additional data in this population is needed to strengthen these models which require prior probability estimates.[33] “Boosting” of an initially low pre-existing antigen response (false negative) with repeated TST testing may have contributed to increased TST positivity postpartum[34]. Although QFT is considered less prone to “boosting”, [6] Esmail et al. recently reported QFT conversion after TST placement may occur in HIV-infection[35], associated with higher baseline median Mtb-Ag response in QFT converters vs. non-converters (0.21 vs. 0.02 IU/ml, p=0.002). In contrast, QFT converters in our study had baseline mean Mtb-Ag responses (0.01 IU/ml) well below the threshold for a positive QFT test (≥0.35 IU/ml). The magnitude of change in mean Mtb-Ag from baseline to postpartum (0.01 to 4.24 IU/ml) in our study suggests these are truly new infections as opposed to “boosting” of borderline QFT positive results.
Strengths of our study include formal screening for active TB through sputum culture, reducing misclassification of women with subclinical TB disease. Our relatively large sample size of longitudinally assessed women provided power to detect significant differences in test performance in both pregnancy and postpartum. Despite the known increased risk of progression from M. tuberculosis infection to disease in association with HIV[36] and continued risk of TB even after ART initiation[37], there are few studies which serially assess LTBI status in HIV-infected individuals. Whether HIV increases Mtb acquisition risk as opposed to progression from infection to disease is unknown.[5] Most studies in HIV-infected adults have assessed TST before and after initiation of ART.[38–40] In this context, TST is unable to discriminate new Mtb infection from immune reconstitution of TST response following ART. The few longitudinal assessments of LTBI in HIV-infected individuals using IGRA have been primarily in low burden settings.[38, 41] Longitudinal IGRA evaluation, such as our study, provides an opportunity to estimate incident Mtb infection in high-risk populations. Molecular fingerprinting studies indicate TB cases in HIV-infected individuals in sub-Saharan Africa are more likely due to new infection than to reactivation.[42, 43] We have previously described a high incidence of IGRA conversion (12.4% from 32 weeks gestation to 12 months postpartum) in a historical cohort of HIV-infected pregnant women prior to widespread availability of ART.[31] The incidence of IGRA conversion (13.4/100 person-years) in this current study, with Mtb-Ag responses well above the threshold for positive QFT among converters, suggest risk for Mtb infection in this cohort of HIV-infected peripartum women is similar to other well-described high-risk groups including South African adolescents[44, 45] and household contacts of known TB cases[46–48]. In our previous work in the same setting, we found 2.4% of HIV-infected pregnant women had culture-confirmed TB and women who reported household members with TB symptoms were more likely to have TB [18]. This suggests the burden of undiagnosed TB in HIV-infected peripartum women, and within their households is high. In addition, mothers visit clinic and hospital in the peripartum period, both settings in which exposure to TB could occur given high HIV and TB prevalence in this region. Further studies are needed to investigate whether the increase in IGRA conversion postpartum is due to increased risk of Mtb acquisition in the peripartum period, or diminished sensitivity of LTBI diagnostics by pregnancy.
TB contributes significant morbidity and mortality to HIV-infected pregnant women and their children,[1] and is the third leading cause of death among women of child-bearing age in high burden areas.[1] Our study provides evidence that pregnancy influences the performance of LTBI diagnostics. We found that QFT was superior to TST in identifying LTBI in HIV-infected pregnant women. The shortcomings of both tests to identify individuals who are most likely to progress to active TB has led to ongoing efforts to develop improved diagnostics that better predict for active TB disease.[49] Our findings suggest that peripartum women and their infants should be included in these evaluations. The development of improved, lower cost, clinic-based testing for LTBI could inform use of isoniazid preventive therapy to potentially impact TB-related morbidity and mortality in HIV-infected peripartum women and their infants in high burden settings.
Supplementary Material
Acknowledgments
We thank the staff at the Ahero County Hospital and Bondo sub-District Hospital antenatal clinics, KEMRI/CDC laboratory personnel, as well as our study staff and participants.
SOURCE OF FUNDING: This work was supported by the National Institute of Allergy and Infectious Diseases and the National Institute of Child Health and Human Development at the National Institutes of Health [K23 AI 120793-01 and T32 AI07140 to SML, K23 AI 85036-01 to DJH, K24 HD054314-06 to GJS, K12 HD000850 to LMC], University of Washington Center for AIDS Research (CFAR) P30AI027757 to SML, UW INTERSECT-Ellison Fellowship to SML, the National Center for Research Resources at the National Institutes of Health [UL1TR000423] and the Firland Foundation.
Footnotes
PRIOR REPORTS: Preliminary study results were presented at the 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention (Vancouver, Canada), July 2015; 46th International Union Against Tuberculosis and Lung Disease World Conference (Cape Town, South Africa), December 2015, 21st International AIDS Conference (Durban, South Africa), July 2016; and 47th International Union Against Tuberculosis and Lung Disease World Conference (Liverpool, United Kingdom), October 2016.
CONFLICTS OF INTEREST: The authors report no conflicts of interest.
SML, DHJ, and GJS designed the study and analyses. SML, LMC, DJH, JK, DM conducted the study. SML and BAR conducted statistical analysis. All co-authors contributed to manuscript writing.
REFERENCES
- 1.Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis. 2012;55:1532–1549. doi: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Getahun H, Sculier D, Sismanidis C, Grzemska M, Raviglione M. Prevention, diagnosis, and treatment of tuberculosis in children and mothers: evidence for action for maternal, neonatal, and child health services. J Infect Dis. 2012;205(Suppl 2):S216–S227. doi: 10.1093/infdis/jis009. [DOI] [PubMed] [Google Scholar]
- 3.Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med. 2012;185:779–784. doi: 10.1164/rccm.201106-1083OC. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Nayak U, Ram M, Bhosale R, Patil S, Basavraj A, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis. 2007;45:241–249. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 5.Lawn SD, Wood R, Wilkinson RJ. Changing concepts of "latent tuberculosis infection" in patients living with HIV infection. Clin Dev Immunol. 2011 doi: 10.1155/2011/980594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–1104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 8.Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2011;56:230–238. doi: 10.1097/QAI.0b013e31820b07ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and infection. N Engl J Med. 2014;370:2211–2218. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathad JS, Bhosale R, Sangar V, Mave V, Gupte N, Kanade S, et al. Pregnancy differentially impacts performance of latent tuberculosis diagnostics in a high-burden setting. PLoS One. 2014;9:e92308. doi: 10.1371/journal.pone.0092308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathad JS, Bhosale R, Balasubramanian U, Kanade S, Mave V, Suryavanshi N, et al. Quantitative IFN-gamma and IL-2 response associated with latent tuberculosis test discordance in HIV-infected pregnant women. Am J Respir Crit Care Med. 2016;193:1421–1428. doi: 10.1164/rccm.201508-1595OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lighter-Fisher J, Surette AM. Performance of an interferon-gamma release assay to diagnose latent tuberculosis infection during pregnancy. Obstet Gynecol. 2012;119:1088–1095. doi: 10.1097/AOG.0b013e3182546aff. [DOI] [PubMed] [Google Scholar]
- 13.Worjoloh A, Kato-Maeda M, Osmond D, Freyre R, Aziz N, Cohan D. Interferon gamma release assay compared with the tuberculin skin test for latent tuberculosis detection in pregnancy. Obstet Gynecol. 2011;118:1363–1370. doi: 10.1097/AOG.0b013e31823834a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chehab BKK, ElFakih R, Zackula R, Minns G. Use of the QuantiFERON-TB Gold assay in pregnant patients. Kansas Journal of Medicine. 2010;3:24–30. [Google Scholar]
- 15.Malhame I, Cormier M, Sugarman J, Schwartzman K. Latent Tuberculosis in Pregnancy: A Systematic Review. PLoS One. 2016;11:e0154825. doi: 10.1371/journal.pone.0154825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenya Ministry of Health (MOH) National Tuberculosis; Leprosy and Lung Disease Unit (NTLD) Kenya NTLD Unit Annual Report 2014. 2014 Available at: http://nltp.co.ke/annual-reports/ [Google Scholar]
- 17.Kenya National AIDS and STI Control Programme (NASCOP) Kenya AIDS Indicator Survey 2012: Final Report. 2014 Available at: http://www.nacc.or.ke/images/documents/KAIS-2012.pdf. [Google Scholar]
- 18.LaCourse SM, Cranmer LM, Matemo D, Kinuthia J, Richardson BA, John-Stewart G, et al. Tuberculosis Case Finding in HIV-Infected Pregnant Women in Kenya Reveals Poor Performance of Symptom Screening and Rapid Diagnostic Tests. J Acquir Immune Defic Syndr. 2016;71:219–227. doi: 10.1097/QAI.0000000000000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modi S, Cavanaugh JS, Shiraishi RW, Alexander HL, McCarthy KD, Burmen B, et al. Performance of Clinical Screening Algorithms for Tuberculosis Intensified Case Finding among People Living with HIV in Western Kenya. PLoS One. 2016;11:e0167685. doi: 10.1371/journal.pone.0167685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Kenya - WHO and UNICEF estimates of national immunization coverage. 2015 Available at: http://www.who.int/immunization/monitoring_surveillance/data/ken.pdf.
- 21.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. 2011 Available at: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf.
- 22.Qiagen. QuantiFERON�-TB Gold in Tube package insert. 2015 Available at http://www.quantiferon.com/irm/content/PI/QFT/2PK/US.pdf. [Google Scholar]
- 23.Cobelens F, Van Deutekom H, Draayer-Jansen I, Schepp-Beelen A, Van Gerven P, Mensen M. Tuberculin skin test reactions by time of reading among Dutch travellers. Int J Tuberc Lung Dis. 2003;7:758–763. [PubMed] [Google Scholar]
- 24.Tuberculin reaction size on five consecutive days. Bull World Health Organ. 1955;12:189–196. [PMC free article] [PubMed] [Google Scholar]
- 25.Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 26.Covelli HD, Wilson RT. Immunologic and medical considerations in tuberculin-sensitized pregnant patients. Am J Obstet Gynecol. 1978;132:256–259. doi: 10.1016/0002-9378(78)90889-x. [DOI] [PubMed] [Google Scholar]
- 27.Kraus TA, Sperling RS, Engel SM, Lo Y, Kellerman L, Singh T, et al. Peripheral blood cytokine profiling during pregnancy and post-partum periods. Am J Reprod Immunol. 2010;64:411–426. doi: 10.1111/j.1600-0897.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 28.Kraus TA, Engel SM, Sperling RS, Kellerman L, Lo Y, Wallenstein S, et al. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol. 2012;32:300–311. doi: 10.1007/s10875-011-9627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raj RS, Bonney EA, Phillippe M. Influenza, Immune System, and Pregnancy. Reprod Sci. 2014;21:1434–1451. doi: 10.1177/1933719114537720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45:1192–1199. doi: 10.1086/522182. [DOI] [PubMed] [Google Scholar]
- 31.Jonnalagadda S, LaCourse SM, Otieno P, Lohman-Payne B, Maleche-Obimbo E, Cranmer LM, et al. Incidence and correlates of tuberculosis IGRA conversion among HIV-infected postpartum women. Int J Tuberc Lung Dis. 2015;19:792–798. doi: 10.5588/ijtld.14.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonnalagadda S, Lohman Payne B, Brown E, Wamalwa D, Maleche Obimbo E, Majiwa M, et al. Latent tuberculosis detection by interferon gamma release assay during pregnancy predicts active tuberculosis and mortality in human immunodeficiency virus type 1-infected women and their children. J Infect Dis. 2010;202:1826–1835. doi: 10.1086/657411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pai M, Dendukuri N, Wang L, Joshi R, Kalantri S, Rieder HL. Improving the estimation of tuberculosis infection prevalence using T-cell-based assay and mixture models. Int J Tuberc Lung Dis. 2008;12:895–902. [PMC free article] [PubMed] [Google Scholar]
- 34.Hecker MT, Johnson JL, Whalen CC, Nyole S, Mugerwa RD, Ellner JJ. Two-step tuberculin skin testing in HIV-infected persons in Uganda. Am J Respir Crit Care Med. 1997;155:81–86. doi: 10.1164/ajrccm.155.1.9001293. [DOI] [PubMed] [Google Scholar]
- 35.Esmail H, Thienemann F, Oni T, Goliath R, Wilkinson KA, Wilkinson RJ. QuantiFERON conversion following tuberculin administration is common in HIV infection and relates to baseline response. BMC Infect Dis. 2016;16:545. doi: 10.1186/s12879-016-1875-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markowitz N, Hansen NI, Hopewell PC, Glassroth J, Kvale PA, Mangura BT, et al. Incidence of tuberculosis in the United States among HIV-infected persons. The Pulmonary Complications of HIV Infection Study Group. Ann Intern Med. 1997;126:123–132. doi: 10.7326/0003-4819-126-2-199701150-00005. [DOI] [PubMed] [Google Scholar]
- 37.Kufa T, Mabuto T, Muchiri E, Charalambous S, Rosillon D, Churchyard G, et al. Incidence of HIV-associated tuberculosis among individuals taking combination antiretroviral therapy: a systematic review and meta-analysis. PLoS One. 2014;9:e111209. doi: 10.1371/journal.pone.0111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pullar N, Steinum H, Bruun J, Dyrhol-Riise A. HIV patients with latent tuberculosis living in a low-endemic country do not develop active disease during a 2 year follow-up; a Norwegian prospective multicenter study. BMC Infect Dis. 2014;14:667. doi: 10.1186/s12879-014-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.French AL, Evans CT, Anastos K, Greenblatt RM, Hershow R, Huebner R, et al. Incidence of tuberculin skin test conversion among HIV-infected and -uninfected women: results of a 6-year study. J Acquir Immune Defic Syndr. 2006;42:592–596. doi: 10.1097/01.qai.0000229995.25493.8b. [DOI] [PubMed] [Google Scholar]
- 40.Girardi E, Palmieri F, Zaccarelli M, Tozzi V, Trotta MP, Selva C, et al. High incidence of tuberculin skin test conversion among HIV-infected individuals who have a favourable immunological response to highly active antiretroviral therapy. AIDS. 2002;16:1976–1979. doi: 10.1097/00002030-200209270-00021. [DOI] [PubMed] [Google Scholar]
- 41.Aichelburg MC, Reiberger T, Breitenecker F, Mandorfer M, Makristathis A, Rieger A. Reversion and conversion of interferon-gamma release assay results in HIV-1-infected individuals. J Infect Dis. 2014;209:729–733. doi: 10.1093/infdis/jit418. [DOI] [PubMed] [Google Scholar]
- 42.Houben RM, Crampin AC, Ndhlovu R, Sonnenberg P, Godfrey-Faussett P, Haas WH, et al. Human immunodeficiency virus associated tuberculosis more often due to recent infection than reactivation of latent infection. Int J Tuberc Lung Dis. 2011;15:24–31. [PubMed] [Google Scholar]
- 43.Middelkoop K, Mathema B, Myer L, Shashkina E, Whitelaw A, Kaplan G, et al. Transmission of tuberculosis in a South African community with a high prevalence of HIV infection. J Infect Dis. 2015;211:53–61. doi: 10.1093/infdis/jiu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Middelkoop K, Bekker LG, Liang H, Aquino LD, Sebastian E, Myer L, et al. Force of tuberculosis infection among adolescents in a high HIV and TB prevalence community: a cross-sectional observation study. BMC Infect Dis. 2011;11:156. doi: 10.1186/1471-2334-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrews JR, Hatherill M, Mahomed H, Hanekom WA, Campo M, Hawn TR, et al. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med. 2015;191:584–591. doi: 10.1164/rccm.201409-1704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill PC, Brookes RH, Fox A, Jackson-Sillah D, Jeffries DJ, Lugos MD, et al. Longitudinal assessment of an ELISPOT test for Mycobacterium tuberculosis infection. PLoS Med. 2007;4:e192. doi: 10.1371/journal.pmed.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pai M, Joshi R, Dogra S, Zwerling AA, Gajalakshmi D, Goswami K, et al. T-cell assay conversions and reversions among household contacts of tuberculosis patients in rural India. Int J Tuberc Lung Dis. 2009;13:84–92. [PMC free article] [PubMed] [Google Scholar]
- 48.Shah M, Kasambira TS, Adrian PV, Madhi SA, Martinson NA, Dorman SE. Longitudinal analysis of QuantiFERON-TB Gold In-Tube in children with adult household tuberculosis contact in South Africa: a prospective cohort study. PLoS One. 2011;6:e26787. doi: 10.1371/journal.pone.0026787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petruccioli E, Scriba TJ, Petrone L, Hatherill M, Cirillo DM, Joosten SA, et al. Correlates of tuberculosis risk: predictive biomarkers for progression to active tuberculosis. Eur Respir J. 2016 doi: 10.1183/13993003.01012-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.