Abstract
Objective
Pediatric patients in Colorado with new onset type 1 diabetes (T1D) presenting with diabetic ketoacidosis (DKA) increased from 29.9% to 46.2% from 1998 to 2012. The purpose of this study was to compare differences between patients with newly diagnosed T1D who presented in DKA with those who did not across three domains: sociodemographic factors, access to medical care, and medical provider factors, aiming to identify potential targets for intervention.
Methods
Sixty-one patients <17 years of age with T1D duration <6 months completed the questionnaire. Groups were compared using Fisher’s exact test or the Kruskal-Wallis test.
Results
Parents of 28% of patients researched their child’s symptoms on the Internet prior to diagnosis. At the first healthcare visit for symptoms of T1D, 23% were not diagnosed. There were no significant differences between groups (DKA vs. non-DKA) in demographics, first healthcare setting for T1D symptoms, provider type at first visit or at diagnosis, insurance status, or specific barriers to care. DKA patients had a longer interval between previous well visit to diagnosis (median 172 vs 263 days, p=0.01). Non-DKA patients were more likely to have blood glucose measured at (p=0.02), and had fewer symptoms prior to (p=0.01) the first visit for diabetes symptoms. Parents of non-DKA patients were more likely to be familiar with symptoms of diabetes (p<0.001) and to suspect diabetes (p=0.01).
Conclusions
Targets for campaigns to prevent DKA include increasing provider glucose and ketone testing, increasing public knowledge about diabetes, and understanding how socio-demographic factors may delay T1D diagnosis.
Keywords: Diabetic ketoacidosis, new onset diabetes, type 1, pediatrics, primary care
Introduction
Diabetic ketoacidosis (DKA) is a life threatening complication of type 1 diabetes mellitus (T1D) and the leading cause of death in children and young adults with T1D [1, 2]. In the US between 2002-2010, approximately 30% of youth newly diagnosed with T1D presented with DKA [3]. Our center has reported that the proportion of patients in Colorado with new onset T1D who present with DKA has increased from 29.9% in 1998 to 35.0% in 2007 and to 46.2% in 2012. The reasons for this trend are unclear, but high cost of co-pay for healthcare visits has been suggested as a contributor [1]. In addition to increased risk for death, the metabolic derangements of DKA, including severe dehydration, cerebral edema, and electrolyte disturbances, can lead to long term morbidity, including cognitive deficits [2, 4, 5, 6]. Moreover, DKA is associated with high healthcare utilization and cost, being responsible for more than 500,000 hospital days for pediatric and adult patients at an estimated annual direct medical expense and indirect cost of 2.4 billion USD [7, 8].
Because early symptoms of diabetes are present before the onset of DKA, DKA at the onset of T1D signifies delayed diagnosis [1, 9]. DKA at diagnosis of T1D is more common in younger children (< 3 years of age) [2], ethnic minority groups, and in children whose families do not have ready access to medical care for social or economic reasons [9].
Therefore, the purpose of this pilot study was to compare the differences between the patients with newly diagnosed T1D who presented in DKA with those who did not across three domains: sociodemographic factors, access to medical care, and medical provider factors. We aimed to identify potential targets for further investigation which could be leveraged to decrease the rate of DKA at the onset of diabetes in pediatric patients.
Methods
This study was approved by the Colorado Multiple Institutional Review Board. All patients 13-17 years of age and all parents of patients 0-12 years of age gave informed consent. Patients 7-12 years of age also gave assent. The survey tool was distributed at the Barbara Davis Center in Aurora, Colorado, on an electronic tablet. Data were collected and managed using the Research Electronic Data Capture (REDCap) hosted at the University of Colorado, Denver [10].
All patients attending the pediatric diabetes clinic between June 8 and July 17, 2015 were screened for eligibility. Those <17 years of age with diagnosis of T1D in the last 6 months were approached for enrollment during their outpatient diabetes visits. Over the course of the study, 119 patients who met criteria for the study were seen at the diabetes center. Sixty four subjects were approached for recruitment based on research study staff availability, all of whom consented to participate. Sixty one completed the survey, and three were not able to be contacted by telephone after the visit to complete the survey. Patients who completed the survey and those who were not approached or did not complete the survey were similar in age, gender, race/ethnicity, and insurance status (data not shown).
The questionnaire was conducted in person or by phone within 30 days of enrollment. Each survey was conducted by one of the authors (BF and LB) to ensure proper interpretation of the questions. The questionnaire was a one-time survey which took approximately fifteen minutes. Patients 10-17 years of age collaborated with their parents or guardians on a single questionnaire. Parents or guardians took the survey for youth < 10 years old. The survey covered three domains: 1. medical provider details (e.g. who was patient’s primary care provider (PCP), questions about each visit with a healthcare provider prior to diagnosis), 2. access to medical care (e.g. no insurance, high copay/deductible, not having a PCP, hard to get an appointment with a PCP), 3. sociodemographic factors. The survey allowed multiple selections as well as a free text field for reported barriers to care. Medical records were used to collect demographics, payer information, date of diabetes diagnosis, and information about the presence of diabetic ketoacidosis. Study staff called private payers to obtain out-of-pocket cost information for each patient.
DKA was defined by pH <7.3 or serum HCO3− <15. The questionnaire asked whether the patient initially presented with DKA, and that information was compared to the medical record. Laboratory records were incomplete for only one patient, but because he was diagnosed with DKA at the outside hospital and managed for several hours with intravenous insulin, he was included in the DKA group. We did not collect information about blood or urine ketone levels because many of the patients began receiving treatment outside our hospital system and ketone levels were not consistently reported. A healthcare provider (HCP) encounter was defined as any visit to a PCP, urgent care, emergency room (ER), or other healthcare setting in which the subject was seen by a pediatrician, family medicine physician, physician assistant, nurse practitioner, or other medical provider. If the HCP did not diagnose diabetes but sent the child directly to the ER where diabetes was diagnosed, it was considered a continuation of a single encounter.
For 8 participants, the date recorded on the survey for the most recent well or sick child visit to the PCP was after the date of diagnosis, and therefore was set to missing.
The distribution of all variables was assessed prior to analysis. Descriptive statistics reported are N (%) for categorical variables, and median (25th, 75th percentiles) for continuous variables because the distributions of all continuous variables were skewed. Groups were compared using Fisher’s exact test or the Kruskal-Wallis test.
Results
Forty four percent of the 61 subjects experienced DKA upon the initial presentation of T1D. There were no significant differences between the groups (DKA vs. non-DKA) in demographic variables: race, gender, age, highest attained parental education level, family income, and insurance status (Table 1).
Table 1.
Demographic variables by DKA or non-DKA at time of diagnosis. Data reported as N (%) unless otherwise specified.
| Variable | Category or Statistic | Non-DKA (n=34) | DKA (n=27) | P |
|---|---|---|---|---|
| Race | Hispanic | 4 (12) | 5(18.52) | 0.904 |
| Non-Hispanic White | 26 (76) | 19 (70) | ||
| Other or unknown | 4 (12) | 3 (11) | ||
| Gender | F | 12 (35) | 13 (48) | 0.43 |
| M | 22 (65) | 14 (52) | ||
| Highest level of parental education |
≥College Graduate | 27 (79) | 15 (56) | 0.06 |
| Insurance type | No insurance | 2 (0) | 2 (4) | 0.40 |
| Private | 19 (56) | 18 (67) | ||
| Public/Medicaid | 12 (35) | 7 (26) | ||
| Military | 1 (3) | 0 (0) | ||
| Household income | $≥65,000 | 25 (74) | 14 (52) | 0.11 |
| Age | Median (25th %ile, 75th %ile) | 8.5 (5,11) | 9 (7,12) | 0.45 |
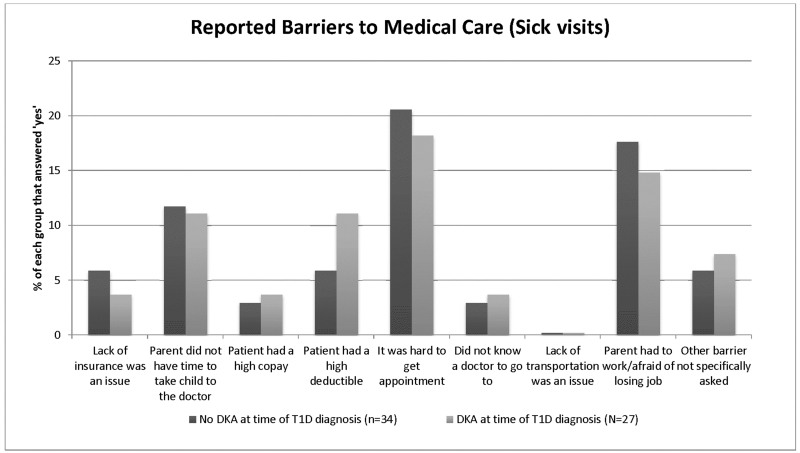
The DKA group had a longer time between the most recent well-child check and diagnosis (Table 2), but groups did not differ across the other medical access variables. The median time from the last well-child check to first seeking care for diabetes associated symptoms was 172 days in the non-DKA group compared to 263 days in the DKA group (p=0.01). Potential barriers to medical access for sick visit care explored in the questionnaire included lack of PCP, lack of time, lack of insurance, having a high deductible or copay, difficulty getting an appointment with a physician, lack of transportation, work-related interference, as well as an “Other” option which allowed a free text response. There were no statistical differences between the groups in regard to reported barriers to care. Difficulty obtaining a healthcare appointment, unable to take off work, and not having time to seek healthcare were the most common barriers to care reported by participants (Figure 1).
Table 2.
Variables related to medical access, by DKA or non-DKA at time of diagnosis. Data reported as N(%) unless otherwise specified.
| Variable | Category or Statistic | Non-DKA | DKA | P |
|---|---|---|---|---|
| Does the patient have a PCP? | Yes | 30(88) | 26(96) | 0.37 |
| Type of PCP | Pediatrician | 21(70) | 17(65) | 0.95 |
| Family Medicine Physician | 6(20) | 6(23) | ||
| NP, PA, Other | 3(10) | 3(12) | ||
| Last well child check to diagnosis (days) | Median (25th %ile, 75th %ile) | 172 (79.5, 269) | 263 (175, 365) | 0.01 |
| Last sick visit (before symptoms of diabetes) to diagnosis (days) |
Median (25th %ile, 75th %ile) | 64.0 (33,150) | 147 (76, 274) | 0.25 |
| Days between noticing something was wrong and visit |
Median (25th %ile, 75th %ile) | 13.0 ( 4 ,30) | 15.0 (7, 30) | 0.49 |
| Number access to care barriers | Median (25th %ile, 75th %ile) | 0.00 (0, 1) | 1.00 (0, 1) | 0.61 |
| Deductible amount ($) | Median (25th %ile, 75th %ile) | 300 (0, 1250) | 350 (0, 2050) | 0.45 |
| Does your family use therapies considered alternative medicine? |
Yes | 11 (32) | 10 (37) | 0.79 |
| Did you seek care from someone other than physician? |
Yes | 1 (3) | 0 (0) | 1.00 |
| Did you look symptoms up on the Internet? | Yes | 9 (26) | 8 (30) | 1.00 |
| How long after suspecting diabetes did you seek care? |
Median (25th %ile, 75th %ile) | 2 (0.5, 7) | 3.50 (0.35, 6) | 0.57 |
Figure 1.
Data reported as (%) of each group that responded “Yes” to having the barriers to care listed. There were no significant differences between the DKA and Non-DKA groups for reported barriers to medical care. Free text responses included, “Didn’t want her to miss school,” “Communication between pediatrician and endocrinologist was not great,” “Parents divorced and parent who had the suspicion of diabetes did not have the patient the week they were diagnosed,” and “Was living away from mother, in Texas with his father.”
Participants without DKA were more likely to have blood glucose measured at the first visit for symptoms of diabetes (91% vs 67%, p=0.02), but also had fewer symptoms prior to the first visit (8 vs 10, p=0.02, Table 3). There were no differences between groups across the other variables reported in Table 3. While there was no difference between the DKA and non-DKA groups, 23% of patients were not diagnosed on the first healthcare encounter. Of those not diagnosed at the first encounter, the median number of encounters was 3 in each group.
Table 3.
Variables related to healthcare provider, by DKA or Non-DKA at time of diagnosis. Data reported as N(%) unless otherwise specified.
| Variable | Category or Statistic | Non-DKA | DKA | P |
|---|---|---|---|---|
| Mention of diabetes at first visit? | Yes | 28 (82) | 17 (63) | 0.14 |
| Blood glucose at first visit? | Yes | 31 (91) | 18 (67) | 0.02 |
| Diagnosed at first visit? | Yes | 28 (82) | 19 (70) | 0.36 |
| Sum of symptoms prior to first visit | Median (25th %ile, 75th %ile) | 8 (6, 9) | 10 (8,11) | 0.02 |
| If not diagnosed at first visit, how many visits? |
Median (25th %ile, 75th %ile) | 3 (2, 3) | 3 (2, 3.5) | 0.88 |
Parents of children without DKA were more likely to be familiar with the symptoms of diabetes and to be suspicious that the child had diabetes (Table 4). Nearly 80% of families in the non-DKA group reported being familiar with the symptoms of diabetes prior to diagnosis, compared to 33% in the DKA group (p<0.001). Furthermore, 65% of parents in the non-DKA group reported having a suspicion of diabetes prior to diagnosis, compared to 30% in the DKA group (p= 0.01). Both groups reported using the internet to look up their child’s symptoms (non-DKA, DKA; 27%, 30%, p= 1.00). Additionally, of the non-DKA subjects who reported having a high suspicion of diabetes, 32% reported checking blood glucose on their own, using their own glucometer or from friend or family member. In contrast, only 4% of the DKA participants who reported suspicion of diabetes used a glucometer to check a blood glucose (p=0.008). There were no significant differences in the role of the provider, the setting of the first visit, or the setting of the diagnosis (Table 5).
Table 4.
Variables related to parental education, by DKA or non-DKA at time of diagnosis. Data reported as N(%) unless otherwise specified.
| Variable | Category or Statistic | Non-DKA | DKA | P |
|---|---|---|---|---|
| Were you familiar with symptoms of diabetes? | Yes | 27 (79) | 9 (33) | < 0.001 |
| Was there any suspicion that the patient had diabetes? |
Yes | 22 (65) | 8 (30) | 0.01 |
| Was patient blood sugar tested with glucometer? | Yes | 11 (32) | 1 (4) | 0.008 |
| Did anything else make you suspect that the patient had diabetes? |
Yes | 10 (29) | 5 (19) | 0.38 |
| Do you have any family members with diabetes? | Yes | 28 (82) | 19 (70) | 0.36 |
| Type of diabetes for family member | None | 6 (18) | 8 (30) | 0.46 |
| T1D | 9 (26) | 3 (11) | ||
| T2D | 14 (41) | 12 (44) | ||
| Multiple family members/types |
5 (15) | 4 (15) | ||
| Do you have a friend or acquaintance with diabetes? |
Yes | 19 (56) | 9 (33) | 0.12 |
Table 5.
Variables related to type of provider and visit setting at the first visit and diagnosis, by DKA or Non-DKA at time of diagnosis. Data reported as N (%) unless otherwise specified.
| Variable | Category or Statistic | Non-DKA | DKA | P |
|---|---|---|---|---|
| Visit setting, first visit | Primary care office | 22 (65) | 15 (56) | 0.87 |
| Urgent care center | 2 (6) | 3 (11) | ||
| Emergency room | 7 (21) | 7 (26) | ||
| Other | 3 (9) | 2 (7) | ||
| Type of provider, first visit | Pediatrician | 17 (50) | 15 (56) | 0.62 |
| Family medicine physician | 6 (18) | 1 (4) | ||
| PA | 3 (9) | 3 (11) | ||
| NP | 1 (3) | 1 (4) | ||
| Emergency physician | 7 (21) | 7 (26) | ||
| Location of diagnosis | Primary care office | 16 (47) | 12 (44) | 0.52 |
| Urgent care center | 1 (3) | 1 (4) | ||
| Emergency room | 14 (41) | 14 (52) | ||
| Other | 3 (9) | 0 (0) |
Discussion
Diagnosing a child with diabetes is a two-step process. The family must first identify symptoms and seek healthcare, and then the provider must make the correct diagnosis. A majority of the time between the onset of symptoms and diagnosis of T1D is spent in this appraisal period, and therefore may play a critical role in delayed diagnosis [11, 12]. Not previously reported in the pathway to diagnosis of T1D was our finding that 28% of families reported searching the child’s symptoms on the Internet, with similar fractions in each group. Despite the children having fewer symptoms and by definition being less ill, parents in the non-DKA group were more likely to have a high suspicion and prior knowledge of diabetes at initial presentation. Campaigns to increase public awareness of the symptoms of T1D have shown success in lowering the incidence of DKA [13]. Public education and increased access to glucose and ketone testing equipment in doctors’ offices have been successful at decreasing the proportion of children presenting in DKA [13, 14]. Therefore an Internet and social media presence of information on health conditions such as T1D could be important tools to increasing public awareness and improving outcomes.
Age at time of presentation, ethnic minority status, lower parental education, lower family income, and lack of adequate health insurance are factors associated with increased risk of DKA for patients newly diagnosed with T1D [1, 3, 15]. However, we did not find demographic differences between the DKA and non-DKA groups, possibly due the small size and predominantly Caucasian makeup of our sample.
While there were no differences in medical access factors between the groups, barriers were common. One in 5 families reporting difficulty in getting a healthcare appointment for the symptoms of diabetes and 1 in 6 reporting being unable to take time off from work represent important access barriers which need to be addressed. Additionally, a longer interval from the last well-child check to T1D diagnosis in the DKA group suggests that these patients were receiving less frequent routine health maintenance examinations. Restricted access to well-child care may also impact sick visit access and they should therefore be investigated in concert.
Despite government mandate that all Americans carry health insurance, lack of coverage remains a potential cause for delay in the diagnosis of T1D in US children, with 2 respondents in each group reported being uninsured and citing insurance status as a barrier to healthcare at the time of diagnosis. In addition, one respondent in each group reported “high copay” as a barrier. Lack of a clear definition for “high copay,” however, could have contributed to underreporting and should be further studied, especially with more Americans now covered on high deductible plans [16].
Notwithstanding the fact that subjects without DKA are by definition less ill upon presentation, they reported fewer symptoms prior to their first visit with a healthcare provider (Table 3), which may indicate that some patients do indeed experience a more rapid evolution of illness, predisposing them to DKA [17].
Twenty percent of children presenting to primary care are not diagnosed at the first clinical encounter, most commonly due to having received alternative diagnoses or awaiting fasting blood tests [11, 12]. Eight to 12% of youth with new onset diabetes receive diagnoses other than diabetes at the initial healthcare contact, resulting in a median delay of 5 days [18, 19]. While there was no difference between our groups, our data were consistent with previous findings, with 23% of all participants’ diabetes initially missed by a healthcare provider. There is clearly a need to improve diagnosis of T1D in pediatric patients.
Of children in the non-DKA group, half of the families who suspected diabetes before it was suggested by a healthcare worker used a glucometer of a friend or family member to aid in the diagnosis of diabetes (Table 2). Patients in the non-DKA group were also more likely to have had a blood glucose checked at the first HCP visit. Increased pre-visit suspicion of diabetes by the parents likely increases the chance that the provider will evaluate for diabetes. Alternatively, an unexpected finding of hyperglycemia, glucosuria, or ketonuria could be the first piece of information which would trigger the provider to consider a diagnosis of diabetes and thus prevent a delay in delivering appropriate care.
Other areas we explored were potential associations between type of provider seen, setting of health care visits, and number of visits before diagnosis of T1D was made. Although pediatricians would be expected to have had more training in T1D in childhood and therefore more familiarity with the diagnosis of T1D in children than other types of providers, we found no difference between the type of provider seen on the first visit and whether the child ultimately presented with DKA (Table 5). Further study will help demonstrate what support will help the spectrum of HCPs more easily make the diagnosis of T1D.
Limitations to this study include the small size and demographic makeup, the retrospective collection of data, and the single center design. These factors may limit the generalizability of our findings. Additionally, it was difficult to verify accurate insurance deductible information from automated insurer systems. As we conducted the survey, most families confidently recounted many details surrounding their child’s diagnosis. Although recruiting as much as six months after diagnosis enabled quick, efficient enrollment, it also could have contributed to recall bias. Recruiting at follow up visits and not capturing all consecutive patients with new onset T1D could contribute to selection bias, although forty four percent of children in this study experiencing DKA is close to what has been recently reported in our region [1].
Conclusion
With over 18,000 new cases of T1D per year in youth aged <20 years and the incidence of T1D in the US increasing by 2.7% annually [20], improving timely diagnosis and reducing the risk of DKA is critical. Our most striking finding was the difference in familiarity of the diabetes symptoms between the two groups. Parents frequently use Internet sources as they consider their child’s symptoms, and many report glucose testing outside of the healthcare setting and prior to seeing a provider. Hence, campaigns to increase awareness and empower the public with knowledge of diabetes represent potential areas for intervention to reduce the incidence of DKA in children who are newly diagnosed with T1D. Because many patients with new onset T1D are initially misdiagnosed, primary and urgent care providers should specifically inquire about polyuria, polydipsia, and weight loss in the setting of vague complaints and have a low threshold to check blood or urine sugar and ketones.
Acknowledgements
Dr. Jeremy Long, The University of Colorado School of Medicine LEADS Program, Jacqueline Shea, Emily Westfall, John Jones, Flor Sepulveda, Colorado Clinical & Translational Sciences Institute (CCTSI) with the Development and Informatics Service Center (DISC) grant support (NIH/NCRR Colorado CTSI Grant Number UL1 RR025780).
Abbreviations
- DKA
Diabetic ketoacidosis
- T1D
Type 1 diabetes
- HCP
Healthcare provider
- PCP
Primary care provider
- ER
Emergency room
Footnotes
Contributors’ Statement Page:
Mr. Baldelli and Mr. Flitter conceptualized and designed the study, prepared the data collection tools, recruited patients, collected the data, interpreted the data, drafted the initial manuscript, and approved the final manuscript as submitted.
Dr. Pyle designed the study, carried out the statistical analyses, interpreted the data, and approved the final manuscript as submitted.
Dr. Maahs designed the study, interpreted the data, and approved the final manuscript as submitted.
Dr. Klingensmith designed the study, interpreted the data, and approved the final manuscript as submitted.
Dr. Slover designed the study, interpreted the data, and approved the final manuscript as submitted.
Dr. Alonso conceptualized and designed the study, oversaw the collection of data and manuscript preparation, interpreted the data, and approved the final manuscript as submitted.
References
- 1.Rewers A, Dong F, Slover RH, et al. Incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado youth, 1998-2012. JAMA. 2015;313(15):1570–1572. doi: 10.1001/jama.2015.1414. [DOI] [PubMed] [Google Scholar]
- 2.Klingensmith GJ, Tamborlane WV, Wood J, et al. Diabetic ketoacidosis at diabetes onset: still an all too common threat in youth. J Pediatr. 2013;162(2):330–334. doi: 10.1016/j.jpeds.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. 2014;133(4):e938–e945. doi: 10.1542/peds.2013-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Realse J, Goettle H, Chase HP. Morbidity and mortality of diabetic ketoacidosis with and without insulin pump care. Diabetes Technol Ther. 2012;14(12):1149–1154. doi: 10.1089/dia.2012.0161. [DOI] [PubMed] [Google Scholar]
- 5.Wolfsdorf JI, Allgrove J, Craig ME, et al. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(Suppl. 20):154–179. doi: 10.1111/pedi.12165. [DOI] [PubMed] [Google Scholar]
- 6.Hsia DS, Tarai SG, Alimi A, et al. Fluid management in pediatric patients with DKA and rates of suspected clinical cerebral edema. Pediatr Diabetes. 2015;16:338–344. doi: 10.1111/pedi.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(12):2739–2748. doi: 10.2337/dc06-9916. [DOI] [PubMed] [Google Scholar]
- 8.Larsson HE, Vehik K, Dabelea D, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347–2352. doi: 10.2337/dc11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rewers A, Klingensmith GJ, Rewers M. Apparent Epidemic of Ketoacidosis in Children Diagnosed with Diabetes, Colorado, 2006-2009. Abstract. Diabetes. 2010 [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usher-Smith JA, Thombson MJ, Zhu H, et al. The pathway to diagnosis of type 1 diabetes in children: a questionnaire study. BMJ Open. 2015;5(3):e006470. doi: 10.1136/bmjopen-2014-006470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usher-Smith JA, Thompson MJ, Walter FM. ‘Looking for the needle in the haystack’: a qualitative study of the pathway to diagnosis of type 1 diabetes in children. BMJ Open. 2013;3(12):e004068. doi: 10.1136/bmjopen-2013-004068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanelli M, Chiari G, Lacava S, Brunella I. Campaign for diabetic ketoacidosis prevention still effective 8 years later. Diabetes Care. 2007;30(4):e12–e12. doi: 10.2337/dc07-0059. [DOI] [PubMed] [Google Scholar]
- 14.King BR, Howard NJ, Verge CF, et al. A diabetes awareness campaign prevents diabetic ketoacidosis in children at their initial presentation with type 1 diabetes. Pediatr Diabetes. 2012;13(8):647–651. doi: 10.1111/j.1399-5448.2012.00896.x. [DOI] [PubMed] [Google Scholar]
- 15.Rewers A, Klingensmith GJ, Davis C, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the Search for Diabetes in Youth Study. Pediatrics. 2008;121(5):e1258–e1266. doi: 10.1542/peds.2007-1105. [DOI] [PubMed] [Google Scholar]
- 16.Sinaiko AD, Mehrotra A, Sood N. Cost-Sharing Obligations, High-Deductible Health Plan Growth, and Shopping for Health Care: Enrollees With Skin in the Game. JAMA Intern Med. 2016;176(3):395–7. doi: 10.1001/jamainternmed.2015.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neu A, Willasch A, Ehehalt S, et al. Ketoacidosis at onset of type 1 diabetes mellitus in children – frequency and clinical presentation. Pediatr Diabetes. 2003;4(2):77–81. doi: 10.1034/j.1399-5448.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 18.Mallare JT, Cordice CC, Ryan BA, et al. Identifying risk factors for the development of diabetic ketoacidosis in new onset type 1 diabetes mellitus. Clin Pediatr. 2003;42(7):591–597. doi: 10.1177/000992280304200704. [DOI] [PubMed] [Google Scholar]
- 19.Lokulo-Sodipe K, Moon RJ, Edge JA, Davies JH. Identifying targets to reduce the incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in the UK. Arch Dis Child. 2014;99(5):438–42. doi: 10.1136/archdischild-2013-304818. [DOI] [PubMed] [Google Scholar]
- 20.Dabelea D, Rewers A, Stafford JM, et al. Trends in the Prevalence of Ketoacidosis at Diabetes Diagnosis: The SEARCH for Diabetes in Youth Study. Pediatrics. 2014;133(4):e938–45. doi: 10.1542/peds.2013-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]