Abstract
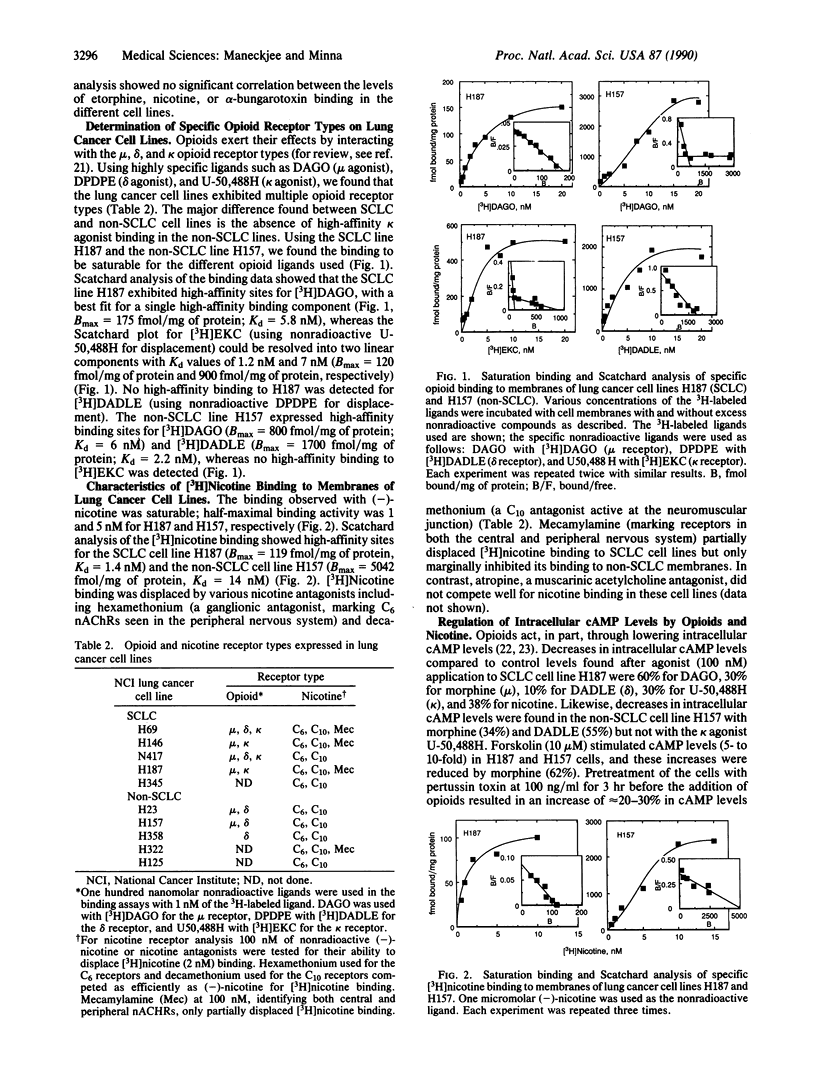
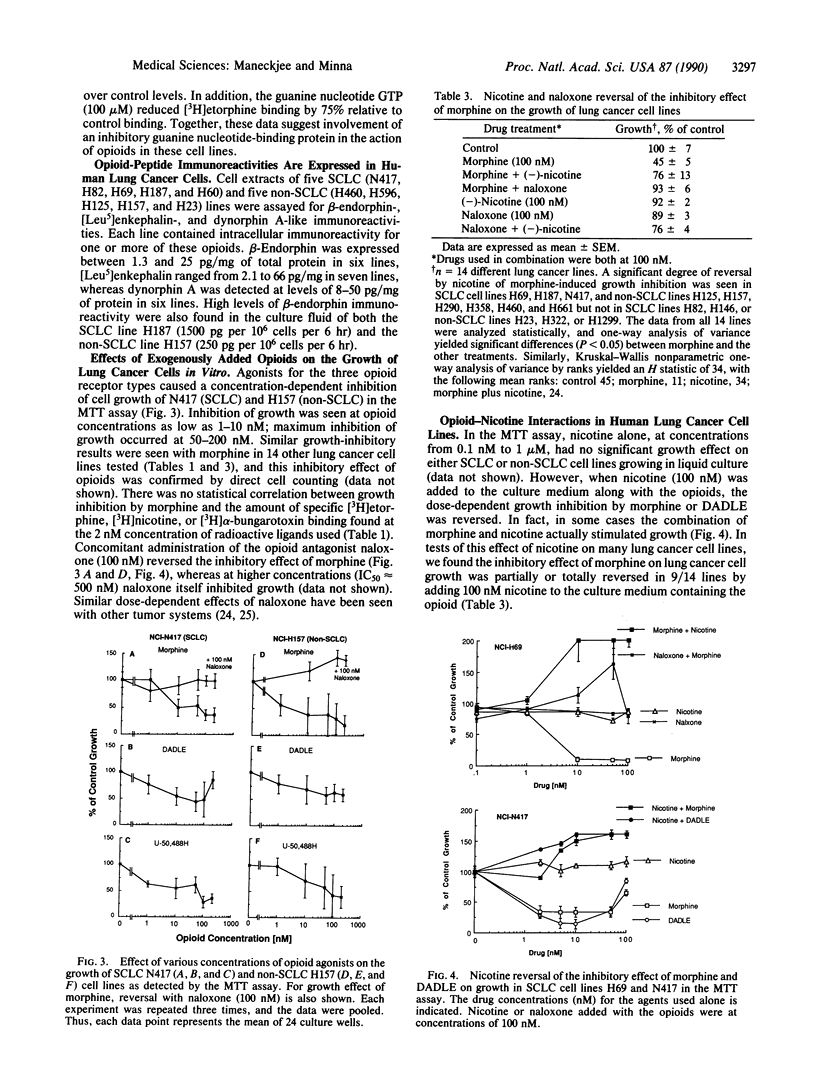
Using specific ligands, we find that lung cancer cell lines of diverse histologic types express multiple, high-affinity (Kd = 10(-9)-10(-10) M) membrane receptors for mu, delta, and kappa opioid agonists and for nicotine and alpha-bungarotoxin. These receptors are biologically active because cAMP levels decreased in lung cancer cells after opioid and nicotine application. Nicotine at concentrations (approximately 100 nM) found in the blood of smokers had no effect on in vitro lung cancer cell growth, whereas mu, delta, and kappa opioid agonists at low concentrations (1-100 nM) inhibited lung cancer growth in vitro. We also found that lung cancer cells expressed various combinations of immunoreactive opioid peptides (beta-endorphin, enkephalin, or dynorphin), suggesting the participation of opioids in a negative autocrine loop or tumor-suppressing system. Due to the almost universal exposure of patients with lung cancer to nicotine, we tested whether nicotine affected the response of lung cancer cell growth to opioids and found that nicotine at concentrations of 100-200 nM partially or totally reversed opioid-induced growth inhibition in 9/14 lung cancer cell lines. These in vitro results for lung cancer cells suggest that opioids could function as part of a "tumor suppressor" system and that nicotine can function to circumvent this system in the pathogenesis of lung cancer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aylsworth C. F., Hodson C. A., Meites J. Opiate antagonists can inhibit mammary tumor growth in rats. Proc Soc Exp Biol Med. 1979 May;161(1):18–20. doi: 10.3181/00379727-161-40479. [DOI] [PubMed] [Google Scholar]
- Beleslin D. B., Krstić S. K., Stefanović-Denić K., Strbac M., Mićić D. Inhibition by morphine and morphine-like drugs of nicotine-induced emesis in cats. Brain Res Bull. 1981 May;6(5):451–453. doi: 10.1016/s0361-9230(81)80017-2. [DOI] [PubMed] [Google Scholar]
- Benowitz N. L. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med. 1988 Nov 17;319(20):1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Benwell M. E., Balfour D. J., Anderson J. M. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J Neurochem. 1988 Apr;50(4):1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Bostwick D. G., Null W. E., Holmes D., Weber E., Barchas J. D., Bensch K. G. Expression of opioid peptides in tumors. N Engl J Med. 1987 Dec 3;317(23):1439–1443. doi: 10.1056/NEJM198712033172304. [DOI] [PubMed] [Google Scholar]
- Brower M., Carney D. N., Oie H. K., Gazdar A. F., Minna J. D. Growth of cell lines and clinical specimens of human non-small cell lung cancer in a serum-free defined medium. Cancer Res. 1986 Feb;46(2):798–806. [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Chneiweiss H., Glowinski J., Premont J. Mu and delta opiate receptors coupled negatively to adenylate cyclase on embryonic neurons from the mouse striatum in primary cultures. J Neurosci. 1988 Sep;8(9):3376–3382. doi: 10.1523/JNEUROSCI.08-09-03376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J. M., Lennon V. A., Lambert E. H., Scheithauer B. Acetylcholine receptors in small cell carcinomas. J Neurochem. 1985 Jul;45(1):159–167. doi: 10.1111/j.1471-4159.1985.tb05488.x. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986 May 22;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Giraud P., Dave J. R., Hotchkiss A. J., Affolter H. U. Nicotinic receptor stimulation activates enkephalin release and biosynthesis in adrenal chromaffin cells. Nature. 1984 Dec 13;312(5995):661–663. doi: 10.1038/312661a0. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Helman L. J., Israel M. A., Russell E. K., Linnoila R. I., Mulshine J. L., Schuller H. M., Park J. G. Expression of neuroendocrine cell markers L-dopa decarboxylase, chromogranin A, and dense core granules in human tumors of endocrine and nonendocrine origin. Cancer Res. 1988 Jul 15;48(14):4078–4082. [PubMed] [Google Scholar]
- Grenhoff J., Svensson T. H. Pharmacology of nicotine. Br J Addict. 1989 May;84(5):477–492. doi: 10.1111/j.1360-0443.1989.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Hecht S. S., Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988 Jun;9(6):875–884. doi: 10.1093/carcin/9.6.875. [DOI] [PubMed] [Google Scholar]
- Hopfield J. F., Tank D. W., Greengard P., Huganir R. L. Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature. 1988 Dec 15;336(6200):677–680. doi: 10.1038/336677a0. [DOI] [PubMed] [Google Scholar]
- Kamerling S. G., Wettstein J. G., Sloan J. W., Su T. P., Martin W. R. Interaction between nicotine and endogenous opioid mechanisms in the unanesthetized dog. Pharmacol Biochem Behav. 1982 Oct;17(4):733–740. doi: 10.1016/0091-3057(82)90355-0. [DOI] [PubMed] [Google Scholar]
- Karras A., Kane J. M. Naloxone reduces cigarette smoking. Life Sci. 1980 Oct 27;27(17):1541–1545. doi: 10.1016/0024-3205(80)90562-7. [DOI] [PubMed] [Google Scholar]
- Kumakura K., Karoum F., Guidotti A., Costa E. Modulation of nicotinic receptors by opiate receptor agonists in cultured adrenal chromaffin cells. Nature. 1980 Jan 31;283(5746):489–492. doi: 10.1038/283489a0. [DOI] [PubMed] [Google Scholar]
- Mansour A., Khachaturian H., Lewis M. E., Akil H., Watson S. J. Anatomy of CNS opioid receptors. Trends Neurosci. 1988 Jul;11(7):308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Murgo A. J. Modulation of murine melanoma growth by naloxone. Cancer Lett. 1989 Feb;44(2):137–142. doi: 10.1016/0304-3835(89)90008-6. [DOI] [PubMed] [Google Scholar]
- Quik M., Geertsen S. Neuronal nicotinic alpha-bungarotoxin sites. Can J Physiol Pharmacol. 1988 Aug;66(8):971–979. doi: 10.1139/y88-160. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Barchas J. D. Small cell carcinoma cell lines contain opioid peptides and receptors. Cancer. 1986 Feb 15;57(4):769–773. doi: 10.1002/1097-0142(19860215)57:4<769::aid-cncr2820570415>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schmidt J. Biochemistry of nicotinic acetylcholine receptors in the vertebrate brain. Int Rev Neurobiol. 1988;30:1–38. doi: 10.1016/s0074-7742(08)60045-8. [DOI] [PubMed] [Google Scholar]
- Scholar E. M., Violi L., Hexum T. D. The antimetastatic activity of enkephalin-like peptides. Cancer Lett. 1987 May;35(2):133–138. doi: 10.1016/0304-3835(87)90036-x. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried J. M., Owens S. E. Response of primary human lung carcinomas to autocrine growth factors produced by a lung carcinoma cell line. Cancer Res. 1988 Sep 1;48(17):4976–4981. [PubMed] [Google Scholar]
- Snyder S. H. Drug and neurotransmitter receptors in the brain. Science. 1984 Apr 6;224(4644):22–31. doi: 10.1126/science.6322304. [DOI] [PubMed] [Google Scholar]
- TerBush D. R., Bittner M. A., Holz R. W. Ca2+ influx causes rapid translocation of protein kinase C to membranes. Studies of the effects of secretagogues in adrenal chromaffin cells. J Biol Chem. 1988 Dec 15;263(35):18873–18879. [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J., Goodman S. R., Rhodes R. E. Opioid receptors and endogenous opioids in diverse human and animal cancers. J Natl Cancer Inst. 1987 Nov;79(5):1059–1065. [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Heroin prolongs survival time and retards tumor growth in mice with neuroblastoma. Brain Res Bull. 1981 Jul;7(1):25–32. doi: 10.1016/0361-9230(81)90094-0. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Naltrexone modulates tumor response in mice with neuroblastoma. Science. 1983 Aug 12;221(4611):671–673. doi: 10.1126/science.6867737. [DOI] [PubMed] [Google Scholar]



