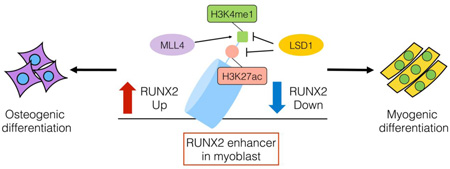
Abstract
Histone methylation dynamics play a critical role in cellular programming during development. For example, specific lysine methyltransferases (KMTs) and demethylases (KDMs) have been implicated in the differentiation of mesenchymal stem cells into various cell lineages. However, a systematic functional analysis for an entire family of KMT or KDM enzymes has not been performed. Here we test the function of all the known and candidate KDMs in myoblast and osteoblast differentiation using the C2C12 cell differentiation model system. Our analysis identified LSD1 as the only KDM required for myogenic differentiation and KDM3B, KDM6A and KDM8 as candidate KDMs required for osteoblast differentiation. We find that LSD1, via H3K4me1 demethylation, represses the master regulator of osteoblast differentiation RUNX2 to promote myogenesis in the C2C12 model system. Finally MLL4 is required for efficient osteoblast differentiation in part by countering LSD1 H3K4me1 demethylation at the RUNX2 enhancer. Together, our findings provide additional mechanisms by which lysine methylation signaling impacts on cell fate decisions.
Graphical abstract
Introduction
Chromatin regulation through the modification of histones plays a critical role during the differentiation of stem and progenitor cells. Changes in histone modifications at critical gene regulatory regions such as promoters and enhancers help mediate cell type specific gene expression programs [1]. In this context, active promoters are marked by Histone H3 lysine 4 tri-methylation (H3K4me3) whereas simultaneous H3 lysine 27 tri-methylation (H3K27me3) is linked to lower expression of developmental genes in Embryonic Stem (ES) cells [2]. Enhancers are marked by H3 lysine 4 mono-methylation (H3K4me1) [3] and H3K27 acetylation (H3K27ac) [4]. H3K27ac at enhancer is correlated with proximal gene expression and its absence is linked to inactive enhancers.
Adult tissue stem cells and progenitor cells are important to maintain or repair adult tissue. Mesenchymal stem cells (MSC) are present in adult tissue such as bone marrow and adipose tissue and can differentiate to osteocytes, adipocytes, and chondrocytes in vitro. Extensive evidence has demonstrated that differentiation of adult tissue stem cells and progenitor cells is regulated by histone methylation [5]. For example, the H3K27 methyltransferase EZH2 inhibits osteogenic differentiation whereas the H3K27 demethylases KDM6A and KDM6B enhance osteogenic differentiation of MSC [6, 7].
Muscle progenitor cells are bi-potent and can differentiate not only to myotubes but also to osteogenic cells[8, 9]. In adult tissue, these bi-potent progenitor cells may contribute to repair of damaged muscle and bone fracture [10]. Both KMTs and KDMs have been implicated in myotube differentiation and consistently genome wide studies have revealed dynamic changes in histone modification states during myotube differentiation [11, 12], However, the full extent by which histone modifiers regulates differentiation of myoblasts is not clear. Mammalian skeletal myogenesis happens both during early embryonic development and in response to damaged mature muscle. During myogenesis, myoblast, which arise from embryonic progenitor cells, differentiate into myocytes, which–differentiate to form myotubes. This process is regulated by key transcription factors, including Pax3, Pax7, MEF, Myf5, MyoD, Mrf4, and Runx2 and these factors are in turn regulated by different chromatin factors [5]. For example, the KMTs G9a and EZH2 both modulate MyoD and MEF transcription during myogenesis [5] and the KDM LSD1 is required for myogenesis [1]. However, the extent that KDMs impact on myogenesis as well as the specific underlying molecular mechanism of enzymes like LSD1 influence this process are not completely understood.
In this study, we aimed to identify KDMs that regulate differentiation of muscle progenitor cells. To this end, we knocked out all known and candidate KDMs in the mouse genome with Cluster Regulatory Interspaced Short Palindromic Repeats (CRISPR)/Cas9 system and examined myogenic and osteogenic differentiation using the C2C12 cell differentiation model system. Our analysis identified the H3K4 demethylase LSD1 as the only KDM required for myogenic differentiation via regulation of expression of myogenic differentiation factors. These activities are mediated via LSD1 demethylation of H3K4me1 at the enhancer region of RUNX2, an osteogenic master regulator that inhibits myotube differentiation. We also identify MLL4, an H3K4 methyltransferase as a positive regulator of myoblast osteogenic differentiation. Together these results provide additional insights into the role of histone methylation in the regulation of stem cell differentiation.
Results
Screen of known and candidate lysine demethylases in regulation of cellular potency in a model system
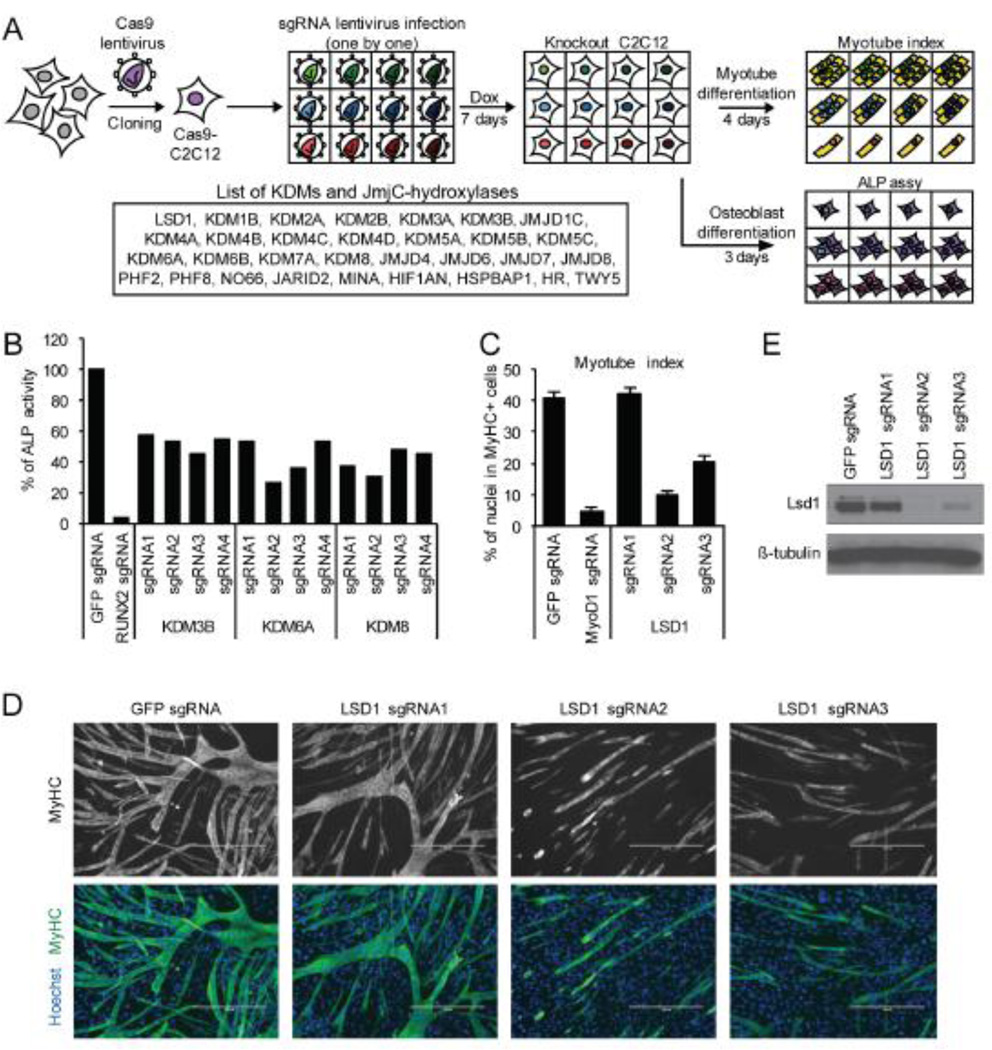
Mouse myoblast C2C12 cells are multi-potent and can be differentiated into various cellular lineages including myotubules and osteoblasts [8]. We used this well established cellular system to explore a role for the known and candidate protein KDMs as well as the related JmjC domain-containing hydroxylases in myogenic and osteogenic differentiation. The CRISPR/Cas9 system was utilized to delete genes in C2C12 cells. As Cas9 could not be efficiently transduced in C2C12 cells when co-expressed with small guide RNAs (sgRNAs), we generated a C2C12 cell line (Cas9-C2C12) in which Cas9 expression could be induced upon doxycycline treatment. Subsequent transduction of sgRNAs into the Cas9-C2C12 cells in the presence of doxycycline allowed for efficient generation of gene-specific knockouts (Fig 1A).
Figure 1.
Summary of KDM knockout screening. (A) Schematic of CRISPR knockout screening for differentiation regulators. Individual sgRNA virus was transduced into Cas9 expressing C2C12 (Cas9-C2C12). Full collection of KDM knockout C2C12 cells were differentiated into myotube or osteoblast. Dox, doxycycline; ALP, alkaline phosphatase. (B) Results of knockout screening for regulators of osteoblast differentiation. ALP activity was detected with luminescent ALP assay kit, calculated by using ALP standard and normalized against protein concentration. RUNX2 knockout was examined as a positive control. Each bar indicates the average of two replicates. Data is a representative of two independent experiment. (C) Knockout screening for regulators of myotube differentiation identified LSD1 as a potential regulator. Myotube index was calculated as percentage of nuclei in MyHC+ myotube. MyoD1 knockout was examined as a positive control. Error bars represent SEM from five random fields. MyHC, myosin heavy chain. (D) Representative image of myotubes of LSD1 knockout C2C12. Myotubes were stained with anti-MyHC antibody and nuclei were stained with Hoechst. GFP sgRNA was used for control. Scale bar indicates 400 µm. (E) Western blot analysis of LSD1 expression in knockout cells. Beta-tubulin level is shown as a loading control.
Treatment of Cas9-C2C12 cells with low serum resulted in myotube differentiation as evaluated by counting myosin heavy chain (MyHC) positive myotubes whereas administration of Bmp4 triggered osteoblast formation, which was detected by measuring ALP activity (see schematic, Fig.1A). To establish a range of differentiation for the Cas9-C2C12 cells, we treated them with a control sgRNA or sgRNAs targeting either Runx2 and Myod1, two master regulators of differentiation that are absolutely required for osteoblast and myotube differentiation, respectively. As expected, Runx2 was required for osteoblast differentiation (Fig. 1B) and Myod1 was required for mytobue differentiation (Fig. 1C).
The CRISPR design tool [13] was used to design a library of sgRNAs against the lysine demethylases LSD1, LSD2, and the JmjC domain-containing family of genes. Two to four independent sgRNA clones were generated to target each gene in the library. Each individual sgRNA virus was transduced into Cas9-C2C12 induced cells to generate the full collection of KDM knock-out cells. The collection was then subject to the osteoblast or myotube differentiation protocol (see Fig. 1A) and the requirement for different KDMs was determined. This analysis identified Kdm3b, Kdm6a and Kdm8 as potential regulators of osteogenic differentiation (Fig. 1B) and Lsd1 as a potential regulator of myogenic differentiation (Fig. 1C and 1D). No other gene in our library impacted on the osteogenic and myogenic potency of C2C12 cells (data not shown)
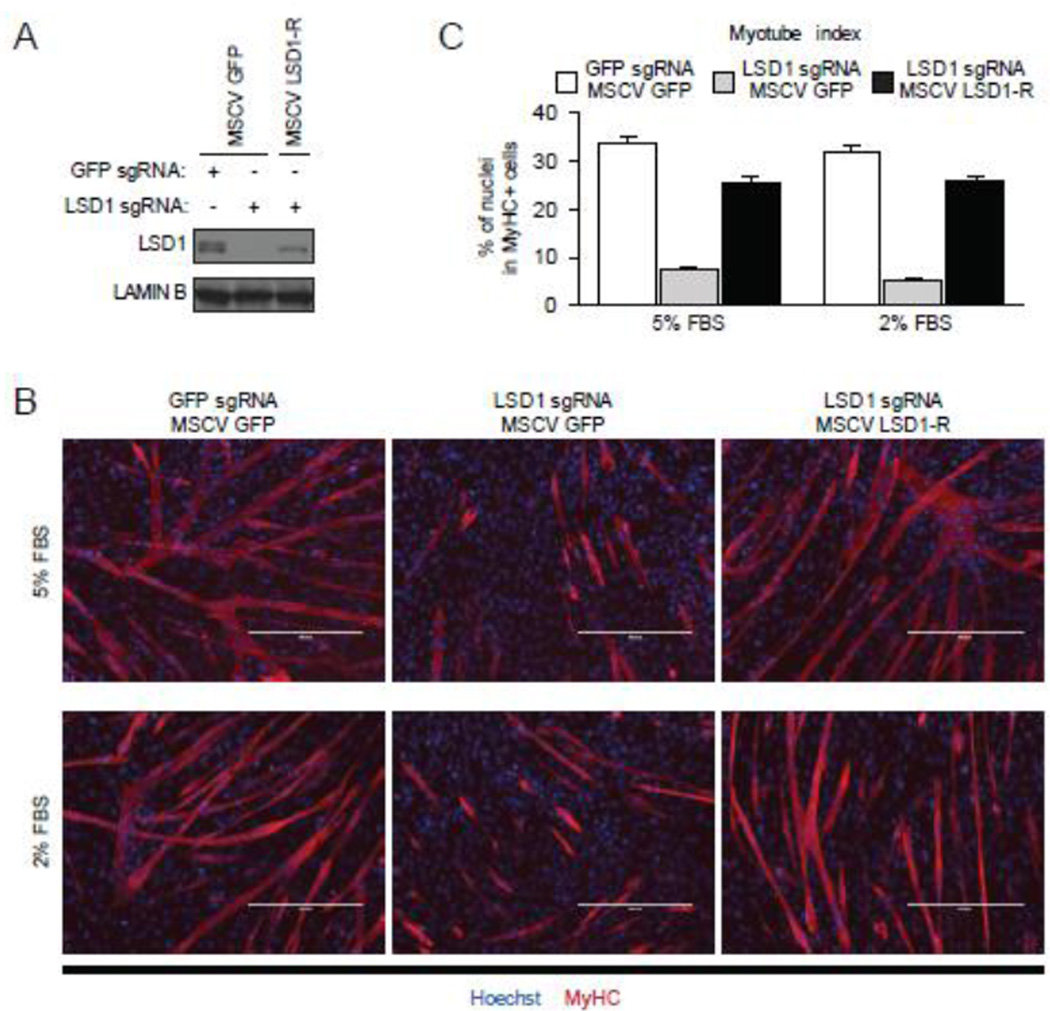
LSD1 has previously been implicated in muscle development [14] and its depletion had the largest impact on C2C12 differentiation amongst the genes we tested. Therefore we further investigated the role of LSD1 in our cellular system. Western Blot analysis demonstrated that the inhibition of myotube differentiation correlated with knockout efficiency of Lsd1 (Fig. 1E). To further test whether the phenotype associated with LSD1 sgRNA treatment was specifically due to depletion of LSD1 versus off-target effects, we generated Cas9-resistant LSD1 by introducing silent mutations within the LSD1 sequence (named LSD1-R) that are critical for Cas9 recognition but do not alter the amino acid sequence (see Methods). Complementation of LSD1-R into LSD1 knockout cells reconstituted LSD1 expression (Figure 2A) as well as the ability of these cells to differentiate into myotubes (Fig. 2B; quantitation shown in Fig. 2C). We note that the dependency on LSD1 for differentiation occurred at two different serum concentrations (Fig. 2C).
Figure 2.
Exogenous expression of LSD1 reconstitutes myotube differentiation of LSD1 knockout cells. (A) Western blot analysis of LSD1 expression in knockout cells reconstituted with Cas9-resistant LSD1 (LSD1-R) showed that exogenous LSD1-R expression was comparable to endogenous LSD1 level. Four days after LSD1 knockout, cells were infected with retrovirus expressing GFP or LSD1-R and maintained in the presence of doxycycline for seven days. Cells were differentiated into myotubes in medium containing 5% FBS or 2% FBS. GFP sgRNA and MSCV GFP were used as control. Lamin B level is shown as a loading control. (B) Representative image of myotubes stained with anti-MyHC antibody. Nuclei were stained with Hoechst. Scale bar indicates 400 µm. (C) Myotube index was calculated as in Figure 1C. Error bars represent SEM based on at least nine random fields from three replicates.
Role of LSD1 in expression of regulatory genes
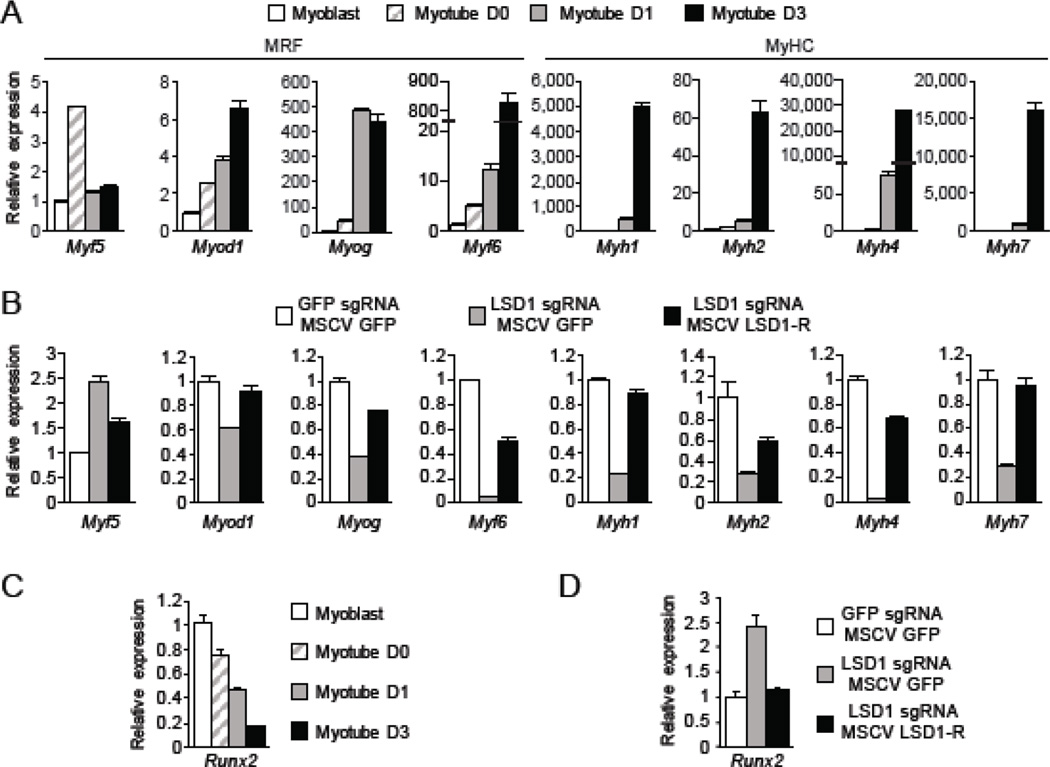
We next examined the expression of the myogenic differentiation factors Myf5, Myod1, Myog, and Myf6 (referred to as myogenic regulatory factors (MRFs) [15]) and the myotube markers Myh1, Myh2, Myh4, and Myh7 (referred to as MyHCs [16]), which represent proteins that are expressed in adult skeletal muscle. As expected, the expression levels of these canonical MRFs and MyHCs increased during differentiation of Cas9-C2C12 cells to myotubes (Fig. 3A). In the absence of LSD1, the early myoblast differentiation factor Myf5 showed increased expression whereas all of the other downstream MRFs and MyHCs showed reduced expression (Fig. 3B). Reconstitution with LSD1-R fully or partially rescued expression for all of these genes, with the increase in Myf5 expression upon LSD1 depletion reduced to close to normal levels (Fig. 3B). In addition to increased expression of MRFs and MyHCs during differentiation, it is important for the expression of other factors that determine alternative lineages to be silenced [16, 17]. We therefore tested expression levels of the key osteogenic differentiation factor Runx2 – specifically a short isoform that is transcribed from the proximal P2 promoter [18]. Runx2 expression levels decrease during myotube differentiation (Fig. 3C). We note that a long isoform expressed from a distal promoter is not detected, suggesting that the proximal promoter is primarily used in C2C12 cells (data not shown). Under myotube differentiation conditions, Runx2 expression was increased in LSD1 knockout cells relative to control cells; complementation of LSD1-R in LSD1 knockout cells restored Runx2 expression to control levels (Fig. 3D). LSD1, as a demethylase of the activation marks H3K4me1 and H3K4me2, is generally believed to function as a transcriptional repressor. Thus, our results suggest a model in which LSD1 is indirectly activating expression of MRFs and MyHCs by repressing a repressor – likely the master regulator Runx2.
Figure 3.
Expression of master regulators of differentiation in LSD1 knockout C2C12. (A) Quantitative RT-PCR analysis of MRF and MyHC expression during myotube differentiation of Cas9-C2C12. Myoblast were cultured in growth medium and maintained less than 50% confluent. The medium was replaced with myotube differentiation medium containing 2% FBS when cells were 90%–100% confluent (Myotube D0). Error bars indicate SEM from three replicates. Data is a representative of two independent experiment. (B) Quantitative RT-PCR analysis of MRF and MyHC expression in LSD1 knockout cells and LSD1-R reconstituted cells. Cells were differentiated in myotube differentiation medium. GFP sgRNA and MSCV GFP were used as control. Error bars indicate SEM from three replicates. Data is a representative of two independent experiment. (C) Quantitative RT-PCR analysis of Runx2 expression during myotube differentiation of Cas9-C2C12. (D) Quantitative RT-PCR analysis of Runx2 expression in LSD1 knockout cells and LSD1-R reconstituted cells. Cells were differentiated into myotube as in (B).
Runx2 inhibits myotube differentiation
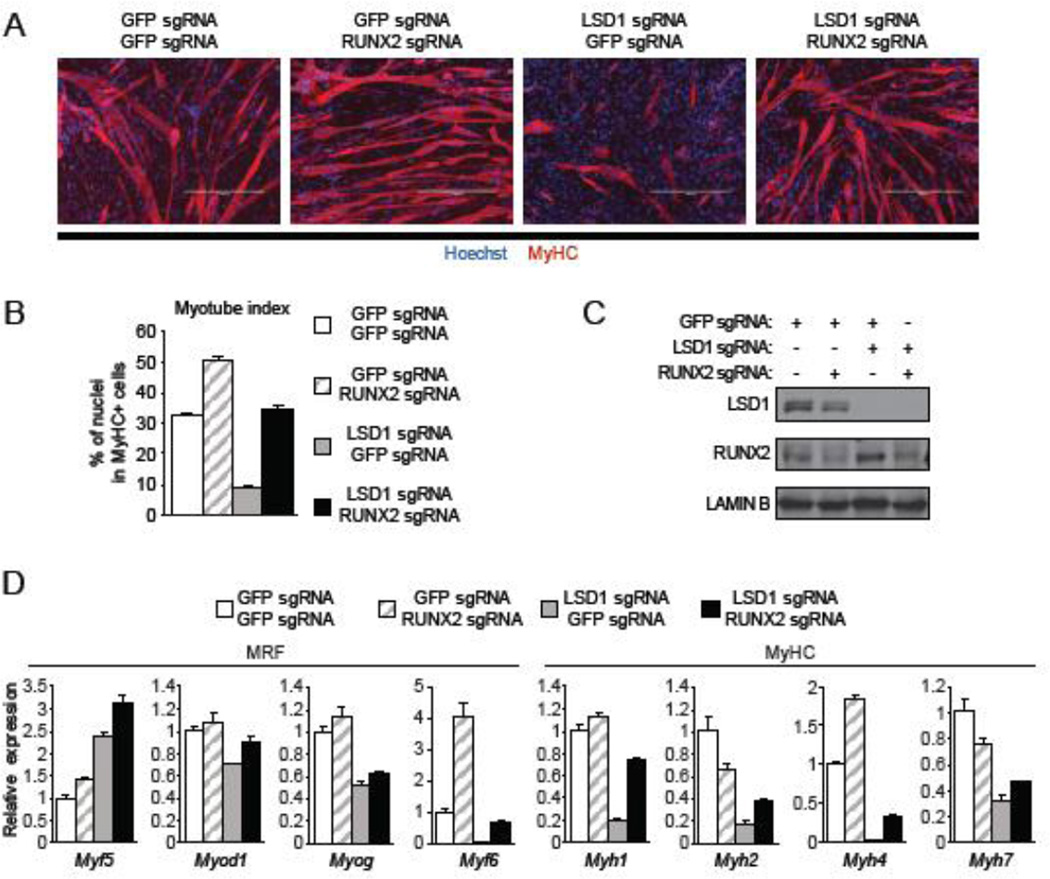
Exogenous overexpression of Runx2 in myoblast inhibits myotube differentiation [19, 20]. These observations and our findings suggest the hypothesis that Runx2 mediates the ability of LSD1 to promote myotube differentiation. To test this idea, we asked whether there was an epistatic relationship between Runx2 and LSD1. In this context, the loss of myogenic differentiation in LSD1 knockout cells was rescued by co-depletion of Runx2 (Figure 4A and 4B). As shown in Figure 4C, depletion of LSD1 resulted in an increase in Runx2 protein levels and CRISPR/Cas9-guided ablation of Runx2 gene resulted in depletion (though not complete loss) of Runx2 protein. The co-depletion of LSD1 and Runx2 rescued expression of Myf6, but not the other MRFs, to control levels relative to LSD1 depletion alone (Figure 4D). Moreover, expression levels for the four MyHC genes were partially rescued by co-depletion of Runx2 with LSD1 compared to LSD1 alone (Figure 4D). Together, these results suggest a model in which the ability of LSD1 to regulate myogenic differentiation is in part mediated via repression of Runx2 expression, which in turn as a regulator of the osteogenic fate is involved in the repression of key myoblast differentiation genes.
Figure 4.
Myogenic differentiation in LSD1 knockout cells was rescued by co-depletion of Runx2. (A) Representative image of myotubes formed by LSD1 and RUNX2 depleted C2C12. Four days after LSD1 knockout, cells were infected with lentivirus expressing RUNX2 sgRNA and maintained in the presence of doxycycline for seven days before myotube differentiation. GFP sgRNA was used as control. Scale bar indicates 400 µm. (B) Myotube index of double knockout cells was calculated as in Figure 1C. Error bars represent SEM based on twelve random fields from tree replicates. (C) Western blot analysis with the indicated antibodies of lysates from double knockout cells. Lamin B level is shown as a loading control. (D) Quantitative RT-PCR analysis of MRF and MyHC expression in double knockout cells differentiated to myotube. GFP sgRNA was used as control. Error bars indicate SEM from three replicates. Data is a representative of two independent experiment.
Lsd1 regulates histone modification on the Runx2 gene
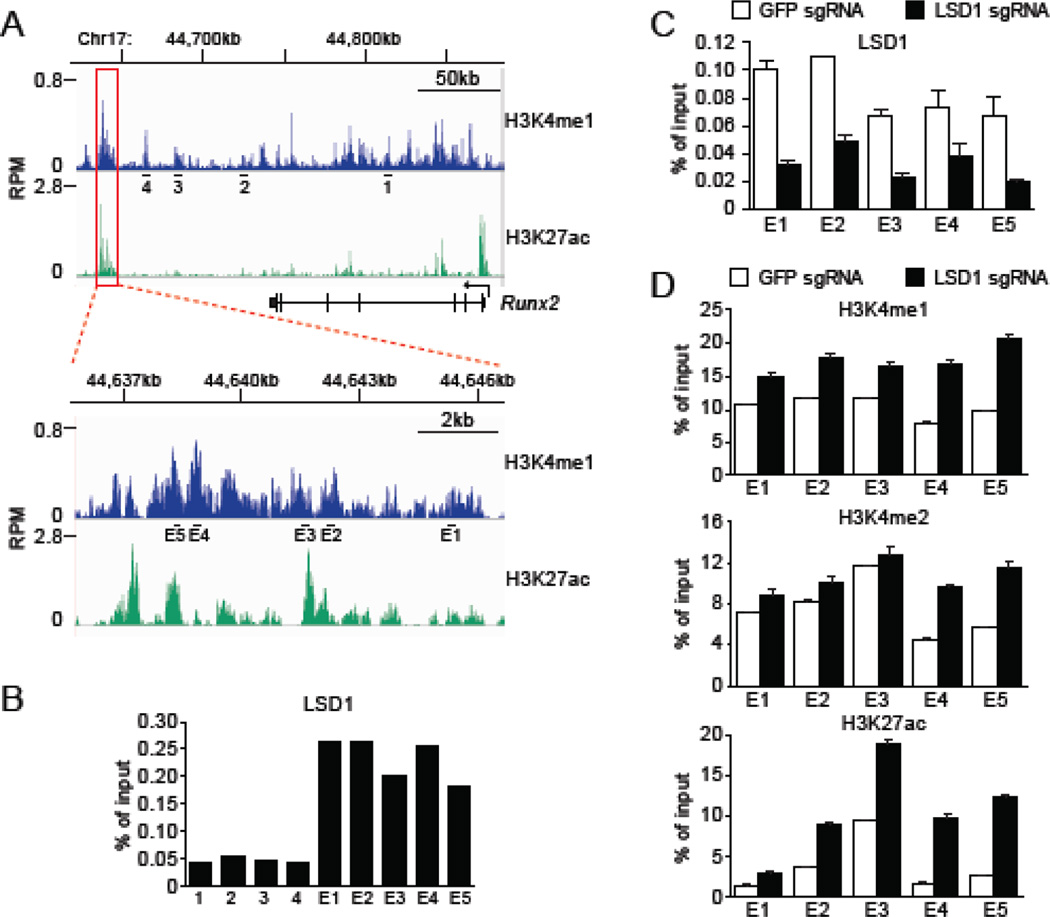
One mechanism by which Lsd1 represses gene expression is via demethylation of H3K4me1/2 at transcriptional enhancers [21, 22]. Thus, we postulated that LSD1 may regulate the Runx2 enhancer in myoblast. A chromatin modification signature associated with enhancers is the presence of H3K4me1 and H3K27ac (histone H3 acetylated at lysine 27). A search of chromatin immunoprecipitation (ChIP) high throughput sequencing (ChIP-seq) data deposited in the GEO for both H3K4me1 (GSM1197187) and H3K27ac (GSM921130) in C2C12 myoblast identified a candidate enhancer region downstream of the Runx2 gene (Figure 5A; indicated by red box). A close up of this region is shown in the lower panel in Figure 5A. We first asked whether LSD1 occupies the Runx2 gene and the candidate downstream enhancer region. As shown in Figure 5B, LSD1 was highly enriched across the candidate enhancer region relative to the Runx2 gene body. As expected, the enrichment of LSD1 ChIP signal across the enhancer was significantly diminished in LSD1-depleted cells (Figure 5C). Moreover, enrichment of H3K4me1, H3K4me2, and H3K27ac signals at the enhancer were all increased in LSD1 knockout cells relative to control cells (Figure 5D). We note that LSD1 depletion had a greater impact on H3K4me1 levels than H3K4me2 levels (Figure 5D).
Figure 5.
LSD1 binds to Runx2 downstream enhancer and regulates histone H3 modification. (A) ChIP-seq data for H3K4me1 and H3K27ac at Runx2 locus in C2C12 myoblast. Public data deposited in GEO (GSM1197187 and GSM921130) were analyzed by using SRAtailor software [31]. Candidate enhancer region downstream of the Runx2 gene is indicated by red box and a close up of this region is shown in the lower panel. Black bars and numbers indicate primers used for ChIP-qPCR in B-D. (B, C) ChIP-qPCR analysis with anti-LSD1 antibody at the Runx2 locus. LSD1 enrichment are shown as % signal/input. (B) Cas9-C2C12 were differentiated into myotube in medium containing 2% FBS. (C) LSD1 knockout cells were examined as negative control for ChIP experiment with anti-LSD1 antibody. Error bars indicate SEM from four replicates of qPCR. (D) ChIP-qPCR analysis with indicated antibody at the Runx2 downstream enhancer in LSD1 knockout cells. GFP sgRNA was used as control. Cells were differentiated in medium containing 2% FBS. Results are shown as % signal/input. Error bars indicate SEM from three replicates of ChIP-qPCR.
The histone lysine methyltransferase MLL4 regulates osteogenic differentiation
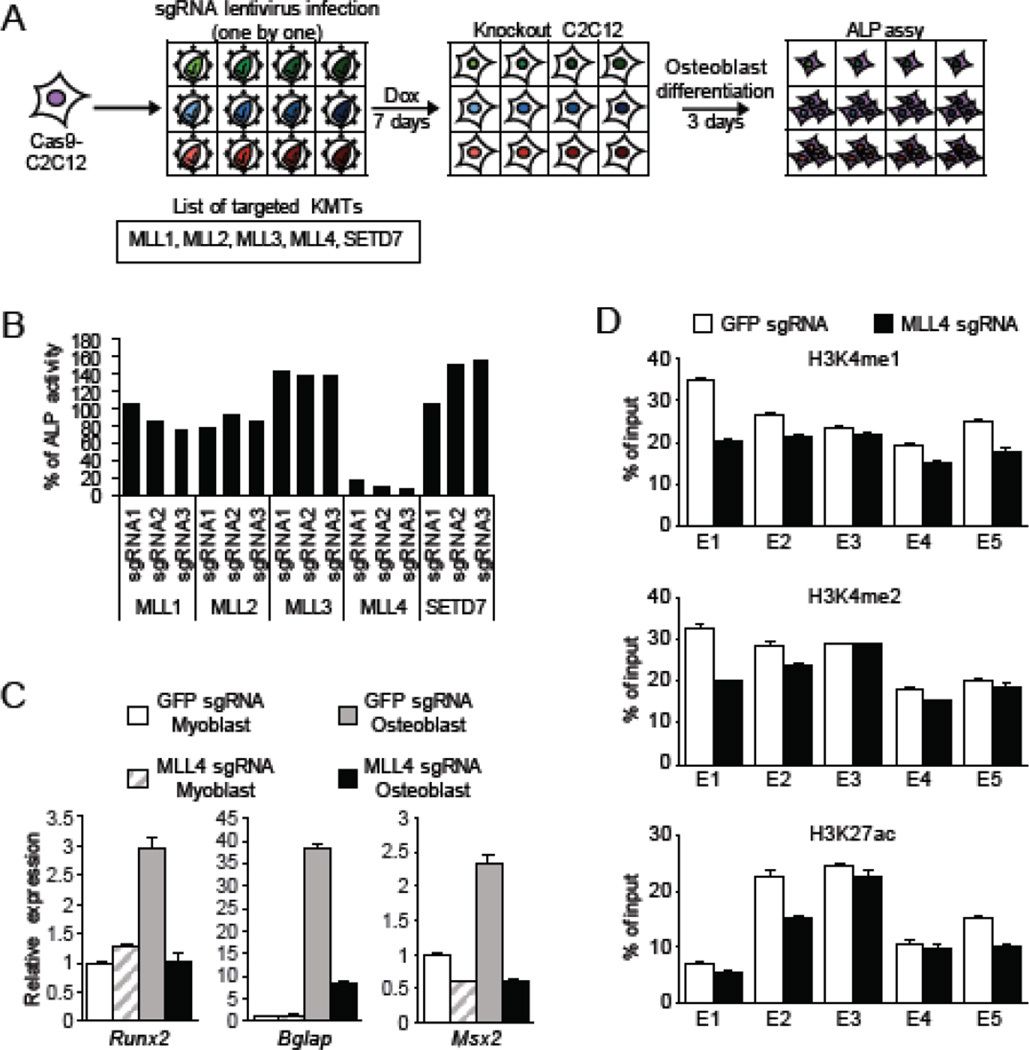
We next postulated that in addition to LSD1, the Runx2 enhancer would also likely be regulated by the enzyme that deposits H3K4me1/2 and that this in turn would regulate osteoblastic differentiation of C2C12 cells. To test this idea, we knocked out using the CRISPR/Cas9 system several known H3K4 methyltransferases and evaluated osteoblast differentiation by ALP (Fig. 6A). As shown in Figure 6B, depletion of MLL4 inhibited osteogenic differentiation. Accordingly, MLL4 depletion also resulted in loss of induction of Runx2 and other osteogenic genes (Fig. 6C). Finally, under conditions that drive osteoblast differentiation H3K4me1 and H3K4me2 levels were reduced at the candidate enhancer of Runx2 in the MLL4 depleted cells relative to control cells (Figure 6D). H3K27ac levels were also slightly reduced upon MLL4 knockdown, most likely as an indirect consequence in the decrease of H3K4me1/2 levels (Figure 6D). Together, these results suggest that in C2C12 cells, MLL4 may in part regulate osteogenic differentiation via its methylation activity at the enhancer of the Runx2 gene.
Figure 6.
MLL4 regulates osteogenic differentiation of C2C12. (A) Schematic of CRISPR knockout of histone H3K4 methyltransferase (KMT). KMT knockout C2C12 cells were differentiated into osteoblast and the differentiation was evaluated by ALP. Dox, doxycycline; ALP, alkaline phosphatase. (B) ALP activity was detected and calculated as in Figure 1B. Each bar indicates the average of two replicates. (C) Quantitative RT-PCR analysis of osteogenic genes in MLL4 knockout C2C12. Myoblast were cultured in growth medium. The medium was replaced with osteoblast differentiation medium containing 100 ng/ml BMP-4 when cells were 50% confluent and cells were differentiated into osteoblast for three days. GFP sgRNA was used as control. Error bars indicate SEM from three replicates. Data is a representative of two independent experiment. (D) ChIP-qPCR analysis with indicated antibody at the Runx2 enhancer in MLL4 knockout C2C12 osteoblast. Cells were differentiated in osteoblast differentiation medium. Primers used for qPCR are indicated in Figure 5A. Results are shown as % signal/input. Error bars indicate SEM from four replicates of qPCR. Data is a representative of two independent experiment.
Discussion
In this study, we identified LSD1 as a regulator of myogenic differentiation in the C2C12 model cell system. Although previous study reported that LSD1 induced myotube differentiation by demethylating H3K9me2 at promoter of myogenic genes [14], only the neural specific isoform can demethylate H3K9me2 in vitro [23]. Here we have provided evidence that a relevant substrate of LSD1 in myoblast-like cells is H3K4me1 and H3K4me2. Based on public ChIP-Seq data [11, 24], we found a large enhancer marked with H3K4me1 and H3K27ac downstream of Runx2 gene. Our data indicates that LSD1 binds to the Runx2 enhancer and demethylates H3K4me1. Although this enhancer is located more than 200 kb apart from Runx2 proximal promoter P2, the enhancer and the promoter reside within the same cohesin-associated loop structure formed between two interacting CTCF sites (see [25]; Figure S1). We propose that this downstream enhancer regulates Runx2 promoter activity. We also observed an increase in acetylated H3K27 at the Runx2 enhancer in LSD1 knockout cells. LSD1 binds to HDAC1 and HDAC2 [21] and LSD1 containing complexes have been implicated in deacetylation of histone H3 [26]. Indeed, artificial recruitment of LSD1 decreases not only H3K4me2 but also H3K27ac at targeted enhancer loci in a cell system [22, 27]. Taken together, our results suggest that LSD1 suppresses Runx2 expression during myotube differentiation by regulating histone modifications at a Runx2 enhancer.
Although previous reports showed that exogenous RUNX2 expression inhibits myotube differentiation, it remained unclear whether endogenous RUNX2 in myoblast inhibits myotube differentiation. Here we showed that knockout of RUNX2 induced myotube differentiation especially when RUNX2 is not suppressed under differentiation condition. This result suggests that for myoblast to differentiate into myotubes RUNX2 must be suppressed, which is achieved at least in part via LSD1.
We also identified MLL4 as regulator of osteogenic differentiation in myogenic progenitor. MLL4 is mammalian homologue of Drosophila trithorax that contributes to the deposition of H3K4me1 at enhancers in vivo [28]. Lee et al reported that MLL3/4 double knockout preadipocytes showed defects in adipogenic differentiation and MyoD induced myogenic differentiation [29]. MLL3 and MLL4 may have redundant function in myogenic differentiation whereas only MLL4 regulates osteogenic differentiation in myoblast. Future studies are needed to investigate whether MLL4 regulates osteogenic differentiation in other progenitor cells and cell systems including osteoblast.
Recent genome wide studies indicate the importance of histone modification at promoter, enhancer and heterochromatin regions during differentiation. Surprisingly, we only identified LSD1 of all the KDMs in myotube differentiation. We also identified KDM3B, KDM6A and KDM8 as regulators of osteogenic differentiation, albeit with a weaker impact than MLL4 showed, but the function of these proteins in this process remains to be determined. In summary, by performing a focused gene editing screen, we have demonstrated the importance of histone modification dynamics at enhancers in a defined cellular differentiation system.
Materials and Methods
Cell, plasmids and antibodies
C2C12 (cat. no. CRL-1772) was purchased from American Type Culture Collection. pCW-Cas9 and pLX-sgRNA were gifts from Eric Lander & David Sabatini (Addgene plasmid #50661 and #50662). Anti-LSD1 antibody (Cell Signaling Technology; cat. no. 2139), anti-beta-tubulin antibody (Millipore; cat. no. 05-661), anti-lamin B antibody (Santa Cruz; cat. no, SC-6217) and anti-RUNX2 antibody (MBL, cat. no. D130-3) were used for western blot. Anti-LSD1 antibody (Cell Signaling Technology; cat. no. 2184), anti-H3K4me1 (Cell Signaling Technology; cat. no. 5326), anti-H3K4me2 (Cell Signaling Technology; cat. no. 9725) and anti-H3K27ac (Cell Signaling Technology; cat. no. 8173) were used for ChIP analysis.
Cell culture
293T cells were cultured in DMEM (Life Technologies; cat. no. 11960069) supplemented with 10% FBS (Life Technologies; cat. no. 26140-079) and 20 mM L-glutamine (Life Technologies; cat. no. 25030-164). Undifferentiated C2C12 cells were maintained in DMEM supplemented with 15% FBS and 20 mM L-glutamine (growth medium).
Vector construction
2 to 4 guide RNAs for each genes ware designed with CRISPR design tool[13] and cloned into pLx-sgRNA as described in Addgene vector information. Target sequences used for sgRNA are listed in Table S1. Full length mouse LSD1 (NM_133872.2) and CRISPR resistant mutant (LSD1-R) were cloned into pMSCV vector that expresses N-terminal flag fusion protein. The following primer was used to generate LSD1-R:
5’-CCGAGACCCCcGAaGGCCGACGGAC.
The lower cases indicate silent mutations.
Virus production and infection
pCW-Cas9 or pLx-sgRNA was co-transfected into 293T cells with pΔ8.2 packing plasmid and pVSV-G envelope plasmid using calcium-phosphate transfection. For retrovirus production, pMSCV vectors were co-transfected with pGag pol packing plasmid and pVSVg envelope plasmid. 48 hours after transfection, medium was collected, filtered with 0.45 µm filter and frozen at −80 °C. C2C12 cells were infected with virus in the presence of 8 µg/ml polybrene (Millipore; cat. no. TR-1003-G). 24 hours after virus infection, cells were treated with appropriate antibiotics.
Cas9-C2C12 cloning and CRISPR knock out
C2C12 expressing Cas9 were selected with 2 µg/ml puromycin (Sigma; cat. no. P9620) and single cells were cloned by limiting dilution. Cas9 expression was induced with 1 µg/ml doxycycline (Sigma; cat. no. D3447) and examined by immunostaining with anti-flag antibody (Sigma; cat. no. F1804) and clones that expressed Cas9 were selected. Myotube differentiation of each clones was examined and a single clone (Cas9-C2C12) that could differentiate into myotube most efficiently was used for further experiments. For knockout experiment, Cas9-C2C12 was infected with sgRNA lentivirus and selected with 10 µg/ml blasticidin (Life technologies; cat. no. A11139) for 7 days in the presence of 1 µg/ml doxycycline. In the double knockout experiment or rescue experiment, cells were infected with second sgRNA lentivirus or MSCV retrovirus 4 days after first infection.
C2C12 differentiation assay
8000 cells were inoculated in a well of 96 well plate and cultured in growth medium. The next day, medium was replaced with DMEM supplemented with 2% FBS or 5% FBS (myotube differentiation medium) for myotube differentiation. After 4 days, cells were fixed and stained with anti-MyHC antibody (Developmental Studies Hybridoma Bank; cat. no. MF20) and anti-mouse IgG Alexa Fluor 488 or Alexa Fluor 555 (Life Technologies; cat. no. A-11029 or A-21424) as described in [17]. Nuclei were stained with Hoechst33258 (Life Technologies; cat. no. H3569). Myotube index was calculated as percentage of nuclei in MyHC positive myotube that had more than 2 nuclei. For quantification, at least five random fields were imaged with EVOS FL Cell Imaging System (Life Technologies). For osteoblast differentiation, growth medium was replaced with DMEM supplemented with 2% FBS containing 75 ng/ml or 100 ng/ml human recombinant BMP-4 (R&D Systems; cat. no. 314-BP-010) (osteoblast differentiation medium). After 3 days, alkaline phosphatase (ALP) activity was detected with luminescent ALP assay kit (Anaspec; cat. no. AS-72122) following the manufacturer’s protocol. Cells in the 96 well plate were lysed with 50 µl lysis buffer for 3 min under agitation and centrifuged at 2,500 g for 5 min at 4 °C. The supernatant was collected and combined with the same volume of chemiluminescent substrate and incubated for 15 min at 4 °C in the dark. Luminescence was measured using a VICTOR3 plate reader (PerkinElmer) and ALP activity was calculated by using ALP standard. Protein concentration was determined by DC Protein Assay Kit (BIO-RAD; cat. no. 5000112) and ALP activity was normalized against protein concentration.
Cell extracts and western blot
Cells were lysed with lysis buffer (50 mM Tris-HCl pH 7.5, 10 mM EDTA, 1% Triton-X, 1% SDS, and protease inhibitors) and sonicated with a Bioruptor Standard (Diagenode). Protein concentration was determined by DC Protein Assay Kit. The same amount of proteins was resolved by SDS-PAGE, transferred to PVDF membrane and analyzed by western blot.
Quantitative RT-PCR Gene Expression Analysis
Real-Time PCR was performed following standard procedures. Briefly, RNA samples were extracted from cells using RNeasy kit (QIAGEN; cat. no. 74104) and reverse transcribed into cDNA using the SuperScript III First Strand Synthesis System (Life Technologies; cat. no. 18080-051). Quantitative real-time PCR analysis was performed on a Roche LightCycler 480 using LightCycler 480 SYBR Green Master Mix (Roche; cat. no. 4887352001). Expression of each genes was normalized to Rplp0 expression. Primers used for real-time PCR analysis were listed in Table S2.
ChIP-qPCR analysis
To analyze histone modifications [30], cells were crosslinked with 1% formaldehyde for 10 min at room temperature and crosslinking was terminated with 125 mM glycine. Cells were harvested using scraper, lysed with lysis buffer (50 mM Tris-HCl pH 7.5, 10 mM EDTA, 1% SDS, protease inhibitors) and sonicated with Bioruptor Standard (Diagenode) for 15 × 30 s pulses with 30 s pause between pulse. The whole cell lysate was diluted with 9 volume dilution buffer (1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl pH 7.5, 167 mM NaCl, protease inhibitors) and incubated with antibody at 4 °C overnight. The same amount of chromatin in each lysate were used for IP. The next day, protein A Dynabeads (Life Technologies; cat. no. 100-02D) were added to the lysate and incubated at 4 °C for 3 hr. Beads were washed with wash buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% Triton X-100), LiCl buffer (10 mM Tris-HCl pH7.5, 250 mM LiCl, 2 mM EDTA, 1% NP-40) and TE buffer. Beads were incubated with elution buffer (1% SDS, 0.1M NaHCO3) at room temperature for 30 min and crosslinking was reversed by heating at 65 °C for 4 hr. Eluted chromatin was treated with Protease K (Life Technologies; cat. no. 25530-049) at 65 °C for 1 hr and DNA was purified with phenol/chloroform/isoamyl alcohol extraction and ethanol precipitation. Purified DNA was subject to quantitative real-time PCR analysis. To analyze LSD1 binding to genome, cells were crosslinked with 2 mM disuccinimidyl glutarate (ProteoChem cat. no. c1104-100mg) for 45 min before formaldehyde treatment and lysed with 0.1% SDS lysis buffer (20 mM Tris-HCl pH 7.5, 2 mM EDTA, 0.1% SDS, 150 mM NaCl, 0.5% Triton X-100, protease inhibitors). Primers used for ChIP-qPCR analysis were listed in Table S3.
Supplementary Material
Highlights.
LSD1 and MLL4 have opposing functions at Runx2 enhancer
LSD1 suppresses Runx2 expression to promote myogenic differentiation
Runx2 deletion rescues LSD1 deletion in myogenic differentiation
Acknowledgments
This work was supported in part by a grants from the NIH to O.G. (R01CA172560) O.G. is a co-founder of EpiCypher, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen T, Dent SY. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat Rev Genet. 2014;15:93–106. doi: 10.1038/nrg3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rada-Iglesias A, Bajpai R, Prescott S, Brugmann SA, Swigut T, Wysocka J. Epigenomic annotation of enhancers predicts transcriptional regulators of human neural crest. Cell Stem Cell. 2012;11:633–648. doi: 10.1016/j.stem.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yahi H, Philipot O, Guasconi V, Fritsch L, Ait-Si-Ali S. Chromatin modification and muscle differentiation. Expert Opin Ther Targets. 2006;10:923–934. doi: 10.1517/14728222.10.6.923. [DOI] [PubMed] [Google Scholar]
- 6.Hemming S, Cakouros D, Isenmann S, Cooper L, Menicanin D, Zannettino A, et al. EZH2 and KDM6A act as an epigenetic switch to regulate mesenchymal stem cell lineage specification. Stem Cells. 2014;32:802–815. doi: 10.1002/stem.1573. [DOI] [PubMed] [Google Scholar]
- 7.Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K, Zhou X, et al. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell. 2012;11:50–61. doi: 10.1016/j.stem.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castiglioni A, Hettmer S, Lynes MD, Rao TN, Tchessalova D, Sinha I, et al. Isolation of progenitors that exhibit myogenic/osteogenic bipotency in vitro by fluorescence-activated cell sorting from human fetal muscle. Stem Cell Reports. 2014;2:92–106. doi: 10.1016/j.stemcr.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu R, Birke O, Morse A, Peacock L, Mikulec K, Little DG, et al. Myogenic progenitors contribute to open but not closed fracture repair. BMC Musculoskelet Disord. 2011;12:288. doi: 10.1186/1471-2474-12-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blum R, Vethantham V, Bowman C, Rudnicki M, Dynlacht BD. Genome-wide identification of enhancers in skeletal muscle: the role of MyoD1. Genes Dev. 2012;26:2763–2779. doi: 10.1101/gad.200113.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asp P, Blum R, Vethantham V, Parisi F, Micsinai M, Cheng J, et al. Genome-wide remodeling of the epigenetic landscape during myogenic differentiation. Proc Natl Acad Sci U S A. 2011;108:E149–E158. doi: 10.1073/pnas.1102223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi J, Jang H, Kim H, Kim ST, Cho EJ, Youn HD. Histone demethylase LSD1 is required to induce skeletal muscle differentiation by regulating myogenic factors. Biochem Biophys Res Commun. 2010;401:327–332. doi: 10.1016/j.bbrc.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Murphy M, Kardon G. Origin of vertebrate limb muscle: the role of progenitor and myoblast populations. Curr Top Dev Biol. 2011;96:1–32. doi: 10.1016/B978-0-12-385940-2.00001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang YY, Li X, Qian SW, Guo L, Huang HY, He Q, et al. Down-regulation of type I Runx2 mediated by dexamethasone is required for 3T3-L1 adipogenesis. Mol Endocrinol. 2012;26:798–808. doi: 10.1210/me.2011-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunadome K, Suzuki T, Usui M, Ashida Y, Nishida E. Antagonism between the master regulators of differentiation ensures the discreteness and robustness of cell fates. Mol Cell. 2014;54:526–535. doi: 10.1016/j.molcel.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Li YL, Xiao ZS. Advances in Runx2 regulation and its isoforms. Med Hypotheses. 2007;68:169–175. doi: 10.1016/j.mehy.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20:8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gersbach CA, Byers BA, Pavlath GK, Garcia AJ. Runx2/Cbfa1 stimulates transdifferentiation of primary skeletal myoblasts into a mineralizing osteoblastic phenotype. Exp Cell Res. 2004;300:406–417. doi: 10.1016/j.yexcr.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 21.Whyte WA, Bilodeau S, Orlando DA, Hoke HA, Frampton GM, Foster CT, et al. Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature. 2012;482:221–225. doi: 10.1038/nature10805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearns NA, Pham H, Tabak B, Genga RM, Silverstein NJ, Garber M, et al. Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nat Methods. 2015;12:401–403. doi: 10.1038/nmeth.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent B, Ruitu L, Murn J, Hempel K, Ferrao R, Xiang Y, et al. A specific LSD1/KDM1A isoform regulates neuronal differentiation through H3K9 demethylation. Mol Cell. 2015;57:957–970. doi: 10.1016/j.molcel.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mousavi K, Zare H, Dell'orso S, Grontved L, Gutierrez-Cruz G, Derfoul A, et al. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol Cell. 2013;51:606–617. doi: 10.1016/j.molcel.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowen JM, Fan ZP, Hnisz D, Ren G, Abraham BJ, Zhang LN, et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell. 2014;159:374–387. doi: 10.1016/j.cell.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;437:432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 27.Mendenhall EM, Williamson KE, Reyon D, Zou JY, Ram O, Joung JK, et al. Locus-specific editing of histone modifications at endogenous enhancers. Nat Biotechnol. 2013;31:1133–1136. doi: 10.1038/nbt.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herz HM, Mohan M, Garruss AS, Liang K, Takahashi YH, Mickey K, et al. Enhancer-associated H3K4 monomethylation by Trithorax-related, the Drosophila homolog of mammalian Mll3/Mll4. Genes Dev. 2012;26:2604–2620. doi: 10.1101/gad.201327.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JE, Wang C, Xu S, Cho YW, Wang L, Feng X, et al. H3K4 mono- and di-methyltransferase MLL4 is required for enhancer activation during cell differentiation. Elife. 2013;2:e01503. doi: 10.7554/eLife.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S, Yang Z, Wilkinson AW, Deshpande AJ, Sidoli S, Krajewski K, et al. The PZP Domain of AF10 Senses Unmodified H3K27 to Regulate DOT1L-Mediated Methylation of H3K79. Mol Cell. 2015;60:319–327. doi: 10.1016/j.molcel.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oki S, Maehara K, Ohkawa Y, Meno C. SraTailor: graphical user interface software for processing and visualizing ChIP-seq data. Genes Cells. 2014;19:919–926. doi: 10.1111/gtc.12190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.