Abstract
We investigated the inhibitory activity of synthetic isocyanurate-based as well as linear mono- and trihydroxamate siderophore-drug conjugates against Candida spp. The conjugated drug was 13C-desketoneoenactin (DE). The MICs of siderophore-drug conjugates were determined in the absence and presence of 2,2′-dipyridyl to restrict iron availability. The ability of various siderophore types to promote growth in an iron-restricted medium was also assayed. Addition of a siderophore portion to the drug strongly impaired the inhibitory activity of DE. However, the activity of the drug conjugates was increased by up to 16-fold in iron-depleted medium for species having their growth strongly promoted by most hydroxamate-type siderophores (C. albicans, C. stellatoidea, and C. pseudotropicalis). The uptake of 55Fe from ferrichrome and from two siderophore-drug conjugates was improved when C. albicans cells were grown in a low-iron medium. In the same assay, unlabeled ferrichrome was able to compete with the uptake of 55Fe from both conjugates, indicating a common mechanism of uptake. A C. albicans strain lacking the siderophore transporter CaSit1/CaArn1 was not able to use ferrichrome or the synthetic ornithine-based trihydroxamate siderophore for growth promotion and was much less susceptible to the siderophore-drug conjugates than its isogenic parent strain. In summary, the ability of some Candida spp. to use ferrichrome-like siderophores for growth promotion explains the selective activity of hydroxamate-drug conjugates, and this activity seems to be related to the presence, in C. albicans, of the siderophore transporter CaSit1/CaArn1. New conjugate designs are necessary to fully restore or improve the initial DE activity.
Iron is an essential nutrient for the growth of nearly all organisms. Although it is an abundant element of the earth's crust, its bioavailability is lowered under oxygen and at neutral pH, in which conditions it forms insoluble ferric hydroxides. The growth of pathogenic microorganisms is also limited by the unavailability of free iron, which is tightly bound to high-affinity proteins like transferrin, lactoferrin, and ferritin, in body fluids. To overcome this availability problem, microorganisms have developed diverse iron acquisition mechanisms. The more common is the secretion and/or use of iron chelators termed siderophores. Siderophore-iron complexes are transported into the cell via specific receptors (11).
The fungal iron transport system has been best studied in the budding yeast Saccharomyces cerevisiae. It is composed of two distinct parts, a reductive pathway and a nonreductive siderophore-dependent pathway. The high-affinity iron permease Ftr1 involved in the reductive pathway (15) has two homologs in C. albicans, encoded by Caftr1 and Caftr2. The permease Caftr1 is required for growth under iron-depleted conditions and, importantly, is required to establish an infection in a systemic-infection mouse model (13). The production of siderophores of the hydroxamate type by C. albicans has also been reported, but their structures are still unknown (7, 16). C. albicans can utilize siderophores that are produced by other microorganisms, and a transporter that mediates the uptake of ferrichrome-type siderophores encoded by the genes Caarn1/Casit1 has been described (1, 5, 6). The expression of both reductive and nonreductive uptake systems is regulated by iron availability. While the expression of these genes is repressed when iron is sufficient, its unavailability will induce their expression.
During microbial infection of a mammalian host, iron availability is lowered and the iron uptake systems of pathogens are induced. The idea of combining an antibiotic with a siderophore to allow drug accumulation in the vicinity of a pathogen or to carry the drug into pathogens through microbial iron uptake systems is an interesting concept. This siderophore-mediated drug delivery (the Trojan Horse approach) has been shown to work in bacteria (9, 14). The recently acquired knowledge on yeast iron transport systems now allows us to investigate the feasibility of the Trojan Horse approach for yeasts.
We show in this paper that this concept is potentially applicable to pathogenic fungi of the genus Candida. We show that the selective activity of the siderophore-drug conjugates on different species depends on their ability to use specific types of siderophores as growth-promoting factors. We also show that the C. albicans ferrichrome receptor CaSit1/CaArn1 may be specifically involved in the activity of hydroxamate siderophore-drug conjugates. This is the first study that associates the selective activity of siderophore-drug conjugates and the specificity of yeast iron transport systems. There is a need to improve drug conjugate designs so that drug activity is not encumbered by the addition of the siderophore moiety.
MATERIALS AND METHODS
Strains and growth conditions.
The Candida species and strains used in this study are listed in Table 1. The reference strains were obtained from the American Type Culture Collection (Manassas, Va.), the genetically defined strains were from Y. Wang (Institute of Molecular and Cell Biology of Singapore) and J. F. Ernst (Institut für Mikrobiologie, Heinrich-Heine-Universität, Düsseldorf, Germany), and the clinical strains were from J. Rex (University of Texas-Houston Medical School, Houston, Tex.). The identity of each of the strains was confirmed by an APIstrip (API 20C AUX, BioMérieux Canada, Inc., St-Laurent, Canada). The growth medium used was YPD (Difco). To generate an iron-limited medium that still allows growth, 100 to 200 μM of the iron chelator 2,2′-dipyridyl (Sigma Chemicals Co.) was added to YPD broth. For testing the growth promotion activity of siderophores, an iron-depleted medium which completely inhibits the growth of the strains was generated by adding 200 μM 2,2′-dipyridyl (220 μM for C. pseudotropicalis) to YPD broth. The MIC of 2,2′-dipyridyl for each Candida strain was determined by varying the concentration of the iron chelator by 10 μM increments over a range from 100 to 250 μM.
TABLE 1.
Yeast strains used in this study
| Strain | Description | Reference |
|---|---|---|
| Candida albicans | ||
| Calb1 | ATCC 10231 | |
| Calb2 | Strain CAI4 (ura3::λimm434/ura3::λimm434) | Fonzi and Irwin (4) |
| Calb3 (Δsit1/arn1) | CAI4 but sit1 Δ::hisG-URA3-hisG/sit1Δ::hisG | Heymann et al. (5) |
| Calb4 (Δftr1Δftr2) | CAI4 but Caftr1::hisG/Caftr1::hisG Caftr2::hisG/Caftr2::hisG | Ramanan and Wang (13) |
| Non-C. albicans Candida spp. | ||
| C. parapsilosis | ATCC 20246 | |
| C. guilliermondii | Clinical strain cg002 | |
| C. krusei | Clinical strain ck001 | |
| C. stellatoidea | Clinical strain cs001 | |
| C. pseudotropicalis | Clinical strain cpt002 |
Siderophores and drug conjugates.
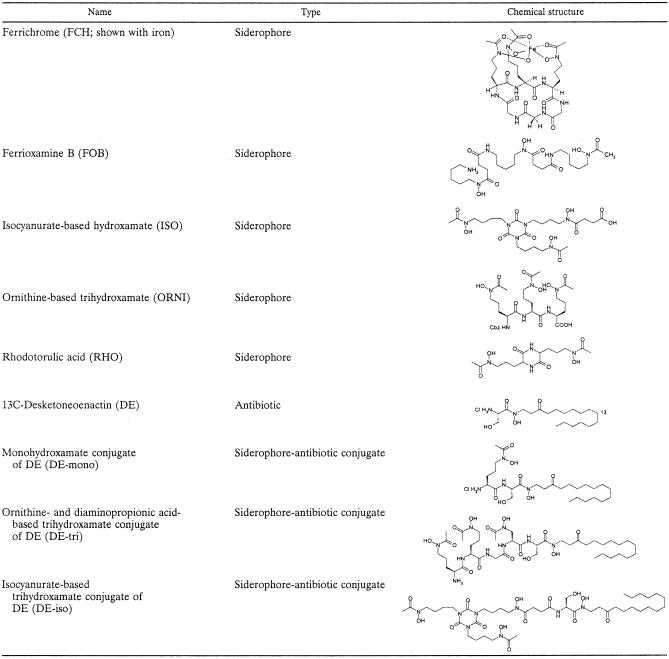
The structures of the siderophores and siderophore-antibiotic conjugates of desketoneoenactin (DE) used in this study are shown in Table 2. Ferrichrome, ferrioxamine B, and rhodotorulic acid were purchased from Sigma Chemical Co. The isocyanurate-based trihydroxamate siderophore (ISO) and the linear tri-δ-N-hydroxy-δ-N-acetyl-l-ornithine-based trihydroxamate siderophore (ORNI) were synthesized at the University of Notre Dame as previously described (3, 8).
TABLE 2.
Structures of antibiotics, siderophores, and siderophore-antibiotics conjugates used in this study
Synthesis of the monohydroxamate conjugate of DE (DE-mono).
To a solution of N5-acetyl-N5-benzyloxy-N2-benzyloxycarbonyl-l-ornithine (0.106 g, 0.26 mmol) in dry dimethylformamide (DMF; 1 ml), prepared as previously described (3), were added benzyloxyneonactin HCl salt (0.130 g, 0.29 mmol), 1-hydroxy-7-azabenzotriazole (HOAt; 0.036 g, 0.26 mmol) and dimethylaminopyridine (DMAP; 0.032 g, 0.26 mmol) at 0°C. The resulting mixture was stirred at room temperature for 4 h and then diluted with CH2Cl2 (50 ml) and water (20 ml). The phases were separated, and the aqueous phase was extracted with CH2Cl2 three times (20 ml). The combined organic phases were washed successively with 10% aqueous citric acid twice (15 ml), water (10 ml), 0.5 M aqueous NaHCO3 twice (15 ml), and brine, and then dried over anhydrous Na2SO4. Filtration followed by removal of the volatiles in vacuo afforded 0.226 g of oily residue, which was purified by flash column chromatography (silica gel, ethyl acetate-hexanes; 2:1) to give 0.096 g (43%) of protected DE-mono as a colorless oil.
Infrared (IR) (undiluted) v: 3408, 3312, 3065, 3034, 2925, 1714, 1652, 1455, 1244 cm−1; 1H nuclear magnetic resonance (NMR) (300 MHz, CD3OD) δ: 7.35 (m, 15 H), 5.08 (bs, 4H), 4.93 (m, 2H), 4.23 (m, 1H), 4.11 (m, 1H), 3.72 (m, 6H), 2.69 (m, 2H), 2.40 (t, J = 7.2 Hz, 2H), 2.04 (s, 3H), 1.80 to 1.60 (m, 4H), 1.45 (quintet, J = 6.9 Hz, 2H), 1.24 (m, 23 H), 0,89 (t, J = 6.6 Hz, 3H); 13C NMR (75 MHz, CD3OD) δ: 211.4, 174.7, 172.5, 158.6, 138.3, 136.7, 135.7, 131.0, 130.8, 130.2, 130.1, 129.8, 129.6, 129.1, 126.9, 77.9, 77.3, 67.8, 62.5, 55.8, 53.7, 45.6, 44.0, 41.8, 40.6, 33.2, 30.94, 30.91, 30.8, 30.7, 30.6, 30.3, 24.8, 24.5, 23.9, 20.7, 14.6; MS (FAB) m/e (rel. int.): 846 [(M+H)+, 40], 828 (3), 755 (6), 613 (8), 484 (73), 362 (100); high-resolution mass spectroscopy (HRMS) (FAB, m/e) calculated for C48H68N4O9 (M)+: 845.5605; found: 845.5033.
Protected DE-mono (85 mg, 0.1 mmol) was dissolved in methanol (4 ml), and the resulting mixture was purged with N2 for 20 min, 10% Pd-C (10 mg) was added, and the resulting mixture was purged with H2 and stirred under a balloon pressure of H2 gas for 6 h. The reaction mixture was then purged with N2, filtered through Celite and concentrated in vacuo to give a colorless oil, which was dissolved in ethyl acetate (6 ml). HCl gas was bubbled through the above solution for 10 min at 0°C. The reaction mixture was then sealed with a rubber septum and stirred at 0°C for an additional 20 min. Removal of the volatiles in vacuo gave a white solid, which was washed with ether to afford 55 mg (96%) of the title compound as a moisture-sensitive white solid.
IR (undiluted) v: 3435, 2924, 1716, 1637, 1467, 1385 cm−1; 1H NMR (500 MHz, CD3OD) δ: 3.90 to 3.60 (m, 7H), 3.49 to 3.44 (m, 1H), 2.78 (m, 1H), 2.50 (m, 1H), 2.18 (s, 3H), 1.71 (m, 3H), 1.55 (m, 3H), 1.40 to 1.25 (m, 20H), 0.90 (t, J = 7.0 Hz, 3H); 13C NMR (125 MHz, CD3OD) δ: 176.6, 173.6, 171.1, 170.5, 63.7, 62.4, 58.7, 55.7, 55.3, 53.8, 49.4, 44.8, 33.1, 33.0, 32.7, 30.78, 30.76, 30.6, 30.5, 30.4, 30.3, 24.8, 24.0, 23.7, 23.4, 20.3, 20.2, 14.5; MS (FAB) m/e (rel. int.): 531 [(M-HCl+H)+, 14], 452 (18), 260 (100), 232 (79), 145 (72), 128 (83); HRMS (FAB, m/e) calculated for C26H50N4O7 (M-HCl+H)+: 531.3758; found: 531.3748.
Synthesis of ornithine- and diaminopropionic acid-based trihydroxamate conjugate of DE (DE-tri).
To a solution of N5-acetyl-N5-benzyloxy-N2-benzyloxycarbonyl-l-ornithinyl-N5-acetyl-N5-benzyloxy-l-ornithinyl-glycinyl-l-3-(N-acetyl-N-benzyloxy)aminoalanine (100 mg, 0.104 mmol), benzyloxyneonactin HCl salt (52 mg, 0.136 mmol), and triethylamine (30 mg, 0.24 mmol) in dry CH2Cl2 (1 ml) as prepared by Ding et al. (2), was added N-ethyl, N′-diethylamino propylcarbodiimide (EDC; 30 mg, 0.156 mmol). The resulting mixture was stirred at room temperature for 12 h and then diluted with ethyl acetate (40 ml) and water (20 ml). The phases were separated, and the aqueous phase was extracted with ethyl acetate (40 ml). The combined organic phases were washed successively with 5% HCl solution (10 ml), 0.5 M aqueous NaHCO3 twice (10 ml), and brine twice (20 ml), then dried over anhydrous Na2SO4.
Filtration followed by removal of the volatiles in vacuo afforded the crude product, which was purified by flash column chromatography (silica gel, CHCl3-MeOH; 25:1) to give 103 g (71%) of protected DE-tri as a white solid: mp 62 to 63°C (CH2Cl2/hexanes). 1H NMR (500 MHz, CD3OD) δ: 8.24 to 8.01 (m, 5H), 7.65 to 7.20 (25H, m), 5.05 to 4.88 (10H, m), 4.35 to 3.52 (18H, m), 2.49 to 2.32 (5H, m), 1.95 to 1.87 (9H, m), 1.80 to 1.20 (29H, m), 0.90 (t, J = 6.5 Hz, 3H); 13C NMR (125 MHz, DMSO) δ: 207.9, 173.1, 171.1, 170.7, 170.3, 169.1, 168.1, 168.0, 136.5, 134.5, 134.4, 129.3, 129.2, 129.0, 128.8, 128.6, 128.4, 128.3, 127.9, 127.8, 127.5, 127.0, 126.9, 90.7, 89.5, 82.7, 82.0, 80.5, 79.5, 79.0, 75.9, 75.8, 75.1, 65.0, 60.6, 51.4, 50.8, 44.6, 44.5, 41.6, 28.4, 28.3, 28.2, 28.0, 22.7, 22.6, 14.3. Anal. (C76H103N9O16) C, H, N.
Protected DE-tri (20 mg, 0.014 mmol) was dissolved in methanol (2 ml), and the resulting mixture was purged with N2 for 20 min, and 10% Pd-C (5 mg) was added. The resulting mixture was purged with H2 and stirred under a balloon pressure of H2 gas for 12 h. The reaction mixture was then purged with N2, filtered through Celite, and concentrated in vacuo to give 12.3 mg (96%) of the title compound as a colorless oil. 1H NMR (600 MHz, CD3OD) δ: 4.45 to 3.30 (m, 18H), 2.89 to 2.40 (m, 4H), 2.25 to 1.21 (m, 37H), 0.89 (t, J = 6.6 Hz, 3H); 13C NMR (150 MHz, CD3OD) δ: 211.6, 175.0, 173.8, 173.7, 171.5, 171.2, 131.0, 129.8, 62.4, 54.3, 53.0, 51.0, 45.0, 43.8, 40.3, 33.2, 31.9, 30.92, 30.91, 30.8, 30.7, 30.6, 30.4, 30.0, 29.7, 24.8, 24.3, 23.8, 20.5, 20.4, 14.6. Anal. (C40H73N9O14) C, H, N.
Synthesis of isocyanurate-based trihydroxamate conjugate of DE (DE-iso).
The synthesis of the side chain differentiated cyanuric acid siderophore platform containing a succinate linker has been described previously (10). The succinate-containing isocyanurate (122.6 mg, 0.145 mmol) was dissolved in acetonitrile (6 ml) and N-hydroxysuccinimide (20 mg, 0.174 mmol) and EDC (31 mg, 0.174 mmol) were added. The solution was stirred for 8 h. The reaction was partitioned between ethyl acetate (30 ml) and water (20 ml). The aqueous layer was extracted with ethyl acetate (3x30 ml) and the combined organic layers were washed with 10% citric acid (20 ml), saturated sodium bicarbonate solution (20 ml), water (20 ml), and dried over sodium sulfate, and filtered, and the solvent was evaporated to provide the N-hydroxysuccinimide active ester. This active ester was dissolved in acetonitrile (6 ml) and benzyloxyneonactin-free amine was added along with DMAP (10 mg). The solution was stirred for 12 h.
The reaction was partitioned between ethyl acetate (30 ml) and water (20 ml). The aqueous layer was extracted with ethyl acetate thrice (30 ml) and the combined organic layers were washed with 10% citric acid (20 ml), saturated sodium bicarbonate solution (20 ml), and water (20 ml). The organic layers were dried over sodium sulfate, filtered, and the solvent was evaporated under reduced pressure. The crude product was purified by column chromatography (1:1 hexanes/ethyl acetate to 10:1 ethyl acetate/methanol) to provide 120 mg of protected DE-iso as a colorless oil in 60% yield: 1H NMR (600 MHz, CDCl3) 7.4 to 7.3 (m, 20H), 5.1 (m, 1H), 4.95 (s, 2H), 4.9 (s, 2H), 4.8 (s, 4H), 4.2 (m, 2H), 3.9 (m, 6H), 3.7 (m, 6H), 3.65 (m, 1H), 3.4 (m, 1H), 2.9 (m, 1H), 2.8 (m, 1H), 2.7 (t, 2H, J = 6.3 Hz), 2.5 (m, 2H), 2.4 (t, 2H, J = 6.3 Hz), 2.0 (s, 6H), 1.6 (m, 12H), 1.2 (m, 22H), 0.9 (t, 3H, J = 6.4 Hz); 13C NMR (150 MHz, CDCl3) δ 173.8, 172.7, 171.2, 149.1, 134.5, 133.8, 129.7, 129.4, 129.3, 129.2, 128.99, 128.96, 76.59, 76.50, 63.0, 52.7, 51.0, 45.0, 44.9, 43.4, 42.73, 42.70, 40.8, 39.5, 32.1, 30.9, 29.86, 29.82, 29.67, 29.60, 29.3, 28.1, 27.6, 25.2, 25.1, 24.3, 24.1, 23.7, 22.9, 20.7, 14.3; IR (undiluted) 3356, 2924, 2849, 1679, 1649, 1456, 1374, 1210 cm−1; FABMS, m/z calcd for C70H98N8O14 [MH]+ 1276, found 1276.
Protected DE-iso (50 mg, 0.039 mmol) was dissolved in methanol (4 ml) and Pd-C (catalytic amount) was added. The reaction was stirred under atmospheric hydrogen for 2 h. The solution was filtered and the solvent was removed under reduced pressure. The title compound was isolated as a colorless oil (34 mg, 99% yield). 1H NMR (600 MHz, CDCl3) δ 5.1 (m, 2H), 3.9 (m, 8H), 3.7 (m, 6H), 3.5 (m, 2H), 2.9 (m, 2H), 2.8 (m, 2H), 2.6 (m, 2H), 2.5 (m, 2H), 2.0 (s, 6H), 1.6 (m, 12H), 1.2 (m, 22H), 0.9 (t, 3H, J = 6.4 Hz); 13C NMR (150 MHz, CDCl3) δ 173.4, 172.5, 171.7, 149.4, 53.8, 47.7, 43.7, 43.2, 42.8, 39.5, 32.1, 29.9, 29.89, 29.87, 29.7, 29.6, 29.5, 29.4, 28.59, 28.57, 24.9, 23.8, 22.9, 20.5, 14.3; IR (undiluted) 3500 to 2500, 2916, 2849, 1687, 1627, 1463, 1374, 1232, 1172 cm−1; FABMS, m/z calculated for C42H74N8O14 [MH]+ 937.7, found 937.7.
Growth promotion assays.
Growth promotion assays were done as follow. The siderophores were diluted to a concentration of 400 μM in 100 μl of YPD broth containing a growth-inhibiting concentration of 2,2′-dipyridyl in the first column of a 96-well microplate and 20 twofold dilutions were made in subsequent wells already containing 50 μl of YPD broth with 2,2′-dipyridyl. The inoculum was prepared by visually adjusting the microbial suspension in saline to a turbidity of a 1.0 McFarland standard. This suspension was diluted 1:100 in YPD broth, yielding an inoculum of ≈104 CFU/ml, and 50 μl was added per well, creating an additional twofold dilution. The final concentration of the siderophores ranged from 200 μM to 0.8 nM. Hemin was also tested in this assay, and ferric chloride was used as a positive control (both products from Sigma Chemical Co.). Incubation was made at 35°C, and the optical density of wells was measured at 595 nm with a PowerWave 200 microplate scanning spectrophotometer and KC4 2.0 software (Bio-Tek Instruments, Inc) after an incubation period of 24 h to evaluate growth.
Susceptibility testing.
Similarly to the growth promotion assay, 20 twofold dilutions of the siderophore-drug conjugates were made in 96-well microplates containing YPD broth (iron-rich condition) or YPD broth supplemented with 2,2′-dipyridyl (iron-low condition). The final concentration of 2,2′-dipyridyl was 100, 150, or 200 μM, and the concentration of the compounds ranged from 200 μM to 0.8 nM. The inoculum was prepared as described above, and plates were incubated for 24 h at 35°C before the optical density of wells was measured at 595 nm. The MIC was defined as the lowest concentration of compound inhibiting at least 80% of the microbial growth observed in wells without drug.
55Fe uptake assays.
Cells from an overnight culture in YPD were used to inoculate fresh YPD broth (iron-rich medium) or YPD broth supplemented with 150 μM of 2,2′-dipyridyl (iron-low medium). The cells were grown for 5 h at 35°C and washed twice with cold phosphate-buffered saline and once with cold 50 mM citrate buffer (pH 6.5). Cells were suspended in 50 mM citrate buffer (pH 6.5) containing 5% (wt/vol) glucose to a final density of ≈2 × 107 cells/ml. The cell suspension was distributed into the wells of a microplate kept on ice (145 μl per well). The 55Fe-labeled compounds were prepared by mixing equimolar quantities of 55FeCl3 (Perkin Elmer) and ferrichrome or siderophore-drug conjugate in 50 mM citrate buffer (pH 6.5). The mixture was allowed to stand at room temperature for 1 h prior to use. Iron was added in the wells as 55Fe-labeled ferrichrome or siderophore-drug conjugate, and the plates were incubated for 60 min at 35°C. Each test was also conducted on ice for 60 min to estimate nonspecific binding of 55Fe to the cell surface. The cells were collected on a GF/C glass microfiber filter (Whatman) with a Minifold I dot-blot system (Schleicher & Schuell) and washed on the filter with 50 mM citrate buffer (pH 6.5) containing 2% EDTA. The iron taken up by the cells was measured by liquid scintillation counting. The specific uptake of 55Fe-labeled siderophores was calculated as counts per minute (cpm) values obtained at 35°C minus background (cpm values obtained on ice).
RESULTS
Growth-promoting activities of natural siderophores and synthetic analogs.
The ability of five sources of iron to reverse the growth inhibition effect of the iron chelator 2,2′-dipyridyl on Candida species was determined in growth promotion assays. The results are presented in Table 3 as the percentage of growth in the presence of 25 μM of siderophore as the sole iron source (or 200 μM for the isocyanurate-based siderophore or rhodotorulate) compared to the control (growth in an iron-rich medium). Although each species seemed to prefer a different set of siderophores, C. albicans, C. pseudotropicalis, and C. stellatoidea, as a whole, were able to use a wider selection of hydroxamate-type siderophores, whereas C. krusei, C. parapsilosis, and C. guilliermondii clearly preferred isocyanurate-based siderophores (Table 3).
TABLE 3.
Growth promotion of Candida species by different hydroxamate siderophores and susceptibility to siderophore-drug conjugates
| Siderophore preference and strain | Growtha of Candida species on:
|
MICb (μM) in iron-rich medium/iron-low medium (ratio) of compound:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| FCH | FOB | ORNI | ISO | RHO | DE | DE-mono | DE-tri | DE-iso | |
| Most hydroxamates | |||||||||
| Calb1 | ++++ | − | − | +++ | − | 0.8/0.2 (4) | 50/3.1 (16) | 25/12.5 (2) | 200/25 (8) |
| Calb2 | ++++ | − | +++ | + | − | 0.8/0.4 (2) | 6.25/1.6 (4) | 25/12.5 (2) | 200/50 (4) |
| C. pseudotropicalis | − | ++++ | +++ | − | − | 1.6/1.6 (1) | 12.5/6.25 (2) | 50/25 (2) | 200/25 (8) |
| C. stellatoidea | + | − | ++ | − | − | 0.8/0.4 (2) | 12.5/6.25 (2) | 50/25 (2) | 200/50 (4) |
| Isocyanurate-based hydroxamate | |||||||||
| C. krusei | − | − | − | ++ | − | 0.2/0.2 (1) | 3.1/3.1 (1) | 25/25 (1) | >200/100 (≥4) |
| C. parapsilosis | − | − | − | ++ | − | 0.2/0.1 (2) | 1.6/1.6 (1) | 12.5/12.5 (1) | 100/50 (2) |
| C. guilliermondii | − | − | + | + | − | 6.25/3.1 (2) | 25/25 (1) | 50/50 (1) | >200/200 (≥2) |
Relative growth in the presence of siderophore (25 μM for ferrichrome [FCH], ferrioxamine B [FOB], and omithine-based trihydroxamate [ORNI] or 200 μM for isocyanurate-based hydroxamate [ISO] and rhodotorulic acid [RHO]) in YPD broth supplemented with 2,2′-dipyridyl compared to growth in YPD broth. −, 0 to 20%; +, 21 to 40%; ++, 41 to 60%; +++, 61 to 80%; ++++, 81 to 100%.
MIC determined in YPD broth (iron-rich medium, first value) and in YPD broth supplemented with 0.15 mM 2,2′-dipyridyl or 0.20 mM for C. pseudotropicalis (iron-low medium, second value).
Of all the siderophores, ferrichrome was the most efficient in promoting the growth of C. albicans in an iron-deficient medium, where it restored up to 95% of microbial growth. The ornithine-based trihydroxamate was the most efficient of the two synthetic hydroxamate siderophores tested, restoring up to 90% of the growth of C. albicans, C. stellatoidea, and C. pseudotropicalis. The synthetic isocyanurate-based siderophore was less efficient in restoring the growth of Candida species in an iron-deficient medium. It took 200 μM of this siderophore to restore the growth of C. albicans (64 and 37% of Calb1 and Calb2 growth in iron-rich medium, respectively), C. krusei (42%), C. parapsilosis (43%), and C. guilliermondii (30%). The dihydroxamate siderophore rhodotorulate did not promote the growth of any of the species tested even at 200 μM. The addition of ferric chloride to the medium restored at least 50% of the growth of all species.
Antifungal activities of the siderophore-drug conjugates.
The inhibitory activities of the siderophore-drug conjugates were measured in both iron-rich and iron-deficient media, and the results are also presented in Table 3. The activity of 13C-desketoneoenactin (DE; MICs, 0.1 to 6.25 μM) (Table 3) was comparable to that measured for amphotericin B, with MICs ranging from 0.2 to 1.6 μM against the different Candida species (data not shown). Addition of a siderophore portion to DE strongly impaired its inhibitory activity. However, iron depletion noticeably improved the MIC of siderophore-DE conjugates against the three species that were able to use a wide variety of hydroxamate-type siderophores (natural or synthetic) in growth promotion assays (C. albicans, C. pseudotropicalis, and C. stellatoidea; Table 3). This was consistent with the idea that iron depletion augments the expression of iron uptake pathways in cells and that siderophore-drug conjugates more efficiently exert their inhibitory effects on cells expressing the appropriate siderophore receptors at their surface. Consequently and inversely, there was no effect of iron depletion on the MICs of DE-mono and DE-tri against species that did not efficiently use most hydroxamate-type siderophores as an iron source (C. krusei, C. parapsilosis, and C. guilliermondii; Table 3). Interestingly, Table 3 also shows that DE itself (i.e., not conjugated) has its own activity improved in iron-low medium. It is noteworthy that DE possesses one hydroxamate group that may contribute to iron binding or recognition by siderophore receptors. This phenomenon was not observed for other nonconjugated drugs such as cilofungin (data not shown).
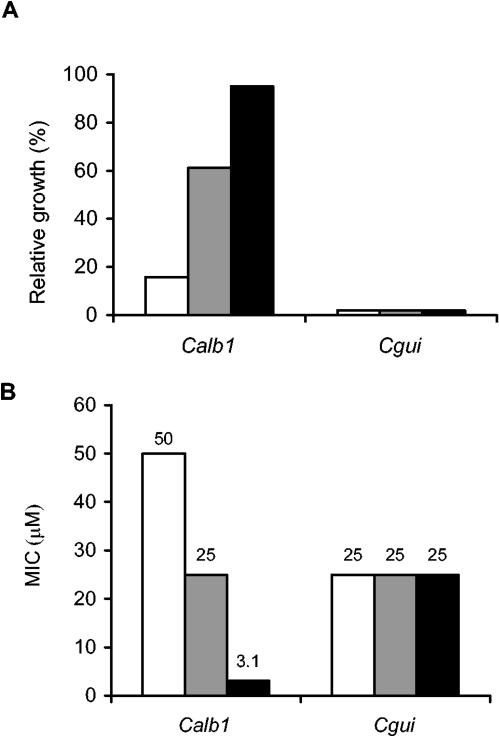
The strongest effect of iron depletion was seen with the monohydroxamate conjugate of 13C-desketoneoenactin (DE-mono), which demonstrated a 16-fold reduction in MIC in iron-deficient medium against C. albicans (Table 3). As seen in Fig. 1, there was an important concentration effect of iron on the inhibitory activity of DE-mono against this species, which can use ferrichrome efficiently for growth promotion. On the other hand, against C. guilliermondii, the growth of which is not promoted by hydroxamate-type siderophores (Fig. 1A), the activity of DE-mono remained constant regardless of the concentration of iron chelator added to the medium (Fig. 1B). The MIC data from Fig. 1 are from a representative experiment: five independent MIC determinations were performed, and the values occasionally differed by one twofold dilution, as expected for the broth microdilution technique.
FIG. 1.
A. Effect of supplemental ferrichrome on growth of Candida species. Relative growth in the presence of ferrichrome (0.5 μM, white bars; 1.5 μM, grey bars; 12.5 μM, black bars) in YPD broth supplemented with 2,2′-dipyridyl compared to growth in YPD broth. B. Effect of iron depletion on the activity of the monohydroxamate conjugate of 13C-desketoneoenactin (DE-mono). MICs were determined in YPD broth (white bars) and in YPD broth supplemented with the iron chelator 2,2′-dipyridyl (0.10 mM, grey bars; 0.15 mM, black bars).
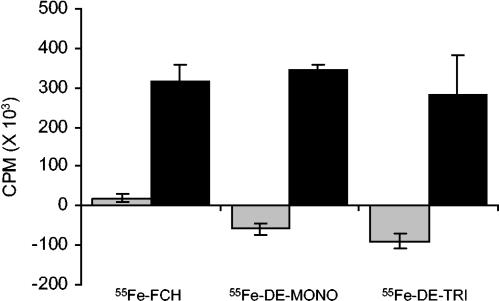
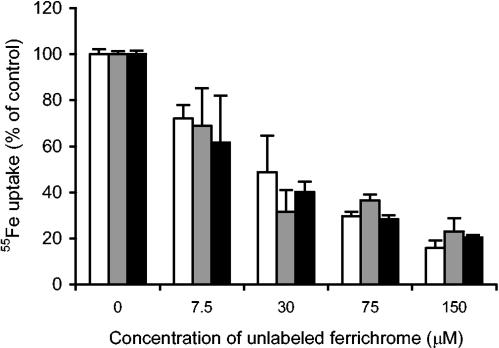
The importance of iron concentration on the inhibitory activity of DE-mono against Calb1 (Fig. 1) most likely represents increased expression of siderophore receptors at the surface of the cells, which in turn increases the association of DE-mono with Candida cells. This view is supported by the results shown in Fig. 2, where the uptake of [55Fe]ferrichrome, [55Fe]DE-mono, and [55Fe]DE-tri by C. albicans was measured. The specific uptake of 55Fe from ferrichrome and the two drug conjugates was significantly increased when cells were grown in iron-low medium compared to that measured with cells grown in iron-rich medium (Fig. 2). This uptake of [55Fe]ferrichrome, [55Fe]DE-mono, and [55Fe]DE-tri was also shown to be reduced in competition with ferrichrome complexed with nonradioactive iron (Fig. 3), demonstrating that DE-mono and DE-tri associate with the cells in the same manner as ferrichrome does.
FIG. 2.
Uptake of 55Fe-labeled compounds. The specific uptake of [55Fe]ferrichrome, [55Fe]DE-mono, and [55Fe]DE-tri by C. albicans Calb1 cells grown in YPD (iron-rich medium; grey bars) or in YPD supplemented with 150 μM of 2,2′-dipyridyl (iron-low medium; black bars) is represented. The data represent uptake measured for 60 min at 35°C minus uptake measured on ice (4°C). Results are presented as mean values ± standard deviation for triplicate independent determinations.
FIG. 3.
Competition of 55Fe-labeled compounds with unlabeled ferrichrome. The uptake of 15 μM [55Fe]ferrichrome (white bars), [55Fe]DE-mono (grey bars), and [55Fe]DE-tri (black bars) in the presence of various concentrations of unlabeled ferrichrome was determined for C. albicans Calb1 cells collected from YPD broth supplemented with 150 μM 2,2′-dipyridyl (iron-low medium). Results are presented as mean values ± standard deviation for triplicate independent determinations.
Growth promotion testing of Candida albicans with defined mutations in the iron transport system.
The ability of two C. albicans iron transport mutant strains to utilize hydroxamate-type siderophores as sources of iron was investigated, and the results are presented in Table 4. As expected, the Δsit1/arn1 strain, which lacks the ferrichrome transporter, was not able to grow when ferrichrome was added as the sole iron source, while its parent strain did grow. The ability of this strain to take up iron from the ornithine-based trihydroxamate siderophore was abolished as well, suggesting that CaSit1/CaArn1 is also implicated in the transport of this synthetic siderophore. Interestingly, the synthetic isocyanurate-based siderophore at 200 μM improved growth of the Δsit1/arn1 strain over that of the parent strain (96% of growth restored for the mutant strain compared to 37% for the parent strain), suggesting that iron uptake from this siderophore does not depend on the siderophore-iron transporter CaSit1/CaArn1.
TABLE 4.
Growth promotion and susceptibility to siderophore-drug conjugates of Candida albicans and iron transport-defective mutants
| Siderophore or iron source | Growtha
|
Compounds | MICb (μM)
|
|||
|---|---|---|---|---|---|---|
| Calb2 (parent) | Calb3 (Δsitl/arn1) | Calb4 (Δftr1 Δftr2) | Calb2 (parent) | Calb3 (Δsitl/arn1) | ||
| Ferrichrome | ++++ | − | ++++ | DE-mono | 1.6 | 12.5 |
| Ornithine-based trihydroxamate | +++ | − | ++++ | DE-tri | 12.5 | 25 |
| Isocyanurate-based hydroxamate | + | ++++ | − | DE-iso | 50 | 100 |
| Hemin | +++ | ++++ | ++++ | |||
| Ferric chloride | ++++ | ++++ | − | |||
See Table 3, footnote a.
MIC determined in YPD broth supplemented with 0.15 mM 2,2′-dipyridyl (iron-low medium).
The Δsit1/arn1 mutant strain used free iron (ferric chloride) as well as its isogenic parent strain, whereas the C. albicans Δftr1Δ ftr2 strain lacking the two high-affinity permeases of the reductive system showed a reduced ability to take up free iron. It took 8-fold more supplemental iron to fully restore the growth of the Δftr1Δftr2 mutant strain to the level of its parental strain (100 μM versus 12.5 μM FeCl3, respectively, data not shown). The growth of the Δftr1Δftr2 mutant strain was still promoted by ferrichrome and the ornithine-based trihydroxamate but not by the isocyanurate-based siderophore. This result supports the idea that iron uptake from the isocyanurate-based siderophore is independent of the siderophore-iron transporter CaSit1/CaArn1, as stated above, while acquisition of iron from the isocyanurate-based siderophore may require the high-affinity permeases of the reductive system that are absent in the Δftr1Δftr2 mutant strain. Hemin, used as an independent control, could also restore the growth of the Δftr1Δftr2 mutant strain, as it did with the Δsit1/arn1 mutant strain. This result is consistent with previous studies that suggested a distinct uptake pathway for hemin (5, 18).
Susceptibility of Candida albicans Δsit1/arn1 mutant strain to siderophore-drug conjugates.
As seen in Table 4, the activity of the siderophore drug conjugates was best against the parent strain Calb2, a strain that used both ferrichrome and the synthetic trihydroxamate siderophores for growth promotion, whereas the MICs of the conjugates were higher against the C. albicans Δsit1/arn1 mutant strain (strain Calb3). This strain lacks the siderophore transporter CaSit1/CaArn1, and its growth was not promoted by either ferrichrome or the synthetic ornithine-based trihydroxamate. Once again, the growth promotion and drug conjugate susceptibility results, this time with a defined Δsit1/arn1 mutant, support the idea that the activity of the hydroxamate-drug conjugates is associated with the function of compatible siderophore receptors at the surface of Candida cells. The relatively low activity of DE-iso against the mutant Δsit1/arn1 that used the isocyanurate-based siderophore more efficiently for growth promotion was not further improved compared to the parent strain. As mentioned before, this may indicate that iron is differently acquired from the isocyanurate-based siderophore and DE-iso. For instance, it was shown that the high-affinity iron transport system involving CaFtr1/CaFtr2 can take up iron from ferrioxamine B without internalization of the siderophore (1, 6).
DISCUSSION
In the present study, synthetic siderophores were attached to the antifungal drug 13C-desketoneoenactin (DE) and the inhibitory activities of these new conjugated compounds against Candida cells were investigated. Although the activity of DE was reduced by conjugation to siderophores, the conjugates' activities were clearly associated with the ability of Candida species and mutants to use the siderophore portion of the conjugates as a source of iron (Tables 3 and 4).
We have more precisely demonstrated that the activity of the monohydroxamate conjugate of 13C-desketoneoenactin (DE-mono) was inversely proportional to the iron content of the medium, the activity being best when the iron content was low (Fig. 1). We know that C. albicans cells grown under iron-rich conditions do not fully express the ferrichrome receptor Casit1/Caarn1 gene, whereas expression of this gene is induced under low-iron conditions (6). It is reasonable to conclude that the improved activity of our siderophore-drug conjugates in iron-depleted conditions may be due to a higher level of expression of the siderophore receptor involved. This idea is supported by the fact that uptake of iron from [55Fe]ferrichrome, [55Fe]DE-mono, and [55Fe]DE-tri was improved when cells were first grown in a low-iron medium (Fig. 2). The improved activity of conjugates in low-iron medium was best observed for Candida species whose growth is strongly promoted by most of the hydroxamate-type siderophores (Table 3). This may indicate that these species possess a receptor that is able to recognize both the synthetic siderophore alone and a similar hydroxamate siderophore conjugated to a drug.
The results on the inhibitory activity of the DE-mono conjugate against the C. albicans Δsit1/arn1 mutant strain also supported this hypothesis (Table 4). We have shown that in low-iron medium, the parent strain is more susceptible to the siderophore-drug conjugate than the Δsit1/arn1 mutant strain, suggesting that the parental CaSit1/CaArn1 protein can serve as a receptor for hydroxamate siderophores as well as for hydroxamate-drug conjugates. The fact that unlabeled ferrichrome competed with the uptake of [55Fe]ferrichrome as well as the uptake of [55Fe]DE-mono and [55Fe]DE-tri (Fig. 3) also supports the hypothesis of a common receptor.
The monohydroxamate conjugate of 13C-desketoneoenactin (DE-mono) was the most active of the conjugates and was the most influenced by the presence of iron when tested against strain Calb1 (Table 3 and Fig. 1). Since the drug 13C-desketoneoenactin already possesses a hydroxamate group, it is possible that DE-mono adopts a conformation that is more efficient than that possible for DE-tri for iron chelation and cell receptor recognition. It would be interesting to design and test the activity of a 13C-desketoneoenactin conjugate showing two additional hydroxamate groups, yielding a true trihydroxamate molecule and hexadentate ligand to verify such a hypothesis.
The observation that the inhibitory activities of the siderophore drug conjugates were reduced compared to that of the drug alone may be explained in two ways. One possibility is that conjugation of the drug may alter the ability of the siderophore to be recognized by the appropriate cell receptor, therefore lowering the amount of drug that comes into contact with cells. Another possibility is that release of the drug from the conjugate may be necessary for optimal interaction with the drug target. Therefore, even if the siderophore-drug conjugate is properly recognized at the cell surface, the activity of the conjugate would be lower than that of the drug alone if release of the drug is not optimal. We are currently working on the mode of conjugation (types of drug-siderophore linkers) to allow release of the drug upon interaction with the microorganism.
The results presented here showed that both synthetic siderophores and siderophore-drug conjugates can be specifically recognized by some Candida species. We can speculate that the ability of conjugates to perform well in an iron-deficient environment similar to that found at the mucosal surface of the mammalian host (17) and the ability of the conjugates to associate with Candida cells through high-affinity iron-transport receptors may represent valuable properties of antimicrobial molecules during therapy. This is supported by the results of Heymann et al. (5) showing that the ferrichrome receptor CaSit1/CaArn1 was required for epithelial invasion and by the finding that high-affinity transport systems have been shown to transport specific substrates more efficiently into cells than simple diffusion when the concentration of the substrate outside the microbial cells is low (12), a situation that may occur between doses of antibiotics in a therapeutic regimen.
Further studies on the proteins implicated in the iron transport systems of C. albicans and non-C. albicans species will help us to understand the selectivity of the inhibitory activities seen for the different drug conjugates. This is the first report that describes the relationship between the inhibitory activities of siderophore-drug conjugates and the iron transport systems of yeasts. It is now possible to envisage the synthesis of new antifungal agents that add siderophores to drugs that do not readily penetrate yeast cells or that are readily pumped out by efflux mechanisms to tackle the problem of drug resistance in pathogenic Candida species. It might also be possible to reduce the relative mammalian cell toxicity of some currently used antifungal agents by conjugation to siderophores to increase their specificity for the cell surface of fungi and yeasts.
Acknowledgments
This work was supported in part by grant 89758-01 from the Natural Sciences and Engineering Research Council of Canada to F.M. The synthetic studies at the University of Notre Dame were supported by the NIH.
REFERENCES
- 1.Ardon, O., H. Bussey, C. Philpott, D. M. Ward, S. Davis-Kaplan, S. Verroneau, B. Jiang, and J. Kaplan. 2001. Identification of a Candida albicans ferrichrome transporter and its characterization by expression in Saccharomyces cerevisiae. J. Biol. Chem. 276:43049-43055. [DOI] [PubMed] [Google Scholar]
- 2.Ding, P., N. M. Niyaz, C. E. Schous, A. Sheinkman, and M. J. Miller. Design and synthesis of a novel siderophore analog for applications in iron transport-mediated drug delivery and syntheses of potent antifungal drug conjugates. Manuscript in preparation.
- 3.Dolence, E. K., C.-E. Lin, M. J. Miller, and S. M. J. Payne. 1991. Synthesis and siderophore activity of albomycin-like peptides derived fron N5-acetyl-N5-hydroxy-l-ornithine. J. Med. Chem. 34:956-968. [DOI] [PubMed] [Google Scholar]
- 4.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heymann, P., M. Gerads, M. Schaller, F. Dromer, G. Winkelmann, and J. F. Ernst. 2002. The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect. Immun. 70:5246-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu, C. J., C. Bai, X. D. Zheng, Y. M. Wang, and Y. Wang. 2002. Characterization and functional analysis of the siderophore-iron transporter CaArn1p in Candida albicans. J. Biol. Chem. 277:30598-30605. [DOI] [PubMed] [Google Scholar]
- 7.Ismail, A., G. W. Bedell, and D. M. Lupan. 1985. Siderophore production by the pathogenic yeast, Candida albicans. Biochem. Biophys. Res. Commun. 130:885-891. [DOI] [PubMed] [Google Scholar]
- 8.Lee, B. H., M. J. Miller, C. A. Prody, and J. B. Neilands. 1985. Artificial siderophores. 2. Syntheses of trihydroxamate analogues of rhodotorulic acid and their biological iron transport capabilities in Escherichia coli. J. Med. Chem. 28:323-327. [DOI] [PubMed] [Google Scholar]
- 9.Miller, M. J., and F. Malouin. 1994. Siderophore-mediated drug delivery: the design, synthesis, and study of siderophore-antibiotic and antifungal conjugates, p. 275-306. In R. Bergeron (ed.), Microbial iron chelates. CRC Press, Boca Raton, Fla.
- 10.Murray, A. P., and M. J. Miller. 2003. The Preparation of a fully differentiated “multiwarhead” siderophore precursor. J. Org. Chem. 68:191-194. [DOI] [PubMed] [Google Scholar]
- 11.Neilands, J. B. 1995. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 270:26723-26726. [DOI] [PubMed] [Google Scholar]
- 12.Nikaido, H. 1994. Porins and specific diffusion channels in bacterial outer membranes. J. Biol. Chem. 269:3905-3908. [PubMed] [Google Scholar]
- 13.Ramanan, N., and Y. Wang. 2000. A high-affinity iron permease essential for Candida albicans virulence. Science 288:1062-1064. [DOI] [PubMed] [Google Scholar]
- 14.Roosenberg, J. M., Y. M. Lin, Y. Lu, and M. J. Miller. 2000. Studies and syntheses of siderophores, microbial iron chelators, and analogs as potential drug delivery agents. Curr. Med. Chem. 7:159-197. [DOI] [PubMed] [Google Scholar]
- 15.Stearman, R., D. S. Yuan, Y. Yamaguchi-Iwai, R. D. Klausner, and A. Dancis. 1996. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 271:1552-1557. [DOI] [PubMed] [Google Scholar]
- 16.Sweet, S. P., and L. J. Douglas. 1991. Effect of iron concentration on siderophore synthesis and pigment production by Candida albicans. FEMS Microbiol. Lett. 64:87-91. [DOI] [PubMed] [Google Scholar]
- 17.Ward, P. P., and O. M. Conneely. 2004. Lactoferrin: Role in iron homeostasis and host defense against microbial infection. BioMetals 17:203-208. [DOI] [PubMed] [Google Scholar]
- 18.Weissman, Z., R. Shemer, and D. Kornitzer. 2002. Deletion of the copper transporter CaCCC2 reveals two distinct pathways for iron acquisition in Candida albicans. Mol. Microbiol. 44:1551-1560. [DOI] [PubMed] [Google Scholar]