Abstract
Introduction: Progress in diagnosis and treatment of patients with intoxication-type inborn errors of metabolism (IT-IEM) such as urea cycle disorders, organic acidurias or maple syrup urine disease is resulting in a growing number of long-term survivors. Consequently, health-related quality of life (HrQoL) of patients is increasingly regarded as a meaningful outcome parameter. To develop the first validated, disease-specific HrQoL questionnaire for IT-IEM, patients and parents were interviewed as content experts to identify major physical and psychosocial constraints and resources.
Methods: Focus group interviews with 19 paediatric IT-IEM patients and 26 parents were conducted in four metabolic centres in Austria, Germany and Switzerland. Disease-specific HrQoL categories were established by qualitative content analysis.
Results: Fourteen disease-specific topics related to the three well-established generic HrQoL dimensions of physical, mental and social functioning were derived from the interview transcripts. Both patients and parents perceived dietary restrictions and social stigmatisation as major burdens. Dietary restrictions and emotional burdens were more important for young (<8 years) patients, whereas cognition, fatigue and social issues were more relevant to older patients (≥8 years). Treatment-related topics had a significant effect on social and emotional HrQoL.
Discussion: By exploring patients’ and parents’ perspectives, 14 HrQoL categories were identified. These new categories will allow the development of a disease-specific, standardised questionnaire to assess HrQoL in paediatric IT-IEM patients. Age-appropriate information on the disease and psychosocial support targeted to patients’ individual burdens are essential to the delivery of personalised care that takes account of physical, mental and social dimensions of HrQoL.
Introduction
Urea cycle disorders (UCD), organic acidurias (OA) and maple syrup urine disease (MSUD) are intoxication-type inborn errors of metabolism (IT-IEM) sharing main clinical and treatment characteristics. Patients must follow a low-protein diet, have to cope with the constant fear of life-threatening metabolic crises and frequently develop neurocognitive impairments.
Considerable progress in recent decades in the diagnosis and treatment of IT-IEM has resulted in a growing number of long-term survivors (de Baulny et al. 2005; Batshaw et al. 2014). The long-term medical care and support of patients and families requires insight into subjective psychosocial conditions and health-related quality of life (HrQoL) (Bullinger 2002). HrQoL is a multidimensional construct that represents “a patient’s perception of the impact of disease and treatment on functioning in a variety of dimensions, including physical, psychological and social domains” (Varni et al. 1999, p. 126). As such, it is a meaningful outcome parameter for clinical trials and the evaluation of the quality and cost-effectiveness of treatment (Bullinger 2002). Three general types of HrQoL assessment measures exist. Generic HrQoL instruments (e.g. the PedsQL (Varni et al. 1999)) compare HrQoL between healthy individuals and patients, chronic generic instruments (e.g. DISABKIDS (The DISABKIDS Group Europe 2006)) serve to assess and compare HrQoL in patients with chronic diseases in general and disease-specific instruments (e.g. the PKU-QOL (Regnault et al. 2015)) address the characteristics of a particular disease or disease group. The latter are therefore the method of choice in clinical trials (Walterfang et al. 2013). Since the concept of HrQoL is based on subjective experience, self-assessment using age-appropriate instruments is the gold standard (Matza et al. 2013). Self and proxy reports are not interchangeable (Upton et al. 2008), but proxy reports are valuable in very young or cognitively impaired patients.
Although interest in psychosocial issues in IT-IEM has recently increased, research is still sparse and has methodological shortcomings (Zeltner et al. 2014). The first disease-specific assessment instrument for individuals with IT-IEM is currently under development by our research group. In this process, patients and parents are involved as “content experts” (Matza et al. 2004). Focus group interviews are the method of choice to identify topics, concerns and resources relevant in everyday life (Matza et al. 2013). This paper presents the qualitative content analysis of focus group and single interviews with IT-IEM patients and their parents. The two main aims of this study were (1) to identify HrQoL topics relevant for paediatric IT-IEM patients and (2) to investigate differences in statement frequencies of topics related to the informant (patient or parent) and to the age of the affected child.
Methods
Subject Recruitment
Physicians from four metabolic centres in Austria, Germany and Switzerland invited IT-IEM patients (≤18 years) and their parents by phone or during routine consultations for participation. If patients were younger than 8 years or unable to participate due to neurocognitive impairment, only their parents were invited.
Focus Groups and Single Interviews
Focus group interviews were conducted by a team of trained moderators with medical or psychological background in each centre. For patients and parents unable to attend, single interviews were arranged either in the hospital, by phone or at home. Focus groups of two to seven participants were composed based on the patient’s age (<8 years, 8–12 years, 13–18 years). Patients younger than 8 years were represented by their parents. Parallel patients’ and parents’ focus groups were conducted for 8–12- and 13–18-year-olds. Each group was led by two trained moderators, who followed a manual specifically adapted for IT-IEM and based on the DISABKIDS methodology (The DISABKIDS Group Europe 2006). Single interviews were conducted by one moderator following the manual. The focus group procedure is presented diagrammatically in Fig. 1.
Fig. 1.
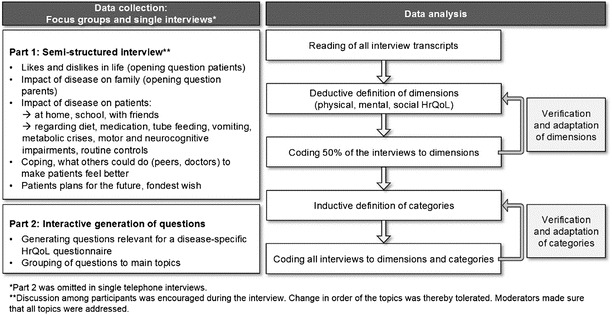
Content of interviews based on the developed manual and content analysis based on Mayring (2010) and Kuckartz (2014)
Data Processing and Analysis
Interviews were transcribed verbatim in German from audio recordings using transcription software (f4, 2014, Dr. Dresing & Pehl GmbH, Marburg, Germany). Statements used in the result section of this paper were translated to English by a professional interpreter. Two coders (N.A.Z., S.L.) analysed the interviews according to the established qualitative data analysis procedure developed by Mayring (Mayring 2010; Kuckartz 2014) using the MAXQDA software (MAXQDA, version 11, 1989–2015, VERBI Software – Consult – Sozialforschung GmbH, Berlin, Germany).
Statements identified in the transcripts were assigned to the three HrQoL core dimensions physical, mental and social HrQoL (World Health Organisation 1947). Based on these core dimensions, categories were inductively defined. The system of categories was elaborated in several analytical cycles (Fig. 1). Coding disagreements were resolved by discussion. Two psychologists not otherwise involved in the study assigned 42 randomly chosen statements to the 14 categories. Inter-rater reliability (Cohen 1988) was “almost perfect” for one (Cohen’s kappa = 0.94) and “substantial” for the second rater (Cohen’s kappa = 0.79) (Landis and Koch 1977).
Educational status of parents was assessed using the ISCED Manual (OECD 1999) which differentiates seven internationally comparable levels. Sex distribution among age groups was examined by fisher’s exact tests. Chi-square tests and standardised residuals were used to detect differences of statement frequencies per category between informant groups (patients/parents) and patients’ age groups. Issues mentioned repeatedly by single participants resulted in multiple coded statements; length of statements was not considered. Stepwise comparisons were calculated between core dimensions, grouped categories and single categories (see Table 2). Analyses were performed using the statistical software package SPSS, version 20.0 for Windows (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as p < 0.05.
Table 2.
System of disease-specific HrQoL categories based on content analysis of the interviews
| Dimension | Group | Category | Description of category |
|---|---|---|---|
| Physical | Symptoms | Metabolic crises/anticipation of crises | Experiencing metabolic crises, anticipation of crises (i.e. infections, hygiene), emergency admissions because of crisis |
| Physical limitations | Motor limitations, organ problems | ||
| Fatigue/nausea/vomiting | Fatigue, nausea and vomiting (events not immediately connected to metabolic crisis and emergency admissions) | ||
| Treatment | Dietary restrictions | Dietary restrictions, regular food intake, low-protein food | |
| Medication/dietetics | Drug and dietetics intake, side effects | ||
| Tube feeding | PEG tube, nasogastric tube | ||
| Routine controls/hospital | Routine controls in the hospital/by the physician, reactions to blood sampling | ||
| Mental | Emotions | Negative emotions | Statements with negative emotional content. Sadness, anger, crying, feeling different than friends, shame (not wanting to make the condition public), wanting the disease gone |
| Positive emotions | Statements with positive emotional content. Being happy, satisfied, feeling good and “normal” with the condition | ||
| Cognitive functioning | Cognitive functioning | Cognitive functioning, especially in school, disturbed concentration, mental disability | |
| Independent living | Independent living | Feeling restricted in conduct of life, choice of employment, planning of leisure time | |
| Social | Social life, social support | Friends | Having friends, social support/social inclusion by friends |
| Family | Statements concerning the family (parents, siblings, grandparents, uncles, aunts) and their handling of the condition | ||
| Stigmatisation/exclusion | Stigmatisation/exclusion | Being treated differently (negative/positive) by peers and society based on the condition: teasing, interrogation regarding condition, being pitied, exclusion (i.e. difficulties finding a school, going on school excursions) |
Results
Sample Characteristics
Thirty (71%) of 42 invited families participated, resulting in a study sample of 19 children and adolescents (n = 9 females, n = 10 males; n = 9 OA, n = 9 UCD, n = 1 MSUD; age range 9.5–16.5 years, mean = 13.0 ± 2.3 years) and 26 parents (n = 25 mothers, n = 1 father). All parents had at least one child affected by IT-IEM (n = 12 females, n = 16 males (from which n = 2 siblings); n = 18 OA, n = 8 UCD; patients’ age range 0.9–16.8 years, mean = 10.3 ± 4.7 years) (see Table 1). Educational status of parents (mean of fathers’ and mothers’ educational status) was mean = 3.64 (±1.12, range 2–6). Distribution of patients’ sex among age groups did not significantly differ for patients (p = 0.18) or parents (p = 0.09).
Table 1.
Number of interview participants in focus groups and single interviews
| Patient’s age | ||||
|---|---|---|---|---|
| <8 years | 8–12 years | 13–18 years | All age groups | |
| Number of patients | – | 9 | 10 | 19 |
| Number of parents | 9 | 9 | 8 | 26 |
| Number of patients in focus group interviews | – | 6 | 8 | 14 |
| Number of patients in single interviews | – | 3 | 2 | 5 |
| Number of parents in focus group interviews | 8 | 8 | 7 | 23 |
| Number of parents in single interviews | 1 | 1 | 1 | 3 |
| Total participants | 9 | 18 | 18 | 45 |
Categorical System
A total of 915 statements addressing patients’ HrQoL were identified from 19 transcripts of five patient and six parent focus groups and five patient and three parent single interviews. Fourteen content categories were defined and allocated to one of the core dimensions (physical, mental and social HrQoL) (Table 2). Frequencies of statements per category for patients and parents are shown in Fig. 2.
Fig. 2.
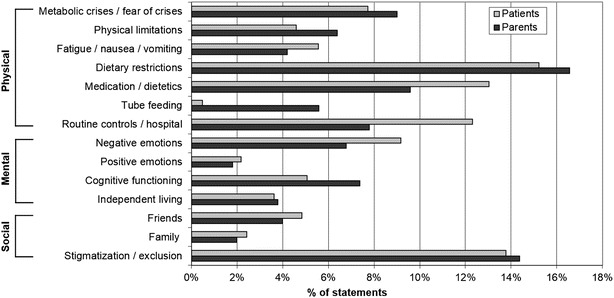
Percentage of statements from total statements per category, for each patient and parent interviews (both for the age of 8–18 years)
Physical Dimension of HrQoL
Symptoms and treatment of the disease relate to the physical dimension of HrQoL. Dietary restrictions (intake of regular food and special low-protein food) were a main topic for both patients and parents (Fig. 2). However, attitudes varied widely from “being used to dietary restrictions” or even having an “aversion to proteins” to complaints about “missing forbidden food and freedom of food choice” and “required organisational effort”. The latter were especially encountered in social situations such as barbecues or school camps. A 16-year-old girl commented: “When I go to school camp, I have to take a second suitcase along. It’s all food. And a woman cooks especially for me”.
According to parents, pre-schoolers did not experience diet as particularly burdensome. In contrast, parents experienced caring for a young child’s diet as most difficult and stressful. They felt disburdened when their child was able to take over responsibility by self-monitoring or indicating need for their sick-day regimen. The mother of a 5-year-old girl stated: “When she was two or three years old, she would often snatch a piece of sausage and put it into her mouth (…). By now I know that she won’t do this anymore, that’s something I can rely on”. However, diet places an incessant strain on family life. Parents felt children used food intake or vomiting to exert pressure or gain attention, as explained by the mother of a 16-year-old: “That’s always some form of pressure of hers, although she often isn’t able to express herself that well; but with food she’s totally in control of me and that she knows very well”. Against this background, parents considered tube feeding supportive, since arguing about food intake became less central: “As soon as he had received the PEG, we were happy. We were no longer at the hospital, the pressure on me was gone, I was no longer under pressure” (mother of a 13-year-old). In contrast, patients may hide their tube: “Absolutely nobody is allowed to see it [the tube] (…) and nobody is allowed to know about it, except close relatives and friends” (mother of a 10-year-old).
Although intake of dietetics and medication was a daily routine, the unpleasant taste of amino acid mixtures remained a major issue, and patients preferred pills over drinks whenever possible. Moreover, the lack of flexibility allowed by a tightly scheduled intake of medication and supplement interfered with everyday life and spontaneous engagement in social situations. Side effects such as diarrhoea or body odour, namely, the fishy odour of carnitine, were additional constraints in social situations.
The majority of patients felt blood sampling (category routine controls/hospital) to be extremely stressful and commented more frequently on this subject than parents (not statistically significant). Adolescents coped better with blood sampling but complained that hospital appointments reduced their leisure time.
Motor deficits and limitations in keeping up with peers were the most burdensome limitations for children with IT-IEM. One of the fondest wishes expressed by non-ambulatory children younger than 8 years was “being able to walk and run like others”. Fatigue/nausea/vomiting were especially important for older patients comparing their performance with healthy peers’. “Because when I overdo it, I have to throw up. I simply don’t manage as much as all the others” (16-year-old male patient working as apprentice).
Metabolic crises were traumatising experiences and caused constant fear of potential new crises, infections and other triggers. Most patients had experienced metabolic crises during infancy, and it was mainly their parents who remembered the details, as the father of a 7-year-old stated: “We had five crises with him, ended up five times in intensive care. Once we were abroad and the physicians said: You know, we can’t do anything, the boy will die”. The mother of a 16-year-old said: “Gastro-intestinal flu: all alarm bells ring, (…) this is a one-way ticket to hospital”. Parents and patients reported always planning their annual leave in proximity to metabolic hospitals they trust to avoid encountering medical staff unfamiliar with the disease.
Comparing Frequencies of Statements
The frequency distribution of patients’ and parents’ statements significantly differed among the categories associated with treatment (χ 2(3) = 25.24, p < 0.05). Patients mentioned tube feeding significantly less frequently than parents.
Comparison of patients’ reports revealed a significant age difference between symptom and treatment statements (χ 2(2) = 11.44, p < 0.05). Patients aged 8 to 12 made more statements about treatment, while 13–18-year-olds talked more frequently about symptoms and limitations. Comparing categories describing symptoms revealed that, in contrast to parents of older children, parents of patients younger than 8 years made no statements related to fatigue/nausea/vomiting (χ 2(4) = 15.18, p < 0.05). In the categories describing treatment, parents of younger children talked more frequently about dietary restrictions and less frequently about medication/dietetics than parents of older children and adolescents (χ 2(6) = 22.19, p < 0.05).
All other comparisons in the physical dimension between different age and respondent groups were not significant.
Mental Dimension of HrQoL
The mental dimension encompasses statements related to emotions, cognition and issues of independent living. Negative emotions were predominantly reported. The most frequent “fondest wish” of the patients was “not to have the disease” or to be “more like normal kids”. The perception of not being able “to do what other kids do” caused frustration. The impression of being “different” in some cases led to feelings of shame, making patients averse to others being informed about their condition. Since having a meal together is of high social significance, IT-IEM patients often felt socially excluded. The mother of a 7-year-old reported: “The kids [at Kindergarten] were not allowed to share [food] with him. This troubled him a lot, the fact that he now suddenly did not belong to the group. He cried bitterly then”.
Cognitive functioning and school performance gained increasing significance for older children and adolescents. Children attending regular schools felt inferior when comparing their school performance with those of their healthy peers. Parents of children with severe cognitive impairment attending special schools worried about their children’s performance but had the impression that the children themselves did not.
Comparing Frequencies of Statements
Parents’ statements regarding emotions, cognitive functioning and independent living significantly differed between age groups (χ 2(2) = 29.70, p < 0.05). In the younger age groups, more statements addressed emotions, while cognitive functioning was more important to the 13–18 years group.
All other comparisons in the mental dimension were not significant.
Social Dimensions of HrQoL
The social dimension refers to the impact of the disease on social life and stigmatisation experiences. Patients and parents generally felt supported by family and close friends. The second highest number of statements was attributed to the social stigmatisation/exclusion category (Fig. 2).
Peers teased patients because of physical or cognitive limitations or side effects of medication. A 9-year-old boy reported: “Actually, my disease is okay, except that some kids make fun of it (…). The medication smells of fish, so they call me fishhead and say that I stink of fish”.
Patients of all age groups considered it difficult to explain their condition in a comprehensible way. “Well, my problem is simply that they keep asking: Why do you have that thing? And I don’t know exactly what I’m supposed to answer” (10-year-old patient). Because of her strict diet, a 16-year-old girl was referred to as “the pickiest person we know” by her schoolmates. “I try to explain to them what it is about. But some of them just don’t want to understand”. Like their children, parents considered it almost impossible to explain the complex, rare disease to others in an understandable way. They felt frustrated because they perceived that their environment had no understanding of their child’s condition. Criticism of or interference with parenting style (e.g. comments that mothers were “too strict” about diet or offers of inappropriate meals) was experienced as stressful. Attempts to avoid social exclusion, for instance, from school camps, exerted intense stress on families (escorting the child, precooking meals). Finding a sensitive social environment was considered of the utmost importance.
Comparing Frequencies of Statements
Compared to 8–12-year-old patients, 13–18-year-old patients commented less on social life and social support (χ 2(1) = 11.53, p < 0.05).
All other comparisons in the social dimension were not significant.
Discussion
This study explored how IT-IEM affects daily life and identified 14 categories relevant for patients’ HrQoL. The evolving categories could coherently be allocated to the core dimensions of physical, mental and social HrQoL, resulting in a structure consistent with well-established generic and chronic disease HrQoL questionnaires (Varni et al. 1999; The DISABKIDS Group Europe 2006; The KIDSCREEN Group Europe 2006). Categories within the social and emotional dimensions are similar to other questionnaires (Vogels et al. 1999; The KIDSCREEN Group Europe 2006), while categories within the physical dimension mostly reflect disease-specific issues. Notably, other questionnaires refer to more than three dimensions (e.g. Varni et al. 1999), while this study kept to the three core dimensions represented in almost all HrQoL questionnaires (Rajmil et al. 2004).
It is an important strength of this study that the individual perspectives of patients were considered and that the proxy reports from parents allowed insight into perspectives of young and/or cognitively impaired patients. Despite international cooperation, owing to the rarity of the diseases, the sample size is limited and patient recruitment was not free of selection bias.
Analyses of group differences demonstrated that the importance of specific HrQoL-related topics varied depending on the patients’ age and between parents and patients. Diet and emotional contents were predominant when patients were young (<8 years), and both patients and parents had to build up knowledge and routine concerning food choice and preparation and intake of medication and dietetics. Furthermore, hospital stays were emotionally burdensome for young children, and families had to deal with uncertainty related to disease progression, neurocognitive development and upcoming metabolic crises. Dealing with diet and uncertainty has been reported to be major burdens for parents with children with metabolic diseases before (Cederbaum et al. 2001; Pelentsov et al. 2015). As coping strategies significantly influence HrQoL in different chronic diseases (Graven and Grant 2013), tailored support may be helpful. Our results suggest that families with young children in particular would benefit from support targeting these uncertainties and coping with diagnosis and treatment. In addition, immediately at diagnosis, patients should be informed about support groups, which have been shown to be important informational resources in rare disorders (Hall 2013; Khangura et al. 2015). Accordingly, the majority of participants in this study greatly appreciated the exchange with others in the context of the interviews and expressed their wish to have this opportunity more frequently. Unfortunately, research shows that few families with children with rare diseases receive psychosocial support at the time of diagnosis (Anderson et al. 2013).
In this study, cognitive functioning and feeling fatigue or less productive gained significance for school-aged and adolescent patients, along with the increasing importance of school performance and social comparison with peers. Importantly, adolescents reported less frequently about social support than younger patients but still encountered experiences of stigmatisation, resulting in an imbalance of constraints and resources. Our results thereby suggest that transitional phases (to school, to adolescence), often combined with social and physical challenges, deserve special attention, as this has been reported before for other inborn errors of metabolism (Packman et al. 2012; Khangura et al. 2015). Sharing meals with others is known to have high social significance; hence, patients living with dietary restrictions experience numerous potentially stigmatising situations (Diesen et al. 2014). Since perceived stigmatisation is a predictor for poorer psychological adjustment (Masnari et al. 2013), it is of great importance that patients are supported in so-called stigma-handling strategies (Diesen et al. 2014), such as social skills training or school-based interventions, in which peers are provided with basic information about the condition, resulting in better understanding (Sharman et al. 2013).
In contrast to parents, patients talked reluctantly about tube feeding. Content of statements indicates tube feeding meant considerable relief for parents in terms of reduced worries and struggles with children about food intake. Consistent with this, previous research showed that tube feeding has a positive impact on parental HrQoL (Fabre et al. 2013). In contrast, feelings of shame and embarrassment seemed to be a reason why patients did not want to talk about their tube in the focus groups – which goes along with the psychosocial impact of tube feeding mentioned in the literature (Burmucic et al. 2006). We are aware of the limitation that the perspectives of very young and/or severely compromised patients on this issue have not been included in this study.
Based on the results of the focus groups, two main clinical implications can be drawn. Firstly, patients with IT-IEM and their parents have significant need for comprehensible explanations and information concerning their disease. Both patients and parents reported difficulties in making the condition understandable to the social environment. For other diseases (e.g. diabetes type 1), this need has been met by age-appropriate, verbal information from medical professionals and attractive paper- or IT-based materials (Murphy et al. 2007; Årsand et al. 2012). Improved understanding of the disease may motivate patients to reach better compliance (Sharman et al. 2013). Being able to explain the disease may help patients and parents to cope better with stigmatising social situations such as the often-experienced exclusion from school or leisure activities. In addition, low-threshold access to information about IT-IEM (e.g. informative homepages, guidelines) and metabolic expert advice would help physicians who are not specialised in the metabolic field in their communication with families.
Secondly, beyond essential medical treatment, psychosocial care should be offered to IT-IEM patients and parents. It has been shown that a child’s well-being is considerably influenced by family variables (Landolt et al. 2002), and although the interviews in this study focused on the well-being of patients, parents spontaneously reported high levels of distress, which require appropriate support.
Remarkably, of the 26 participating parents, only one was male. This highly unequal gender distribution narrows the breadth of the parent perspective but seems to reflect real-life patterns. In many families, it is still mothers who are the primary health carer. In their traditional role as major income earner of the family, fathers are less present in the hospital setting and take other functional roles in caring for the child, such as seeking information (Yeh 2004). The underrepresentation of fathers in research focusing on children with chronic diseases is a common fact that it is important to note (Goldstein et al. 2013).
Studies in other diseases clearly revealed that physicians underestimate the impact of disease and treatment on emotional and social domains (Srikrishna et al. 2009). Treatment issues were a main concern for patients in this study because they hampered everyday life and made the disease perceivable for others. Thus, the fondest wishes of the patients to “be normal” and “not have the disease” should motivate physicians and dieticians to consider even more highly that the most feasible and practical treatment protocol serves patients and families best. Side effects of medication, such as an unpleasant smell, diarrhoea and frequent intake of disliked amino acid mixtures, may impair emotional and social well-being more than expected. Additionally, fear of blood sampling made hospital appointments extremely stressful for many patients. It is well established that, besides local anaesthetics, psychological techniques (e.g. relaxation techniques, developmentally appropriate distraction) are highly effective in reducing pain and anticipatory fear (Duff 2003). It has been shown in other chronic paediatric diseases that addressing social issues and emotional distress during regular hospital follow-ups is supportive and that the use of HrQoL questionnaires (Santana and Feeny 2013) significantly increases meaningful patient-physician communication and patient well-being (Velikova et al. 2004).
Conclusion
The categories identified describe HrQoL of IT-IEM patients and will allow the construction of a disease-specific HrQoL questionnaire. Care for IT-IEM patients can be improved by providing appropriate information about the disease and individualised psychosocial support to ameliorate the multifaceted effects of the disease on physical, mental and social well-being.
Acknowledgements
We thank all patients and parents who participated in the study and shared their experiences of living with IT-IEM. We are indebted to Prof. Monika Bullinger, Hamburg, for her valuable input regarding the design of the study. Furthermore, we gratefully acknowledge all colleagues involved as focus group moderators, during transcription and inter-rater reliability testing: Tilla Aegerter, Michael Ertl, Anna Giammarco, Ann-Christin Haag, Ornella Masnari, Miriam Michel, Katharina Nitsche, Corinne Pellegrino and Sabine Weber.
The study was supported by radiz – Clinical Research Priority Program for Rare Diseases from the University of Zurich – and by Milupa Metabolics, Friedrichsdorf, Germany.
Sentence Take-Home Message
Taking patients’ and parents’ perspectives into account allows the definition of meaningful follow-up parameters and the development of personalised interventions.
Compliance with Ethical Guidelines
Conflict of Interest
Nina A. Zeltner and Martina Huemer have received research grants from Milupa Metabolics. Markus A. Landolt, Matthias R. Baumgartner, Sarah Lageder, Julia Quitmann, Rachel Sommer, Daniela Karall, Chris Mühlhausen, Andrea Schlune and Sabine Scholl-Bürgi declare that they have no conflict of interest.
Informed Consent
All procedures were followed in accordance with the Helsinki Declaration of 1975, as revised in 2000 and approved by the ethical committee in Zurich, Switzerland (KEK-ZH Nr. 2012.0020), and the local ethical committees in Hamburg, Düsseldorf and Innsbruck. Informed consent was obtained from all participants/their legal representatives to be included in the study.
Details of the Contributions of Individual Authors
N.A.Z. was involved in designing the study, collected and analysed the data and drafted the manuscript. M.A.L. was involved in designing the study, gave advice on data collection and analysis and critically reviewed the manuscript. M.R.B. was involved in designing the study, contributed patient data and critically reviewed the manuscript. S.L. assisted in collecting and analysing the data. J.Q. was involved in designing the study. R.S. was involved in designing the study and collecting the data. C.M., A.S., D.K. and S.S. contributed patient data. M.H. provided the original concept of the study, coordinated the study and revised the manuscript. All authors read and approved the final version of the manuscript.
Footnotes
Competing interests: None declared
Contributor Information
Nina A. Zeltner, Email: nina.zeltner@kispi.uzh.ch
Markus A. Landolt, Email: markus.landolt@kispi.uzh.ch
Matthias R. Baumgartner, Email: matthias.baumgartner@kispi.uzh.ch
Sarah Lageder, Email: sarah.lageder@windowslive.com.
Julia Quitmann, Email: j.quitmann@uke.de.
Rachel Sommer, Email: r.sommer@uke.de.
Daniela Karall, Email: daniela.karall@tirol-kliniken.at.
Chris Mühlhausen, Email: muehlhausen@uke.de.
Andrea Schlune, Email: andrea.schlune@med.uni-duesseldorf.de.
Sabine Scholl-Bürgi, Email: sabine.scholl-buergi@tirol-kliniken.at.
Martina Huemer, Email: martina.huemer@kispi.uzh.ch.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Anderson M, Elliott EJ, Zurynski YA. Australian families living with rare disease: experiences of diagnosis, health services use and needs for psychosocial support. Orphanet J Rare Dis. 2013;8:22. doi: 10.1186/1750-1172-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Årsand E, Frøisland DH, Skrøvseth SO, et al. Mobile health applications to assist patients with diabetes: lessons learned and design implications. J Diabetes Sci Technol. 2012;6:1197–1206. doi: 10.1177/193229681200600525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batshaw ML, Tuchman M, Summar M, Seminara J. A longitudinal study of urea cycle disorders. Mol Genet Metab. 2014;113:127–130. doi: 10.1016/j.ymgme.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullinger M (2002) Assessing health related quality of life in medicine. An overview over concepts, methods and applications in international research. Restor Neurol Neurosci 20:93–101 [PubMed]
- Burmucic K, Trabi T, Deutschmann A, et al. Tube weaning according to the Graz model in two children with Alagille syndrome. Pediatr Transplant. 2006;10:934–937. doi: 10.1111/j.1399-3046.2006.00587.x. [DOI] [PubMed] [Google Scholar]
- Cederbaum JA, LeMons C, Rosen M, et al. Psychosocial issues and coping strategies in families affected by urea cycle disorders. J Pediatr. 2001;138:S72–S80. doi: 10.1067/mpd.2001.111839. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioural sciences. 2. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- De Baulny HO, Benoist JF, Rigal O, et al. Methylmalonic and propionic acidaemias: management and outcome. J Inherit Metab Dis. 2005;28:415–423. doi: 10.1007/s10545-005-7056-1. [DOI] [PubMed] [Google Scholar]
- Diesen PS, Wiig I, Grut L, Kase BF. Betwixt and between being healthy and ill: the stigma experienced by young adults with phenylketonuria. Scand J Disabil Res. 2014;17:321–334. doi: 10.1080/15017419.2014.941003. [DOI] [Google Scholar]
- Duff AJA. Incorporating psychological approaches into routine paediatric venepuncture. Arch Dis Child. 2003;88:931–937. doi: 10.1136/adc.88.10.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre A, Baumstarck K, Cano A, et al. Assessment of quality of life of the children and parents affected by inborn errors of metabolism with restricted diet: preliminary results of a cross-sectional study. Health Qual Life Outcomes. 2013;11:158. doi: 10.1186/1477-7525-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein H, Akre C, Belanger RE, Suris JC. Detached, distraught or discerning? Fathers of adolescents with chronic illness: a review of the literature. Int J Adolesc Med Health. 2013;25:109–117. doi: 10.1515/ijamh-2013-0018. [DOI] [PubMed] [Google Scholar]
- Graven LJ, Grant JS. Coping and health-related quality of life in individuals with heart failure: an integrative review. Heart Lung. 2013;42:183–194. doi: 10.1016/j.hrtlng.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Hall JG. The role of patient advocacy/parent support groups. S Afr Med J. 2013;103:1020–1022. doi: 10.7196/SAMJ.6976. [DOI] [PubMed] [Google Scholar]
- Khangura SD, Tingley K, Chakraborty P, et al. Child and family experiences with inborn errors of metabolism: a qualitative interview study with representatives of patient groups. J Inherit Metab Dis. 2015 doi: 10.1007/s10545-015-9881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuckartz U (2014) Qualitative Inhaltsanalyse. Methoden, Praxis, Computerunterstützung., 2nd edn. Beltz Juventa, Weinheim
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- Landolt MA, Grubenmann S, Meuli M. Family impact greatest: predictors of quality of life and psychological adjustment in pediatric burn survivors. J Trauma. 2002;53:1146–1151. doi: 10.1097/00005373-200212000-00019. [DOI] [PubMed] [Google Scholar]
- Masnari O, Schiestl C, Rössler J, et al. Stigmatization predicts psychological adjustment and quality of life in children and adolescents with a facial difference. J Pediatr Psychol. 2013;38:162–172. doi: 10.1093/jpepsy/jss106. [DOI] [PubMed] [Google Scholar]
- Matza LS, Swensen AR, Flood EM, et al. Assessment of health-related quality of life in children: a review of conceptual, methodological, and regulatory issues. Value Health. 2004;7:79–92. doi: 10.1111/j.1524-4733.2004.71273.x. [DOI] [PubMed] [Google Scholar]
- Matza LS, Patrick DL, Riley AW, et al. Pediatric patient-reported outcome instruments for research to support medical product labeling: report of the ISPOR PRO good research practices for the assessment of children and adolescents task force. Value Health. 2013;16:461–479. doi: 10.1016/j.jval.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Mayring P. Qualitative Inhaltsanalyse, Grundlagen und Techniken. 11. Weinheim/Basel: Beltz; 2010. [Google Scholar]
- Murphy HR, Wadham C, Rayman G, Skinner TC. Approaches to integrating paediatric diabetes care and structured education: experiences from the Families, Adolescents, and Children’s Teamwork Study (FACTS) Diabet Med. 2007;24:1261–1268. doi: 10.1111/j.1464-5491.2007.02229.x. [DOI] [PubMed] [Google Scholar]
- OECD (1999) Classifying educational programmes: manual for ISCED-97 implementation in OECD countries, 1999th edn. OECD Organisation for Economic Co-operation and Development, Paris
- Packman W, Mehta I, Rafie S, et al. Young adults with MSUD and their transition to adulthood: psychosocial issues. J Genet Couns. 2012;21:692–703. doi: 10.1007/s10897-012-9490-1. [DOI] [PubMed] [Google Scholar]
- Pelentsov LJ, Laws TA, Esterman AJ. The supportive care needs of parents caring for a child with a rare disease: a scoping review. Disabil Health J. 2015 doi: 10.1016/j.dhjo.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Rajmil L, Herdman M, Fernandez de Sanmamed M-J, et al. Generic health-related quality of life instruments in children and adolescents: a qualitative analysis of content. J Adolesc Health. 2004;34:37–45. doi: 10.1016/S1054-139X(03)00249-0. [DOI] [PubMed] [Google Scholar]
- Regnault A, Burlina A, Cunningham A, et al. Development and psychometric validation of measures to assess the impact of phenylketonuria and its dietary treatment on patients’ and parents’ quality of life: the phenylketonuria – quality of life (PKU-QOL) questionnaires. Orphanet J Rare Dis. 2015;10:59. doi: 10.1186/s13023-015-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana M-J, Feeny D. Framework to assess the effects of using patient-reported outcome measures in chronic care management. Qual Life Res. 2013 doi: 10.1007/s11136-013-0596-1. [DOI] [PubMed] [Google Scholar]
- Sharman R, Mulgrew K, Katsikitis M. Qualitative analysis of factors affecting adherence to the phenylketonuria diet in adolescents. Clin Nurse Spec. 2013;27:205–210. doi: 10.1097/NUR.0b013e31829555d5. [DOI] [PubMed] [Google Scholar]
- Srikrishna S, Robinson D, Cardozo L, Gonzalez J. Is there a discrepancy between patient and physician quality of life assessment? Neurourol Urodyn. 2009;28:179–182. doi: 10.1002/nau.20634. [DOI] [PubMed] [Google Scholar]
- The DISABKIDS Group Europe . The DISABKIDS questionnaires. Quality of life questionnaires for children with chronic conditions. Lengerich: Pabst Science; 2006. [Google Scholar]
- The KIDSCREEN Group Europe . The KIDSCREEN questionnaires. Quality of life questionnaires for children and adolescents. Lengerich: Pabst Science; 2006. [Google Scholar]
- Upton P, Lawford J, Eiser C. Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res. 2008;17:895–913. doi: 10.1007/s11136-008-9350-5. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22:714–724. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- Vogels TC, Verrips GHW, Koopman HM et al (1999) TACQOL manual parent and child form 6–11 years. Leiden Center for Child and Pediatrics LUMC-TNO
- Walterfang M, Bonnot O, Mocellin R, Velakoulis D. The neuropsychiatry of inborn errors of metabolism. J Inherit Metab Dis. 2013;36:687–702. doi: 10.1007/s10545-013-9618-y. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (1947) World Health Organisation Constitution. WHO, Geneva
- Yeh C-H. Gender differences in the use of coping strategies among Taiwanese parents whose children have cancer. Cancer Nurs. 2004;27:100–107. doi: 10.1097/00002820-200403000-00002. [DOI] [PubMed] [Google Scholar]
- Zeltner NA, Huemer M, Baumgartner MR, Landolt MA. Quality of life, psychological adjustment, and adaptive functioning of patients with intoxication-type inborn errors of metabolism – a systematic review. Orphanet J Rare Dis. 2014;9:159. doi: 10.1186/s13023-014-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


