Abstract
Fluorescent Pseudomonas, aerobic, Gram-negative bacteria possess many traits that make them well suited as biocontrol and growth promoting agents. Our study revealed that isolates vary in mechanisms involved in the antagonist interactions against pathogen and growth stimulatory effects on host plant. Most of the potential antagonistic fluorescent Pseudomonas identified were avid iron chelators (P233, P201, 176, P76 and, P76). Wide variation in ACCd enzyme production was observed. ACCd enzyme assay tested P141 > P247 > P126, as potential ACCd enzyme producer. Cynogenic fluorescent Pseudomonas isolates P76 and P124 exerted strong inhibitory against S. rolfsii. However, another cynogenic fluorescent Pseudomonas P179 had no influence against R solani and S. rolfsii which remains unexplained. Noticeable crop specific plant growth stimulation exerted by different fluorescent Pseudomonas was observed on wheat (P124), chickpea (P72), lathyrus (P85, P216), greengram (P11), blackgram (P99, P233); bottlegourd (P248, P167); rice (P176, P247).
Keywords: ACCd enzyme, Confrontation assay, Fluorescent Pseudomonas, HCN, PGPR, Siderophore
Introduction
Aerobic gram negative Fluorescent Pseudomonas spp. have emerged as the largest and potentially most promising group of plant growth promoting rhizobacteria involved in the bio-control of plant diseases (Weller et al. 2002; Fravel 2005). A large number of secondary metabolites, growth hormones, antibiotics and chelating compounds such as siderophores (Choudhary et al. 2009; Beneduzi et al. 2012) are known to be released by these fluorescent pseudomonads. They maintain soil health by employing a wide variety of mechanisms including nitrogen fixation, enhanced solubilization of phosphate and phytohormone production (such as auxin and cytokinin). Plant growth-promoting rhizobacteria (PGPR) competitively colonize plant roots and stimulate plant growth and/or reduce the incidence of plant disease. Fluorescent Pseudomonas applied to seed or soil provides excellent control against plant pathogens (De La Fuente et al. 2006; Lagzian et al. 2013). Pseudomonas spp. produce an arsenal of antimicrobials (including hydrogen cyanide, HCN), pyoluteorin, phenazines, pyrrolnitrin, siderophores, cyclic lipopeptides and 2,4-diacetylphloroglucinol (DAPG) (Thomashow and Weller 1991; Weller 2007). This is considered as an indirect strategy to promote plant growth as well as the ability to induce systemic resistance in plants (Santoyo et al. 2012; Glick 2014). In this study, we evaluated the fluorescent isolates for siderophore and HCN production. PGPR and their interactions with plants are exploited commercially (Podile and Kishore 2007) and hold great promise for sustainable agriculture. Applications of these associations were investigated in Wheat (Triticum aestivum), Chickpea (Cicer arientinum), Lathyrus (Lathyrus sativus), Greengram (Vigna radiata), Blackgram (Vigna mungo), Bottlegourd (Lagenaria siceraria) and Rice (Oryza sativa) through seed bacterization.
Materials and methods
Microorganisms and culture conditions
The experimental material consisted of purified twenty-four isolates (Table 1) of fluorescent Pseudomonas spp. from soils (rhizospheric and non-rhizospheric) of different geographical locations of Chhattisgarh. Isolation of fluorescent pseudomonads was done by adopting serial dilution method on King’s B (KB) medium. Isolates were characterized on the basis of biochemical tests as per the procedures outlined in Bergey’s Manual of Systematic Bacteriology (Sneath 1986). Glycerol stock of isolates were maintained in the culture collections of the Department of Plant Molecular Biology and Biotechnology, Indira Gandhi Krishi Vishwavidyalaya, Raipur, Chhattisgarh, India and revived on KMB slants when required. Fungal pathogens Rhizoctonia solani and Sclerotium rolfsii were isolated from naturally infected sick soils of rice and chickpea and maintained on PDA slants.
Table 1.
Fluorescent Pseudomonas spp. isolates used in the present study
| S. no. | Isolates | Origin/location | |
|---|---|---|---|
| 1 | P5 | Fluoresecent Pseudomonas | Fenugreek soil, IGKV Horticulture, Raipur |
| 2 | P6 | Pseudomonas putida | Cashew tree soil, IGKV Horticulture, Raipur |
| 3 | P11 | Pseudomonas putida | Brinjal plant soil, IGKV Horticulture, Raipur |
| 4 | P67 | Pseudomonas fluorescens | Charama |
| 5 | P72 | Pseudomonas putida | Chhati |
| 6 | P76 | Pseudomonas putida | Mustard field soil, chhati |
| 7 | P85 | Pseudomonas aeruginosa | Cow dung soil, Darba |
| 8 | P99 | Fluoresecent Pseudomonas | Jagtara |
| 9 | P124 | Fluoresecent Pseudomonas | Kanker forest-3 |
| 10 | P126 | Pseudomonas putida | Kanker forest soil |
| 11 | P129 | Pseudomonas putida | Kanker forest soil |
| 12 | P141 | Pseudomonas putida | Kirda |
| 13 | P143 | Pseudomonas putida | Kodebor |
| 14 | P151 | Pseudomonas putida | Kurud |
| 15 | P161 | Pseudomonas putida | VIP nursery soil, Raipur |
| 16 | P167 | Pseudomonas putida | Purur |
| 17 | P176 | Pseudomonas putida | Rice field, fallow land soil, Raipur |
| 18 | P179 | Fluoresecent Pseudomonas | Rice-lathyrus field soil, Raipur |
| 19 | P201 | Pseudomonas putida | VIP road, Raipur |
| 20 | P205 | Pseudomonas putida | Babool tree soil, VIP road, Raipur |
| 21 | P216 | Fluoresecent Pseudomonas | Bamboo tree soil, VIP road, Raipur |
| 22 | P233 | Fluoresecent Pseudomonas | Badi, Tekabheta |
| 23 | P247 | Fluoresecent Pseudomonas | Laichopi, Raweli |
| 24 | P248 | Pseudomonas aeruginosa | Maize, Parsada |
Siderophore production
Qualitative and quantitative estimation of siderophore production was done by CAS assay (Schwyn and Neilands 1987). Specific tests were carried out for the identification of hydroxamate and catecholate types of siderophores following the standard methods (Arnow 1937). For qualitative estimation, chrome azurol S solution was prepared and added to melted King’s B agar medium in the ratio 1:15. Spot inoculation at the centre of the CAS plate was done from actively growing cultures of Pseudomonas. Colonies exhibiting an orange halo after 3 days incubation (28 ± 2 °C) were considered positive for siderophore production and the diameter of the orange halo was measured. Simultaneously succinate medium (broth) was also used for qualitative estimation of siderophore production on the basis of fluorescence observed after 3 days incubation (28 ± 2 °C).
Quantitative spectrophotometric assay for siderophore production (liquid assay)
For siderophore quantification, actively growing cultures of Pseudomonas was inoculated to 20 mL King’s B broth in 100 mL flasks and incubated for 3 days at 28 ± 2 °C. The bacterial cells were removed by centrifugation at 3000 rpm for 5 min. 0.5 mL of the culture supernatant was then mixed with 0.5 mL of CAS solution and 10 µl shuttling reagent (sulfosalicyclic acid). After 20 min of incubation, the absorbance of colour obtained was determined using spectrophotometer at 630 nm. Un-inoculated King’s B broth was used as blank while reference solution was prepared by adding CAS dye and shuttle solution to King’s B and absorbance was recorded. Values of siderophore released in King’s B was expressed as percent siderophore units and calculated using the formula: (A r − A s)/A r × 100; where A r is the absorbance of reference solution and A s is the absorbance of samples.
Hydroxyquinoline mediated siderophore test
Isolates were inoculated on King’s B medium supplemented with a strong iron chelater 8- Hydroxyquinoline (50 mg/L) (De Brito et al. 1995) and incubated at 28 ± 2 °C for 48–72 h. Only those bacteria that produce a more avid iron chelator will grow.
Arnow’s assay
Arnow’s assay was used for qualitative determination of catechol type of siderophore. Actively growing cultures of Pseudomonas were inoculated to 20 mL King’s B broth in 50 mL tubes and incubated for 3 days at 28 ± 2 °C. The bacterial cells were removed by centrifugation at 3000 rpm for 5 min. Three milliliter of the culture supernatant was then mixed with 0.3 mL of 5 N HCl solution, 1.5 mL of Arnow’s reagent (10 g NaNO2, 10 g Na2MoO4·2H2O dissolved in 50 mL distilled water) and 0.3 mL of 10 N NaOH. After 10 min the presence or absence of pink colour was observed and noted.
Tetrazolium test
This test is based on the capacity of hydroxamic acid to reduce tetrazolium salt by hydrolysis of hydroxymate groups using a strong alkali. The reduction and release of alkali shows red colour to a pinch of tetrazolium salt when 1–2 drops of 2 N NaOH and 0.1 mL of test sample is added. Instant appearance of a deep red colour indicated the presence of hydroxamate siderophore.
FeCl3 test
One milliliter of the culture supernatant was mixed with freshly prepared 0.5 mL of 2% aqueous FeCl3 and observed for the presence and absence of deep red colour.
Confrontation assay
Fluorescent Pseudomonas isolates were multiplied on King’s B broth and incubated for two days at 28 °C till the fluorescent pigment appeared in the broth. Petri-plates containing pre-sterilized potato dextrose agar (PDA) medium was inoculated with plant pathogenic fungi Sclerotium rolfsii or Rhizoctonia solani (in the center) and incubated at 25 °C for three days till the fungus completely covered the entire plate. Bipartite interactions were performed following a simple confrontation assay technique as proposed by Kotasthane et al. (unpublished results), wherein edge of glass funnel was deployed for deposition of bio-agent surrounding pre-inoculated fungal pathogen.
HCN production
The production of HCN was estimated by method of (Wei et al. 1991). The cultures were grown on KM plates supplemented with 4.4 g/L glycine as a precursor and the filter paper strips soaked in saturated picric acid solution were exposed to the growing Pseudomonas isolates. The plates were incubated for 7 days at 28 ± 2 °C and observations were recorded as change in the colour of filter paper to brown as positive indicator for HCN production.
Quantitative estimation of ACC deaminase activity
ACC deaminase activity was determined by measuring the production of α-ketobutyrate and ammonia generated by the cleavage of ACC by ACC deaminase (Honma and Shimomura 1978; Penrose and Glick 2003). Pseudomonas isolates were grown in 5 mL of trypticase soya broth at 28 °C until they reached stationary phase. The cells were collected by centrifugation, washed twice with 0.1 M Tris–HCl (pH 7.5), re-suspended in 2 mL of modified DF minimal medium supplemented with 2 mM final concentration of ACC. Incubated at 28 °C with shaking for another 36–72 h. The induced bacterial cells were harvested by centrifugation at 3000g for 5 min, washed twice with 0.1 M Tris–HCl (pH 7.5), and re-suspended in 200 μL of 0.1 M Tris–HCl (pH 8.5). The cells were labilized by adding 5% toluene (v/v) and then vortexed at the highest speed for 30 s. Fifty microlitre of labilized cell suspension was incubated with 5 μL of 0.3 M ACC in an microcentrifuge tube at 28 ± 2 °C for 30 min. The negative control for this assay consisted of 50 μL of labilized cell suspension without ACC, while the blank consisted of 50 μL of 0.1 M Tris–HCl (pH 8.5) with 5 μL of 0.3 M ACC. The samples were then mixed thoroughly with 500 μL of 0.56 N HCl by vortexing and the cell debris was removed by centrifugation at 12,000g for 5 min. A 500 μL aliquot of the supernatant was transferred to a glass test tube, mixed with 400 μL of 0.56 N HCl and 150 μL of DNF solution (0.1 g 2,4-dinitrophenylhydrazine dissolved in 100 mL of 2 N HCl); and incubated at 28 °C for 30 min. One milliliter of 2 N NaOH was added to the sample before the absorbance at 540 nm was measured. The concentration of α-ketobutyrate in each sample was determined by comparison with a standard curve generated as follows: A stock solution of 100 mmol/L α-ketobutyrate (Sigma-Aldrich Co., Mumbai, India) was prepared in 0.1 mol/L Tris–HCl (pH 8.5) and stored at 4 °C. Just prior to use stock solution is diluted with same buffer to make a 10 mmol/L solution from which a standard concentration curve is generated. Each 500 μL α-ketobutyrate solutions of 1, 5, 10, 15, 20, 25, 30, 35, 40, 45 and 50 µM (prepared from stock solution) were mixed, respectively, with 400 μL of 0.56 N HCl and 150 μL DNF solution. One milliliter of 2 N NaOH was added and the absorbance at 540 nm was determined as described above. The values for absorbance versus α-ketobutyrate concentration (µM) were used to prepare a standard curve.
Estimation of PGPR activity
The cultures of fluorescent Pseudomonas spp. were inoculated in 100 mL conical flask containing 25 mL King’s B broth and incubated at 28 ± 2 °C for 48 h. For field trials seed bacterization was done with stock cultures (used for confrontation assays) of selected fluorescent Pseudomonas isolate. Slurry for seed bacterization was prepared @ 5 mL of bacterial culture +3 g of talcum powder/kg of seed. Care was taken for uniform coating of all the seeds, which were dried in shade. Seeds of each of Wheat (GW-272), Chickpea, Lathyrus (KH-014), Greengram (puspa vishal), Blackgram (T-U-94-2), Bottlegourd (Lagenaria siceraria), Rice (swarna) were planted in pots containing soil mixed with sand and compost in the ratio of 3:1:1. Plants growth were measured for root and shoot length.
Statistical analysis
The design of all phenotype assays and plant growth experiments was a completely randomized block with three replications per treatment. Data of all biochemical tests and plant growth experiments were subsequently analyzed by ANOVA followed by Duncan’s test using WASP (Web Agri Stat Package) software (http://icargoa.res.in/wasp/index.php). Critical difference at 0.05 level of significance was calculated for the observed values along with average and standard deviation. Duncan’s test controls the Type I comparison wise error rate and as per Duncan’s grouping, means with the same letter are not significantly different. Duncan’s test can be used irrespective of whether F is significant or not and compares all possible pairs of treatment means.
Results
Isolates were characterized on the basis of biochemical tests as per the procedures outlined in Bergey’s Manual of Systematic Bacteriology (Sneath 1986) and tests reported by (Blazevic et al. 1973) that specifically differentiate the fluorescent Pseudomonas into P. aeruginosa, P. putida and P. fluorescence (Table 1).
Qualitative and quantitative assay for siderophore production
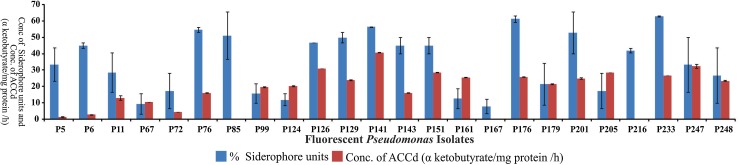
Twenty-four isolates of fluorescent Pseudomonas were screened by different siderophore assay. All fluorescent Pseudomonas isolates produced siderophore on iron deficient succinate medium with variable chromogenic response and were also +ve for siderophore production on CAS agar plate assay and hydroxamate type of siderophore assay (tetrazolium and FeCl3 test). Siderophores are also viewed as contingent antibiotics, the selective advantage is easily verified by placing a bacterial culture in a medium containing a strong iron chelator 8-hydroxyquinoline (50 mg/L); only those bacteria that produce a more avid iron chelator grow. All fluorescent Pseudomonas isolates except P5, P11, P67, P72, P99, P126, P161, P167, P179 and P205 were +ve in HQ test. Fluorescent pseudomonas isolates P6, P85 and P233 produce a more avid iron chelator and produced high % siderophore units in quantitative test. Arnow’s assay (test for catechol type of siderophore) tested +ve for 22 isolates except P85 and P216. Fluorescent pseudomonas isolates P233 > P176 > P141 > P76 > P201 were all avid iron chelators and tested +ve for all siderophore tests (Fig. 1; Table 2).
Fig. 1.
Percent Siderophore units and ACCd enzyme producing ability of different fluorescent Pseudomonas
Table 2.
Siderophore, ACCd enzyme, HCN producing ability and inhibitory effect of fluorescent Pseudomonas isolates against R solani and S. rolfsii
| Treatment | Siderophore | ACC (α ketobutyrate/mg protein/h) | HCN | Confrontation assay (% inhibition) | |||
|---|---|---|---|---|---|---|---|
| % Siderophore units | HQ Test | R. solani | S. rolfsii | ||||
| 1 | P5 | 33.54bcdefg ± 10.21 | – | 1.48 ± 0.23 | – | 38.90abc ± 11.10 | 19.45ef ± 2.75 |
| 2 | P6 | 45.1abcde ± 1.77 | *** | 2.9 ± 0.15 | – | 51.10ab ± 1.10 | 35.55abcde ± 6.65 |
| 3 | P11 | 28.67cdefgh ± 11.97 | – | 13.11 ± 1.29 | – | 34.45abc ± 12.75 | 27.25bcdef ± 9.45 |
| 4 | P67 | 9.48gh ± 6.15 | – | 10.51 ± 0.06 | – | 37.25abc ± 9.45 | 27.80bcdef ± 0.05 |
| 5 | P72 | 17.40fgh ± 10.74 | – | 4.42 ± 0.07 | – | 42.20abc ± 3.30 | 26.10cdef ± 6.60 |
| 6 | P76 | 54.79ab ± 1.46 | * | 16.24 ± 0.05 | +++ | 49.45ab ± 7.25 | 49.40a ± 5.00 |
| 7 | P85 | 51.15abcd ± 14.48 | *** | nd | – | 40.00abc ± 6.70 | 43.35abc ± 4.45 |
| 8 | P99 | 15.93gh ± 5.93 | – | 19.75 ± 0.21 | – | 29.45bcd ± 7.25 | 33.85abcde ± 8.35 |
| 9 | P124 | 11.98gh ± 3.65 | * | 20.37 ± 0.25 | +++ | 43.85abc ± 11.65 | 35.00abcde ± 7.20 |
| 10 | P126 | 46.77abcde ± 0.10 | – | 31.05 ± 0.046 | – | 36.10abc ± 8.30 | 17.21ef ± 15.0 |
| 11 | P129 | 49.9abcd ± 3.23 | * | 23.94 ± 0.32 | – | 42.20abc ± 2.20 | 8.33f ± 2.78 |
| 12 | P141 | 56.46ab ± 0.21 | * | 40.87 ± 0.08 | – | 40.00abc ± 6.70 | 23.30cdef ± 7.80 |
| 13 | P143 | 45abcde ± 5.00 | * | 16.27 ± 0.14 | – | 42.75abc ± 1.65 | 27.80bcdef ± 11.10 |
| 14 | P151 | 45abcde ± 5.00 | * | 28.64 ± 0.26 | – | 50.00ab ± 0.05 | 21.65def ± 10.55 |
| 15 | P161 | 12.71gh ± 6.05 | – | 25.61 ± 0.05 | – | 22.20cd ± 0.05 | 10.00f ± 1.10 |
| 16 | P167 | 7.92h ± 4.49 | – | nd | – | 35.00abc ± 5.00 | 41.65abcd ± 2.75 |
| 17 | P176 | 61.36a ± 1.98 | * | 25.89 ± 0.21 | – | 52.75ab ± 8.35 | 32.20abcde ± 7.70 |
| 18 | P179 | 21.56efgh ± 12.81 | – | 21.61 ± 0.27 | +++ | 4.44d ± 2.22 | 10.01f ± 7.79 |
| 19 | P201 | 52.82abc ± 12.82 | * | 25.02 ± 0.37 | – | 55.00a ± 5.00 | 25.00cdef ± 7.20 |
| 20 | P205 | 17.39fgh ± 10.73 | – | 28.56 ± 0.02 | – | 4.44d ± 2.22 | 22.20def ± 0.05 |
| 21 | P216 | 41.98abcdef ± 1.35 | ** | nd | – | 44.45abc ± 5.55 | 47.20ab ± 8.30 |
| 22 | P233 | 62.92a ± 0.42 | *** | 26.75 ± 0.05 | – | 45.55abc ± 12.55 | 32.80abcde ± 6.10 |
| 23 | P247 | 33.34bcdefg ± 16.67 | ** | 32.61 ± 1.22 | – | 41.65abc ± 8.35 | 22.80def ± 5.00 |
| 24 | P248 | 26.875defgh ± 16.88 | ** | 23.55 ± 0.272 | – | 36.65abc ± 25.55 | 47.20ab ± 2.80 |
| Max. | 62.915a ± 0.42 | 55.00a ± 5.00 | 49.40a ± 5.00 | ||||
| Min. | 9.48gh ± 6.15 | 4.44d ± 2.22 | 8.33f ± 2.78 | ||||
| CD (0.01%) | 34.198 | – | 27.70 | ||||
| CD (0.05%) | 25.230 | 25.39 | 20.45 | ||||
| CV | 4.247 | 32.08 | 34.60 | ||||
| Fcal | 2.17 | 2.03 | |||||
Values are average of three replications; values after ± represents standard error; CV, coefficient of variance; CD, critical difference; Values are significant at 1 and 5% levels; As per Duncan’s grouping means with the same letter are not significantly different; HQ hydroxyquinoline test; *** Luxuriant/high growth; ** medium growth; * low growth; –, no growth; nd, not determined
Screening of 1-aminocyclopropane-1-carboxylic acid deaminase (ACC Deaminase) containing fluorescent Pseudomonas isolates
Test for ACC deaminase activity revealed wide variation in quantified amount of α-ketobutyrate produced by different fluorescent Pseudomonas isolates (Fig. 1; Table 2) which allowed us to classify them as high, medium and low ACC deaminase enzyme producing groups. Group of fluorescent Pseudomonas isolates produced µmol α ketobutyrate/mg protein/h in the range of 40.87 ± 0.08 to 25.02 ± 0.37 and 23.94 ± 0.32 to 10.51 ± 0.06 were placed in high and medium ACC deaminase producing groups. Isolate P141 was the highest enzyme producer followed by P247 and P126 (Fig. 1; Table 2). Three isolates P216, P5, P72 were identified as low ACC deaminase producers.
Hydrogen cyanide production by isolates of fluorescent Pseudomonas spp.
Out of 24 Pseudomonas isolates only three isolates P67, P124 and P179 turned the strip brown confirming +ve for HCN production.
In vitro antagonistic activity of fluorescent Pseudomonasisolates againstR. solaniandS. rolfsii
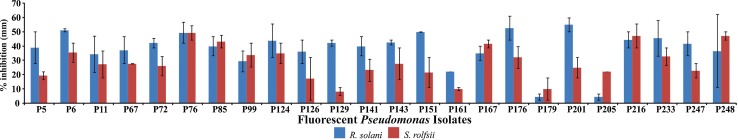
Confrontation assays were performed to assess antagonistic potential of 24 fluorescent Pseudomonas isolates in vitro against R. solani and S. rolfsii (Fig. 2; Table 2). Differences in growth inhibitions of R. solani and S. rolfsii ranged from 4.44 to 55 and 8.325 to 49.4%, respectively. Confrontation assays revealed isolate P76 exerting antagonism against both R solani and S rolfsii, where as fluorescent Pseudomonas isolates (P201, 176, P6, P151 and P248, P216, P85, P167) exerted pathogen specific antagonism against R. solani and S. rolfsii, respectively. Isolate P205 and P129 showed the lowest inhibitory effect on R. solani and S. rolfsii, respectively. Variation in quantitative inhibitory data was significant at 0.01 and 0.05% level for S. rolfsii and 0.05% for R. solani.
Fig. 2.
Inhibitory effect of fluorescent Pseudomonas isolates against R solani and S. rolfsii
Correlation between siderophore production and in vitro antagonistic activity of fluorescent Pseudomonas isolates against R. solani and S. rolfsii
Some correlation between inhibitory effects and the ability to produce siderophore (quantitative assay) was observed. All potential fluorescent pseudomonas isolates identified following confrontation assays (P201, 176, P6, P151, P76 and P248, P216, P85, P167, P76 effective against R. solani and S. rolfsii, respectively) were high siderophore producers except P167 and P248. Fluorescent pseudomonas isolates P6, P85 and P233 produce a more avid iron chelator and produced high % siderophore units in quantitative tests of which P6 and P233 exhibited antagonistic activity against R solani. Similarly some correlation was also observed between antagonism and ability to produce HCN by fluorescent Pseudomonas. Isolates P76, P124, 179 were cynogenic of which P76 and P124 exerted strong inhibitory effects during confrontation assays against S. rolfsii, whereas P179 expressed no influence against these two soil borne fungal pathogens which remains unexplained.
Plant growth promoting response of rice, wheat, greengram, blackgram, lathyrus, chickpea, bottlegourd following seed bacterization with fluorescent Pseudomonas isolates
From pot experiments, plant growth-attributing characters such as root and shoot lengths were recorded for seven crops (rice, wheat, bottlegourd, lathyrus, chickpea, greengram, blackgram). Significantly greater amount of root and coleoptile growth stimulation was recorded in the seedlings of seven different crops derived following seed bacterization with 24 different fluorescent Pseudomonas isolates as compared to untreated control (Figs. 3, 4; Tables 3, 4). Effects amongst fluorescent Pseudomonas treatments to stimulate plant growth also varied. Plants of seven crop derived from bacterized seed had more stimulatory effects on coleoptile elongation than root length. Fluorescent Pseudomonas isolates P176 stimulated coleoptiles elongation on all seven crops tested. Stimulation of coleoptile elongation by fluorescent Pseudomonas isolates was more predominant in wheat followed by chickpea, lathyrus, greengram, blackgram, bottlegourd and rice.
Fig. 3.

Plant growth promoting expression of bottle gourd seedling following seed bacterization with fluorescent Pseudomonas isolate
Fig. 4.
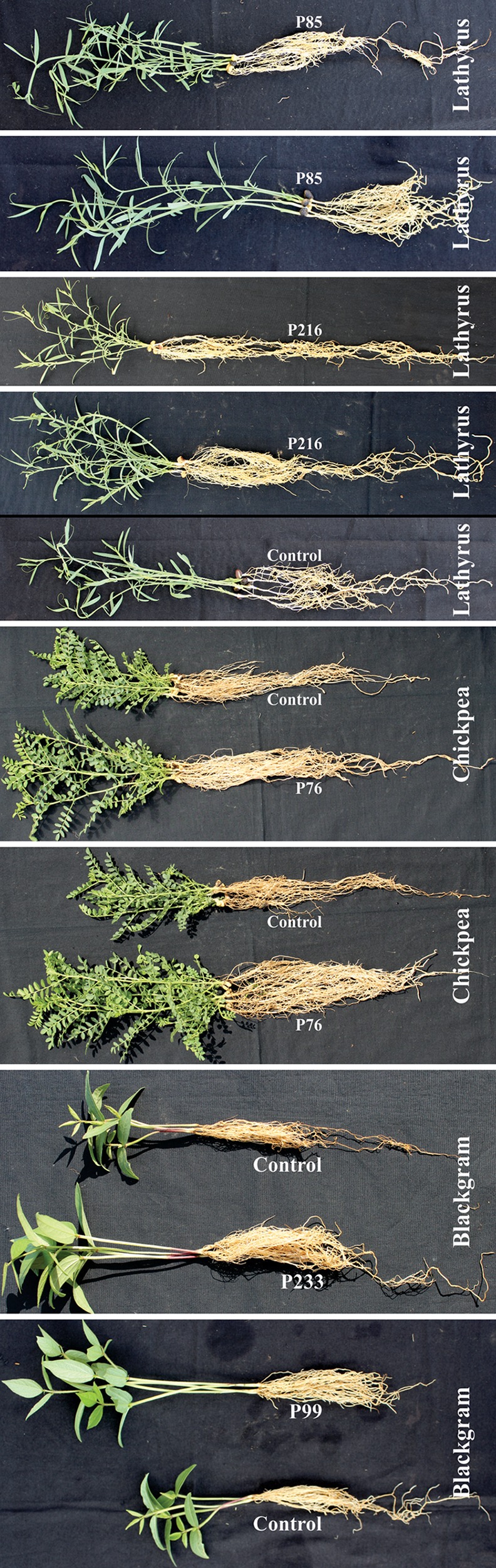
Plant growth promoting expression of bottle gourd seedling following seed bacterization with fluorescent Pseudomonas isolate black gram, chick pea and lathyrus seedling following seed bacterization with fluorescent Pseudomonas isolate
Table 3.
Plant growth promoting response of rice, wheat and bottle gourd seedlings following seed bacterization with fluorescent Pseudomonas isolates
| S. no. | Treatment | Rice | Wheat | Bottlegourd | |||
|---|---|---|---|---|---|---|---|
| Root length (cm) | Shoot length (cm) | Root length (cm) | Shoot length (cm) | Root length (cm) | Shoot length (cm) | ||
| 1 | Control | 9.325efg ± 1.135 | 20.3hijk ± 1.498 | 30.2bcdefg ± 0.742 | 31.73jk ± 0.4107 | 43.9efg ± 12.27 | 17.6efgh ± 1.254 |
| 2 | P5 | 12.2abcdef ± 1.301 | 20.775ghijk ± 0.771 | 28.42defgh ± 1.369 | 33.04ij ± 0.6838 | 45.7defg ± 6.74 | 14.33fgh ± 0.794 |
| 3 | P6 | 10.25cdefg ± 0.487 | 21.65fghij ± 1.713 | 27.68efgh ± 1.685 | 30.77k ± 1.0157 | 42.83efgh ± 0.82 | 16.4efgh ± 0.589 |
| 4 | P11 | 8.5fg ± 0.951 | 21.225fghijk ± 0.684 | 31.86bcdef ± 1.999 | 33.78hi ± 0.6555 | 44.5efg ± 6.01 | 11.1fgh ± 0.733 |
| 5 | P67 | 14.5ab ± 2.480 | 23.225abcdefg ± 0.390 | 27.24fgh ± 1.259 | 36.04efg ± 0.5142 | 54cdefg ± 8.01 | 12.75fgh ± 0.777 |
| 6 | P72 | 10defg ± 0.970 | 19.25jk ± 0.811 | 27.56fgh ± 1.755 | 36.35defg ± 0.9332 | 83.3b ± 1.15 | 15fgh ± 1.780 |
| 7 | P76 | 8.525fg ± 0.581 | 21.85efghi ± 0.835 | 30.66bcdefg ± 0.897 | 36.75cdef ± 0.8353 | 70.3bc ± 2.77 | 36.73bcd ± 5.981 |
| 8 | P85 | 12.175abcdef ± 1.035 | 24.45abcd ± 0.362 | 27.19gh ± 1.119 | 37.25bcde ± 0.8825 | 41.83fgh ± 12.44 | 40.68bc ± 7.366 |
| 9 | P99 | 9.2efg ± 0.426 | 23.6abcdef ± 0.942 | 27.69efgh ± 1.102 | 38.84ab ± 0.4621 | 70.8bc ± 2.64 | 56.73a ± 5.496 |
| 10 | P124 | 10.3cdefg ± 2.358 | 22.775abcdefgh ± 0.536 | 36.52a ± 2.766 | 38.18abcd ± 1.1289 | 87.7b ± 7.23 | 58.93a ± 9.433 |
| 11 | P129 | 10.825bcdefg ± 0.409 | 20.135ijk ± 0.613 | 28.12efgh ± 1.907 | 38.55abc ± 0.5677 | 69.88bc ± 9.66 | 24.53def ± 4.514 |
| 12 | P126 | 9.775efg ± 0.559 | 22.35bcdefghi ± 0.598 | 30.07bcdefg ± 1.316 | 36.89bcdef ± 0.8538 | 64.95bcdef ± 8.54 | 50.65ab ± 12.99 |
| 13 | P141 | 7.15g ± 0.891 | 22.15cdefghi ± 0.366 | 26.56gh ± 1.240 | 39.78a ± 0.9069 | 67.83bcd ± 11.11 | 11.4fgh ± 1.023 |
| 14 | P143 | 7.625g ± 1.004 | 19.025k ± 1.424 | 31.14bcdefg ± 1.775 | 35.9efg ± 0.2994 | 59.18cdefg ± 4.64 | 37.4bcd ± 3.447 |
| 15 | P151 | 13.625abcd ± 2.755 | 24.725ab ± 0.201 | 30.86bcdefg ± 1.150 | 35.58efgh ± 0.6486 | 75.63bc ± 6.82 | 20.85efg ± 0.296 |
| 16 | P161 | 12.5abcde ± 0.631 | 23.325abcdefg ± 0.239 | 32.28abcde ± 1.513 | 35.83efg ± 0.4839 | 42.4efgh ± 9.24 | 12.58fgh ± 0.862 |
| 17 | P167 | 9.8defg ± 0.715 | 23.15abcdefg ± 0.744 | 24.38h ± 1.906 | 34.45ghi ± 0.4381 | 73.5bc ± 16.98 | 63.75a ± 5.883 |
| 18 | P176 | 10defg ± 1.772 | 24.95a ± 0.833 | 34.51ab ± 1.602 | 38.27abcd ± 0.6350 | 65.6bcde ± 10.00 | 37.5bcd ± 11.76 |
| 19 | P179 | 7.8g ± 0.356 | 22defghi ± 0.082 | 28.61defgh ± 1.749 | 36.09efg ± 0.3703 | 20.33h ± 1.13 | 3.95h ± 0.466 |
| 20 | P201 | 8.625fg ± 2.138 | 21.4fghijk ± 1.485 | 29.44cdefg ± 1.610 | 38.59abc ± 0.9865 | 52.88cdefg ± 7.83 | 15.4fgh ± 1.362 |
| 21 | P205 | 7.55g ± 0.144 | 22.325bcdefghi ± 0.725 | 29.75cdefg ± 1.447 | 36.81cdef ± 0.3945 | 42.7efgh ± 5.66 | 14.175fgh ± 0.312 |
| 22 | P216 | 8.7efg ± 1.079 | 24.675abc ± 1.071 | 27.41fgh ± 1.529 | 35.16fgh ± 0.2973 | 60cdefg ± 9.37 | 11.55fgh ± 0.689 |
| 23 | P233 | 13.9abc ± 2.200 | 24.325abcde ± 0.531 | 32.82abcd ± 1.641 | 35.86efg ± 0.3638 | 41.58gh ± 4.30 | 30.98cde ± 12.056 |
| 24 | P247 | 16a ± 1.567 | 22.3bcdefghi ± 1.219 | 33.79abc ± 3.066 | 37.28bcde ± 0.4847 | 44.5efg ± 11.85 | 7.14gh ± 0.865 |
| 25 | P248 | 10defg ± 0.799 | 20.825 ghijk ± 1.314 | 29.48cdefg ± 1.389 | 38.12abcd ± 0.5951 | 112.73a ± 4.10 | 36.7bcd ± 3.604 |
| Max. | 16a ± 1.567 | 24.95a ± 0.833 | 36.52a ± 2.766 | 39.78a ± 0.9069 | 112.73a ± 4.10 | 63.75a ± 5.883 | |
| Min. | 7.15g ± 0.891 | 19.025k ± 1.424 | 24.38h ± 1.906 | 30.77k ± 1.0157 | 20.33h ± 1.13 | 3.95h ± 0.466 | |
| CD 0.01 | 5.077 | 3.390 | 6.165 | 2.636 | 30.907 | 20.522 | |
| CD 0.05 | 3.825 | 2.568 | 4.652 | 1.987 | 23.295 | 15.464 | |
| C V | 26.234 | 8.167 | 12.461 | 4.372 | 27.961 | 41.673 | |
| F.cal** | 2.987 | 3.412 | 2.814 | 9.65 | 5.559 | 10.136 | |
Values are average of three replications; values after ± represents standard deviation; CV, coefficient of variance; CD, critical difference; ** Values are significant at 1 and 5% levels; As per Duncan’s grouping means with the same letter are not significantly different
Table 4.
Plant growth promoting response of lathyrus, chickpea, green gram and black gram seedlings following seed bacterization with fluorescent Pseudomonas isolates
| S. no. | Treat | Lathyrus | Chickpea | Greengram | Blackgram | ||||
|---|---|---|---|---|---|---|---|---|---|
| Root length (cm) | Shoot length (cm) | Root length (cm) | Shoot length (cm) | Root length (cm) | Shoot length (cm) | Root length (cm) | Shoot length (cm) | ||
| 1 | Control | 18.185bcdefg ± 0.679 | 13.365i ± 1.094 | 28.333bcdef ± 0.726 | 16.333mn ± 0.498 | 17.28bcde ± 2.10 | 13.7h ± 1.747 | 16.435ef ± 0.733 | 12.625mnop ± 0.3637 |
| 2 | P5 | 20.565abcde ± 0.957 | 16.635cdefg ± 0.835 | 29bcdef ± 6.449 | 14.933n ± 0.536 | 15.39de ± 2.58 | 14.665fgh ± 0.830 | 18.565bcdef ± 1.115 | 15.815defgh ± 0.3044 |
| 3 | P6 | 19.415abcde ± 0.853 | 19.135abc ± 0.382 | 20.533fg ± 0.088 | 21.066defghi ± 0.636 | 18.04bcd ± 1.80 | 18.225abcd ± 1.19 | 20.985abcd ± 1.401 | 18.025ab ± 0.5456 |
| 4 | P11 | 22.575ab ± 1.424 | 19.835ab ± 0.521 | 29.833bcde ± 0.441 | 22.866bcde ± 0.754 | 18.03bcd ± 0.44 | 20.385a ± 0.987 | 19.925bcde ± 1.858 | 16defg ± 0.3969 |
| 5 | P67 | 19.385bcdef ± 2.496 | 13.885hi ± 0.922 | 27.333cdef ± 1.202 | 21.5cdefg ± 1.510 | 14.72de ± 0.48 | 17.625bcde ± 0.468 | 20.215bcde ± 1.540 | 13.375jklmnop ± 0.742 |
| 6 | P72 | 19.085bcdef ± 1.464 | 16.2defgh ± 0.450 | 38.85a ± 6.438 | 25.7a ± 0.115 | 17.47bcde ± 1.12 | 16.1defgh ± 1.022 | 20.225bcde ± 2.244 | 13.165lmnop ± 0.4625 |
| 7 | P76 | 12.785 g ± 0.585 | 13.15i ± 0.157 | 36.833ab ± 2.920 | 21.266defgh ± 0.504 | 13.99e ± 2.29 | 14.8fgh ± 0.452 | 19.15bcde ± 1.187 | 14.575hijk ± 0.3172 |
| 8 | P85 | 18.285bcdef ± 2.483 | 20.815a ± 0.780 | 28.833bcdef ± 0.833 | 23.233bcd ± 0.536 | 16.22cde ± 0.68 | 14.985fgh ± 1.236 | 20.665abcde ± 0.959 | 16.8bcde ± 0.3007 |
| 9 | P99 | 22.35ab ± 3.126 | 17.765bcdef ± 0.677 | 34.166abc ± 2.351 | 21.7cdefg ± 0.751 | 15.82cde ± 1.29 | 19.125abc ± 0.859 | 19.765bcde ± 0.251 | 18.615a ± 0.3912 |
| 10 | P124 | 21.375abcd ± 3.196 | 20.2ab ± 0.774 | 26.833cdef ± 4.086 | 23.833abc ± 0.167 | 17.95bcd ± 0.77 | 17.615bcde ± 0.750 | 18.635bcdef ± 0.884 | 15.235fghi ± 0.6759 |
| 11 | P126 | 16.375defg ± 1.830 | 18.1bcde ± 0.174 | 23defg ± 2.021 | 20.75efghi ± 1.010 | 15.99cde ± 1.49 | 15.325efgh ± 0.820 | 17.425def ± 0.574 | 12.5nop ± 0.3048 |
| 12 | P129 | 16.465defg ± 1.723 | 18.015bcde ± 0.923 | 27.666cdef ± 0.882 | 25.066ab ± 0.924 | 15.69cde ± 2.80 | 16.865cdefg ± 1.170 | 19.715bcde ± 1.178 | 14.025ijklm ± 0.4498 |
| 13 | P141 | 15.665efg ± 2.070 | 14.8ghi ± 1.093 | 33.166abc ± 2.242 | 18.933hijkl ± 0.470 | 22.15a ± 0.76 | 16.4defg ± 0.524 | 19.65bcde ± 0.991 | 17.515abc ± 0.4502 |
| 14 | P143 | 17.55bcdefg ± 0.484 | 15.285fghi ± 0.739 | 33.65abc ± 3.839 | 21defghi ± 1.732 | 18.49abcd ± 0.84 | 19.175abc ± 1.301 | 22.135abc ± 2.148 | 17.685abc ± 0.4719 |
| 15 | P151 | 13.965fg ± 0.380 | 16.8cdefg ± 1.365 | 31.433abcd ± 2.599 | 21.066defghi ± 0.581 | 19.59abc ± 1.48 | 20.235a ± 1.473 | 16.465ef ± 0.802 | 12.335op ± 0.4575 |
| 16 | P161 | 15.775efg ± 1.730 | 13.115i ± 0.984 | 30.5abcde ± 2.180 | 22.166cdef ± 0.928 | 15.94cde ± 0.38 | 16.185defg ± 0.648 | 16.785def ± 1.574 | 13.275klmnop ± 0.2087 |
| 17 | P167 | 18.735bcdef ± 0.671 | 16.85cdefg ± 1.646 | 22efg ± 4.726 | 16.833lmn ± 0.899 | 18.63abcd ± 1.27 | 16.465defg ± 0.390 | 17.835def ± 1.218 | 13.885ijklmn ± 0.3319 |
| 18 | P176 | 20.525abcde ± 2.771 | 18.785abcd ± 1.573 | 24.25defg ± 3.320 | 19.5ghijk ± 1.155 | 17.08bcde ± 0.23 | 19.675ab ± 0.338 | 22.7ab ± 2.257 | 17.165bcd ± 0.6932 |
| 19 | P179 | 16.7cdefg ± 1.198 | 13.365i ± 0.546 | 16.75g ± 1.299 | 18.25jklm ± 0.722 | 20.37ab ± 0.17 | 16.065defgh ± 0.228 | 17.75def ± 2.027 | 16.5cdef ± 0.3857 |
| 20 | P201 | 19.915bcde ± 3.689 | 16.065efgh ± 0.933 | 24.4defg ± 3.114 | 19.733ghijk ± 0.536 | 16.47bcde ± 1.08 | 16.935cdef ± 0.388 | 19.885bcde ± 1.499 | 16.75bcde ± 0.4392 |
| 21 | P205 | 18.615bcdef ± 0.742 | 16.525defg ± 0.545 | 23.933defg ± 0.788 | 20.166fghij ± 0.601 | 16.60bcde ± 1.80 | 14.45gh ± 0.149 | 16.95def ± 1.551 | 14.175ijkl ± 0.3099 |
| 22 | P216 | 25.7a ± 2.957 | 15.185fghi ± 0.508 | 29.866bcde ± 4.332 | 20.766efghi ± 0.865 | 18.00bcd ± 1.41 | 16.965cdef ± 0.889 | 19.675bcde ± 2.523 | 14.7ghij ± 0.8147 |
| 23 | P233 | 15.715efg ± 1.047 | 14.65ghi ± 0.899 | 28.666bcdef ± 0.441 | 22.5cdef ± 1.155 | 17.84bcde ± 1.07 | 18.065abcd ± 0.268 | 24.825a ± 2.510 | 15.585efgh ± 0.8166 |
| 24 | P247 | 19.315bcdef ± 1.818 | 19.825ab ± 1.567 | 31.4abcd ± 2.358 | 18.733ijkl ± 0.617 | 16.00cde ± 0.97 | 16.65defg ± 0.388 | 18.125cdef ± 1.196 | 15.7efgh ± 0.4449 |
| 25 | P248 | 21.93abc ± 2.049 | 20.705a ± 0.755 | 26.033cdef ± 2.338 | 17.366klm ± 0.857 | 16.09cde ± 1.12 | 15.2efgh ± 0.228 | 14.585f ± 0.716 | 16.8bcde ± 0.6334 |
| Max. | 25.7a ± 2.957 | 20.815a ± 0.780 | 38.85a ± 6.438 | 25.7a ± 0.115 | 22.15a ± 0.76 | 20.385a ± 0.987 | 24.825a ± 2.510 | 18.615a ± 0.3912 | |
| Min. | 12.785g ± 0.585 | 13.115i ± 0.984 | 16.75g ± 1.299 | 14.933n ± 0.536 | 13.99e ± 2.29 | 14.665fgh ± 0.830 | 14.585f ± 0.716 | 12.335op ± 0.4575 | |
| CD 0.01 | 7.243 | 3.447 | 11.443 | 3.190 | – | 3.268 | 5.700 | 1.857 | |
| CD 0.05 | 5.467 | 2.590 | 8.584 | 2.391 | 3.923 | 2.464 | 4.298 | 1.401 | |
| C V | 20.760 | 10.994 | 18.490 | 7.075 | 16.217 | 10.361 | 15.917 | 6.495 | |
| Fcal** | 2.274 | 7.27 | 2.835 | 9.781 | 1.688 | 4.488 | 2.115 | 14.069 | |
Values are average of three replications; values after ± represents standard deviation; CV, coefficient of variance; CD, critical difference; ** Values are significant at 1 and 5% levels; as per Duncan’s grouping means with the same letter are not significantly different
Potential isolates stimulating plant growth (coleoptiles elongation and/or root length) in different crops were as follows
Wheat (GW-272): P124 stimulated coleoptiles elongation by 20.23% and root length by 17.30%; Chickpea (Cicer arientinum): P72 stimulated coleoptiles elongation 36.45% and root length by 27.08%; Lathyrus (KH-014): P85 stimulated coleoptiles elongation by 35.78% and P216 stimulated root length by 29.22%; Greengram (puspa vishal): P11 stimulated coleoptiles elongation by 32.81%; Blackgram (T-U-94-2): P99 stimulated coleoptiles elongation 32.16% and P233 stimulated root length by 33.8%; Bottlegourd (Lagenaria siceraria): P248 stimulated coleoptiles elongation 72.39% and P167 root length by 68.83%; Rice (swarna): P176 stimulated coleoptiles elongation 16.56% and P247 stimulated root length by 41.69%. Fluorescent Pseudomonas isolates stimulating both coleoptiles elongation and/or root length were P141 on greengram; P6, P143, P176 and P233 on blackgram; P76, P99, P124 and P167 on bottlegourd; and P151, P233 on rice. Fluorescent Pseudomonas isolates stimulating only root length were: P72, P129, P141 and P151 on bottlegourd; and P67 and P247 on rice.
Discussion
Understanding the mechanisms involved in the antagonist interactions between bacteria, pathogen and host plant is important for efficient utilization of these natural resources in crop health management (Thomashow and Weller 1991). In soil, plant roots normally coexist with bacteria and fungi which may produce siderophores capable of sequestering the available soluble iron and hence interfere with plant growth and function. Siderophore production confers competitive advantages to PGPR that can colonize roots and exclude other microorganisms from this ecological niche (Haas and Défago 2005). Under highly competitive conditions, the ability to acquire iron via siderophores may determine the outcome of competition for different carbon sources that are available as a result of root exudation or rhizo deposition (Crowley 2006). Siderophores production by strains of Pseudomonas spp. for plant disease control is of great interest because of its possibilities in the substitution of chemical pesticides. In this study, we have compared the ability of several fluorescent Pseudomonads to produce suderophores, cyanogenesis and antagonism in plate assay. All potential fluorescent Pseudomonas isolates identified following confrontation assays were high siderophore producers except P167, P248. Of the potential antagonistic fluorescent Pseudomonas isolates P233, P201, P176, were effective against R. solani where as P76 against both R solani and S. rolfsii were avid iron chelators and high siderophore producers. Similarly, microbial cyanogenesis has been demonstrated in a few bacterial species (belonging to the genera Pseudomonas, Chromobacterium, Rhizobium and several cyanobacteria (Blumer and Haas 2000). Glycine has generally been used as a precursor of cyanide in fungi and bacteria (Brysk et al. 1969; Wissing 1974) and cyanogenesis is one of the mechanisms of antagonism and biocontrol properties (Haas and Défago 2005; Lanteigne et al. 2012). In this investigation identified cynogenic isolates P76 and P124 exerted strong inhibition against S. rolfsii where as cynogenic P179 was ineffective against R solani and S. rolfsii remains unexplained. Our study revealed that isolates vary in the mechanisms and ability to inhibit pathogens. Plant growth-promoting bacteria use a number of different mechanisms to promote the growth of plants (Glick 2012), but enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing strains are the key bacterial trait which relives plants from deleterious effects of ethylene by cleaving ACC, into ammonia and –ketobutyrate (Honma and Shimomura 1978) and also facilitates plant growth (Glick et al. 2007; Glick 2014). ACC deaminase activity revealed wide variation in ACCd enzyme production in the range of 40.87 ± 0.08 to 25.02 ± 0.37 and 23.94 ± 0.32 to 10.51 ± 0.06 µmol α ketobutyrate/mg protein/h and P141 > P247 > P126, were potential ACCd enzyme producer. Fluorescent pseudomonas are one of the most abundant bacteria in the rhizosphere of many plants (Botelho and Mendonça-Hagler 2006), have large capacity to produce phytohormones, mainly auxins (Patten and Glick 2002a, b; Khalid et al. 2005) and secondary metabolites, such as antibiotics (Bergsma-Vlami et al. 2005), thus they are able to improve plant growth and plant health (Belimov et al. 2009a, b). Seed (of crops) biopriming with different isolates of fluorescent pseudomonas with ability to produce different levels of ACCd enzyme and siderophore were correlated with plant growth promoting effects. More stimulatory effects on coleoptile elongation than root length were observed. Our combined in vitro and pot experiment show the potential of isolate P176 to be developed as a commercial bio-inoculant as it stimulated coleoptiles elongation on all seven crops tested. Noticeable effects of plant growth stimulation were observed more on legume crops than on cereals. Potential isolates stimulating plant growth (coleoptiles elongation and or root length) specific to different crops were as follows: Both coleoptile elongation and root length:- Wheat (P124), Chickpea (P72); Only coleoptile elongation:- Greengram (P11), Lathyrus (P85), Blackgram (P99), Bottlegourd (P248), Rice (P176); Only root length:- Lathyrus (P216), Blackgram (P233), Bottlegourd (P167), Rice (P247). Nonetheless, this study and the results are particularly useful for identifying likely candidates for bio-control and for making educated guesses concerning the mechanisms by which they induce plant growth.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Arnow LE. Colorimetric determination of the components of 3, 4-dihydroxyphenylalanine-tyrosine mixtures. J Biol Chem. 1937;118:531–537. [Google Scholar]
- Belimov AA, Dodd IC, Hontzeas N, et al. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 2009;181:413–423. doi: 10.1111/j.1469-8137.2008.02657.x. [DOI] [PubMed] [Google Scholar]
- Belimov AA, Dodd IC, Safronova VI et al (2009b) ACC deaminase-containing rhizobacteria improve vegetative development and yield of potato plants grown under water-limited conditions. Asp Appl Biol 163–169
- Beneduzi A, Ambrosini A, Passaglia LMP. Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol. 2012;35:1044–1051. doi: 10.1590/S1415-47572012000600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma-Vlami M, Prins ME, Staats M, Raaijmakers JM. Assessment of genotypic diversity of antibiotic-producing Pseudomonas species in the rhizosphere by denaturing gradient gel electrophoresis. Appl Environ Microbiol. 2005;71:993–1003. doi: 10.1128/AEM.71.2.993-1003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazevic DJ, Koepcke MH, Matsen JM. Incidence and identification of Pseudomonas fluorescens and Pseudomonas putida in the clinical laboratory. Appl Microbiol. 1973;25:107–110. doi: 10.1128/am.25.1.107-110.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer C, Haas D. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch Microbiol. 2000;173:170–177. doi: 10.1007/s002039900127. [DOI] [PubMed] [Google Scholar]
- Botelho GR, Mendonça-Hagler LC. Fluorescent Pseudomonads associated with the rhizosphere of crops: an overview. Brazilian J Microbiol. 2006;37:401–416. doi: 10.1590/S1517-83822006000400001. [DOI] [Google Scholar]
- Brysk MM, Lauinger C, Ressler C (1969) Biosynthesis of Cyanide from [2-14 C 15 N] Glycine in Chromobacterium violaceum. Biochim Biophys Acta (BBA)-General Subj 184:583–588 [DOI] [PubMed]
- Choudhary DK, Prakash A, Wray V, Johri BN. Insights of the fluorescent pseudomonads in plant growth regulation. Curr Sci. 2009;97:170–179. [Google Scholar]
- Crowley DE (2006) Microbial siderophores in the plant rhizosphere. In: Iron nutrition in plants and rhizospheric microorganisms. Springer, Berlin pp 169–198
- De Brito AM, Gagne S, Antoun H. Effect of compost on rhizosphere microflora of the tomato and on the incidence of plant growth-promoting rhizobacteria. Appl Environ Microbiol. 1995;61:194–199. doi: 10.1128/aem.61.1.194-199.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente L, Landa BB, Weller DM. Host crop affects rhizosphere colonization and competitiveness of 2, 4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology. 2006;96:751–762. doi: 10.1094/PHYTO-96-0751. [DOI] [PubMed] [Google Scholar]
- Fravel DR. Commercialization and implementation of biocontrol 1. Annu Rev Phytopathol. 2005;43:337–359. doi: 10.1146/annurev.phyto.43.032904.092924. [DOI] [PubMed] [Google Scholar]
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1–15. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169:30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. In: New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research. Springer, Berlin pp 329–339
- Haas D, Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- Honma M, Shimomura T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem. 1978;42:1825–1831. [Google Scholar]
- Khalid A, Tahir S, Arshad M, Zahir ZA. Relative efficiency of rhizobacteria for auxin biosynthesis in rhizosphere and non-rhizosphere soils. Soil Res. 2005;42:921–926. doi: 10.1071/SR04019. [DOI] [Google Scholar]
- Lagzian A, Saberi Riseh R, Khodaygan P, et al. Introduced Pseudomonas fluorescens VUPf5 as an important biocontrol agent for controlling Gaeumannomyces graminis var. tritici the causal agent of take-all disease in wheat. Arch Phytopathol Plant Prot. 2013;46:2104–2116. doi: 10.1080/03235408.2013.785123. [DOI] [Google Scholar]
- Lanteigne C, Gadkar VJ, Wallon T, et al. Production of DAPG and HCN by Pseudomonas sp. LBUM300 contributes to the biological control of bacterial canker of tomato. Phytopathology. 2012;102:967–973. doi: 10.1094/PHYTO-11-11-0312. [DOI] [PubMed] [Google Scholar]
- Patten CL, Glick BR. Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can J Microbiol. 2002;48:635–642. doi: 10.1139/w02-053. [DOI] [PubMed] [Google Scholar]
- Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol. 2002;68:3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Podile AR, Kishore GK (2007) Plant growth-promoting rhizobacteria. In: Plant-associated bacteria. Springer, Berlin pp 195–230
- Santoyo G, del Orozco-Mosqueda MC, Govindappa M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Biocontrol Sci Technol. 2012;22:855–872. doi: 10.1080/09583157.2012.694413. [DOI] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Sneath PHA (1986) Bergey’s manual of systematic bacteriology, vol 2. William & Wilkins, Baltimore, USA
- Thomashow LS, Weller DM (1991) Role of antibiotics and siderophores in biocontrol of take-all disease of wheat. In: The rhizosphere and plant growth. Springer, Berlin pp 245–251
- Wei G, Kloepper JW, Tuzun S. Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology. 1991;81:1508–1512. doi: 10.1094/Phyto-81-1508. [DOI] [Google Scholar]
- Weller DM. Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology. 2007;97:250–256. doi: 10.1094/PHYTO-97-2-0250. [DOI] [PubMed] [Google Scholar]
- Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS. Microbial populations responsible for specific soil suppressiveness to plant pathogens 1. Annu Rev Phytopathol. 2002;40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. [DOI] [PubMed] [Google Scholar]
- Wissing F. Cyanide formation from oxidation of glycine by a Pseudomonas species. J Bacteriol. 1974;117:1289–1294. doi: 10.1128/jb.117.3.1289-1294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]