Abstract
The chemically modified tripeptide glycyl-prolyl-glycine-amide (GPG-NH2) inhibits replication of human immunodeficiency virus (HIV) type 1 (HIV-1) in vitro, probably by interfering with capsid formation. The aim of the present study was to determine whether the metabolites glycyl-proline (GP-OH), glycine (G-OH), prolyl-glycine-amide (PG-NH2), proline (P-OH), and glycine-amide (G-NH2) from proteolytic cleavage may inhibit the replication of HIV-1 in vitro. PG-NH2 has previously been shown to have a modest effect on HIV-1 replication. In the present study we show that G-NH2 exhibits a pronounced inhibitory effect on HIV-1. This effect was not due to a decrease in cell proliferation or viability and could not be shown for herpes simplex virus type 1. The G-NH2 concentration that inhibited virus replication by 50% (IC50) was equimolar to that of GPG-NH2 and ranged from 3 to 41 μM. Transmission electron microscopy revealed that the effect of G-NH2 on HIV-1 morphology was equivalent to that of GPG-NH2 and showed disarranged capsid structures, indicating interference with capsid formation. Serial passage of HIV-infected cells with G-NH2 for more than 20 subcultivations did not decrease the susceptibility to the compound. The results from this study suggest that GPG-NH2 might act as a prodrug and that G-NH2 is an active antiretroviral metabolite.
Combination therapy comprising several antiretroviral drugs has become the standard treatment for human immunodeficiency virus (HIV)-infected patients. These drugs can be divided into four classes: (i) nucleoside or nucleotide reverse transcriptase inhibitors, (ii) nonnucleoside reverse transcriptase inhibitors, (iii) protease inhibitors, and (iv) fusion inhibitors. Despite the numerous different drugs, therapy is associated with severe side effects, poor compliance, and the development of resistance. Moreover, the rates of transmission of drug-resistant HIV strains are increasing. Long-term, probably life-long, treatment is required, and consequently, there is a need for new, safer antiretroviral drugs (6, 16).
Short, chemically modified peptides such as glycyl-prolyl-glycine-amide (GPG-NH2) can inhibit the replication of HIV type 1 (HIV-1) in vitro (15). Electron microscopy studies have indicated a possible interaction of GPG-NH2 with capsid formation and virus assembly (7), thus indicating a potential new class of antiretroviral drug.
Since digested proteins and peptides are enzymatically cleaved in the gut to facilitate the uptake of dipeptides and free amino acids (reviewed by Mariotti et al. [12]), we wanted to establish whether any metabolite of GPG-NH2 from such cleavage shows an antiretroviral effect. Several different classes of proteolytic enzymes may metabolize short peptides such as GPG-NH2, for example, (i) aminopeptidases, which act at the N terminus and liberate single amino acids; (ii) carboxypeptidases, which act at the C terminus and liberate single amino acids; (iii) dipeptidylpeptidases, which act at the N terminus and liberate dipeptides; and (iv) prolyl oligopeptidase, which cleaves peptide bonds on the carboxyl end of a proline (3). Proteolytic cleavage of GPG-NH2 may result in five fragments: glycine (G-OH), prolyl-glycine-amide (PG-NH2), glycyl-proline (GP-OH), proline (P-OH), and glycine-amide (G-NH2). In the initial screening of peptides for their antiretroviral effects, PG-NH2 was tested and showed only a modest, if any, effect on HIV-1 replication (15). The aim of this study was to reveal whether any potential metabolite from the proteolytic cleavage of GPG-NH2 can inhibit the replication of HIV-1. The cleavage products were tested for their antiretroviral effects in vitro. GP-OH, PG-NH2, P-OH, and G-OH did not show any inhibitory effect on HIV-1. G-NH2, on the other hand, was effective against HIV-1 but not herpes simplex virus type 1 (HSV-1). To confirm that the antiretroviral properties of G-NH2 were not due to any effect on the cells, the proliferation and viabilities of the treated cells were tested. Transmission electron microscopy (TEM) of G-NH2-treated cells indicated that the effect of G-NH2 on the viral core structure resembles the effect previously shown with GPG-NH2.
In selection studies it has been shown that resistance to GPG-NH2 cannot be generated even after 30 passages (1). In the present study, 22 passages with G-NH2 were performed, and no emergence of resistant mutants could be detected.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells (PBMCs) from healthy blood donors were purified by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation and cultured in RPMI 1640 medium (Gibco, Paisley, United Kingdom) supplemented with 10% heat-inactivated fetal calf serum (FCS; Sigma, St. Louis, Mo.) and 0.1% penicillin-streptomycin (AstraZeneca, Södertälje, Sweden, and Sigma, Steinheim, Germany). Cells used for HIV-1 culture were stimulated with phytohemagglutinin (PHA; 2.5 μg/ml; Becton Dickinson Microbiology Systems, Sparks, Md.) for 3 to 5 days. The medium was then supplemented with proleukin (200 IU/ml; Chiron, Amsterdam, The Netherlands), hydrocortisone (5 μg/ml; Sigma, St. Louis, Mo.), and polybrene (2.24 μg/ml; Sigma, St. Louis, Mo.). H9 and ACH-2 cells were cultured in the same medium but without proleukin or hydrocortisone and with 20 and 10% FCS, respectively. African green monkey kidney (GMK AH1) cells were cultured in Eagle minimum essential medium (Gibco) supplemented with 2% calf serum and antibiotics. All cells were incubated at 37°C with 5% CO2 and 95% humidity.
IC50 determinations and selection of drug resistance.
Stocks of clinical HIV-1 isolates were prepared by collecting the supernatants from infected cultures of PBMCs after one passage. The 50% tissue culture infective dose (TCID50) endpoint for each clinical isolate was determined by infecting PBMCs (600,000 cells/ml) with serial, 10-fold dilutions of the virus (in duplicate). The medium was changed on day 7, and the experiment was terminated on day 14. After termination the amounts of p24 antigen were determined by an in-house p24 antigen enzyme-linked immunosorbent assay essentially as described previously (8). Recombinant p24 (Protein Sciences Corp., Meriden, Conn.) at fixed concentrations served as a standard, and the detection limit of the assay was 0.5 ng/ml.
The drug susceptibilities of the clinical isolates were evaluated essentially as described previously (9). PBMCs (600,000 cells/ml) were infected with non-syncytium-inducing clinical isolates at 100 TCID50s for 2 h at 37°C, resuspended in fresh medium containing various concentrations (1, 4, 16, 64, and 256 μM) of test compound, and incubated at 37°C (all peptides and amino acids were provided by Bachem, Bubendorf, Switzerland). Infected cultures without drug served as controls, and each isolate was plated in duplicate. Medium was changed at days 5 to 7 postinfection, and the supernatants were monitored for their p24 antigen contents (by the in-house enzyme-linked immunosorbent assay described above) until the termination of incubation at day 14. When the virus yield reached approximately 80% of the maximum (days 5 to 9), the drug concentration that inhibited virus replication by 50% (IC50) was calculated by plotting the amount of p24 antigen against the drug concentration. We used H9 cells (500,000 cells/ml) to determine the IC50s for HIV-1IIIB. Selection of resistance to G-NH2 and subsequent sequencing of the p24 gene were performed as described previously (1), with an initial G-NH2 concentration of 100 μM and a final concentration of 500 μM.
HSV-1 production assay.
The KOS 321 strain of HSV-1 was inoculated on GMK cells at 3.4 × 102 PFU/ml in medium containing methylcellulose, and GPG-NH2 or G-NH2 (20, 100, or 500 μM) was added. Mock-treated cultures and cultures treated with acyclovir (100 μM; Zovirax, Wellcome, London, United Kingdom) served as controls. After 3 to 4 days the cultures were stained with crystal violet to visualize the plaques, and the number of plaques was counted.
Proliferation.
PBMCs (600,000 cells/ml) were cultured for 96 h in RPMI 1640 medium supplemented with 10% inactivated FCS, antibiotics, and PHA (2.5 μg/ml). The cells were treated with GPG-NH2, G-OH, or G-NH2 at different concentrations (10, 50, 100, or 500 μM); and [3H]thymidine (1 μCi/100,000 cells; Amersham Pharmacia Biotech, Freiburg, Germany) was added to the cultures 8 h before termination of the experiment. Lysed cells were harvested on a nitrocellulose filter, and radioactivity (counts per minute) was recorded on a β-scintillation counter (Direct Beta Counter; Packard Instrument Company, Meriden, Conn.). Untreated cells and cells treated with the immunosuppressive agent cyclosporine (1 μg/ml; Sandimmun; Novartis, Täby, Sweden) served as controls.
Viability.
PBMCs (600,000 cells/ml) were cultured with 2 mM G-NH2 or GPG-NH2 for 14 days. After 7 and 14 days, viable and disrupted cells were counted in a Bürcher chamber after the cells were stained with trypan blue solution (0.4%; Sigma, St. Louis, Mo.). Untreated cells served as controls.
TEM of virus assembly.
HIV-1-infected ACH-2 cells, untreated or treated with 100 μM G-NH2, were fixed with freshly prepared 2.5% glutaraldehyde in phosphate buffer and were postfixed in 1% osmium tetroxide. The cells were embedded in Epon and poststained with 1% uranyl acetate. Sections were made approximately 60 nm thick to accommodate the volume of the core structure parallel to the section plane. Duplicate sample preparations were made. Specimens were analyzed with a Zeiss CEM 902 electron microscope, equipped with a spectrometer to enhance image contrast, at an accelerating voltage of 80 kV. A liquid nitrogen-cooling trap of the specimen holder was used throughout. Evaluation of morphology was performed in a blinded manner with a series of electron micrographs to depict different categories of virus morphology. The specific focus was on the packing of the virus core structure. Statistical evaluation was performed by using the chi-square distribution.
RESULTS
IC50 determinations and selection of drug resistance.
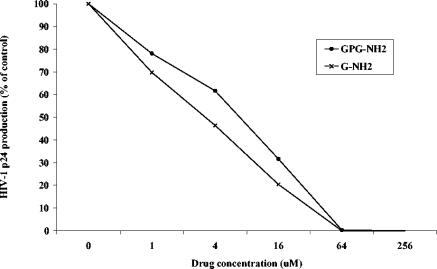
To evaluate whether the potential metabolites from proteolytic cleavage of GPG-NH2 can inhibit the replication of HIV-1, GP-OH, PG-NH2, G-OH, and G-NH2 were tested for their antiretroviral effects in vitro. H9 cells were infected with HIV-1SF-2 and cultured in medium containing 100 μM test compound. The metabolites GP-OH, PG-NH2, and G-OH had no effect on HIV-1 replication (Fig. 1). On the other hand, G-NH2 inhibited the replication of HIV-1. To evaluate the IC50 of G-NH2, various concentrations of the test compound (1, 4, 16, 64, or 256 μM) were added to HIV-1IIIB-infected H9 cells. The IC50 was calculated by plotting the amount of p24 against the drug concentration, and it was found that G-NH2 inhibited HIV-1 replication at approximately the same concentration as GPG-NH2. IC50s ranged from 7.4 to 21 μM for GPG-NH2 and from 3.2 to 22 μM for G-NH2 (Table 1; Fig. 2).
FIG. 1.
Effect of the metabolites and GPG-NH2 on HIV-1 replication. Each test compound was added to HIV-1-infected H9 cells at 100 μM, and the level of production of p24 was measured. The data represent mean values and standard deviations for three to four samples.
TABLE 1.
Sensitivities of HIV-1 strains to GPG-NH2 and G-NH2
| HIV-1 strain | IC50 (μM [range])
|
|
|---|---|---|
| GPG-NH2 | G-NH2 | |
| IIIB | 16a (7.4-21) | 11a (3.2-22) |
| Clinical isolatesb | 14 (2.7-24) | 23 (14-41) |
The IC50s are averages of six tests.
Seven clinical isolates were tested.
FIG. 2.
Production of p24 plotted against escalating drug concentration after treatment of HIV-1IIIB-infected H9 cells with G-NH2 or GPG-NH2. The IC50s were 3.2 μM for G-NH2 and 11 μM for GPG-NH2.
To further ascertain the antiretroviral effect of G-NH2, seven non-syncytium-inducing clinical isolates were tested for their susceptibilities to GPG-NH2 and G-NH2 in vitro. Both compounds were effective against all isolates tested, with IC50s ranging from 2.7 to 25 μM for GPG-NH2 and from 14 to 41 μM for G-NH2.
Four amino acids in their natural and amidated forms (phenylalanine, proline, serine, and tryptophan) were tested but did not show any antiretroviral effect on HIV-1IIIB in H9 cells.
After 22 passages in vitro, the concentration of G-NH2 was increased from 100 to 500 μM. However, no effect on susceptibility to G-NH2 was found. The IC50s at passage 22 were 4.8 μM for treated HIV-1 and 5.7 μM for untreated HIV-1. Sequencing of the HIV-1 p24 gene did not reveal any mutations after 22 passages.
HSV-1 production.
HSV-1KOS 321 was cultured in GMK cells containing GPG-NH2 or G-NH2 (20, 100, or 500 μM). The number of plaques was counted, and the plaque counts for the treated cultures were compared with those for mock-treated cultures or cultures treated with acyclovir (100 μM). No inhibitory effect of either GPG-NH2 or G-NH2 was found, and treatment with each compound at 500 μM resulted in plaque count reductions of only 5 and 9%, respectively. Treatment with acyclovir resulted in a 98% reduction in plaque counts.
Proliferation.
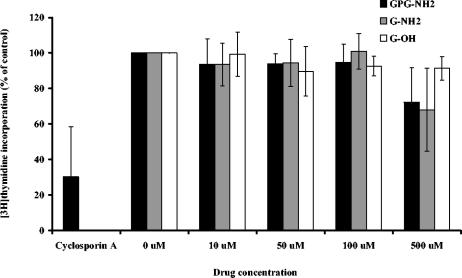
PBMCs were treated with GPG-NH2, G-OH, or G-NH2 at different concentrations (10, 50, 100, or 500 μM); and [3H]thymidine incorporation was measured after 4 days. No significant decrease in DNA synthesis could be found after treatment with the respective test compound at concentrations up to 500 μM (Fig. 3). Treatment with cyclosporine resulted in 70% reduced thymidine incorporation into DNA.
FIG. 3.
Effects of treatment with GPG-NH2, G-NH2, G-OH, or cyclosporine (1 μg/ml) on the proliferation of PHA-stimulated PBMCs after 4 days. Incorporation of [3H]thymidine was measured in a β-scintillation counter and plotted (as a percentage of the thymidine incorporation for the control) against the drug concentration. The data represent mean values and standard deviations for three to four tests (n = 6).
Viability.
PBMCs were treated with 2 mM GPG-NH2 or G-NH2 and were cultured for 14 days. After 7 days, dead cells were stained with trypan blue solution, and all cells were counted under a light microscope. No decrease in viability was found after treatment with GPG-NH2 or G-NH2 (the proportions of viable cells were 102 and 100% of those for the untreated control, respectively). Even after 14 days no decrease in viability compared to that for the control was found.
TEM studies of virus assembly.
HIV-1-infected ACH-2 cells, untreated or treated with 100 μM G-NH2, were analyzed for capsid morphology by TEM. The micrographs of virus specimens were divided into two groups: one group had immature and mature virus core structures, and one group had aberrant core structures. The latter category included particles with partially filled, tubular, irregular, or empty cores. Virus particles (n = 389) from untreated cells were morphologically evaluated; 80% showed normal cores, whereas 20% had aberrant core structures (Fig. 4a). Of 738 virus particles from G-NH2-treated cells, 42% were determined to have normal core structures and 58% were determined to have aberrant core structures (Fig. 4b, c, and d). A highly significant difference (P < 0.001) was observed between the two groups. Virus particles from G-OH-treated cells showed morphologies equivalent to those of control virus particles (data not shown).
FIG. 4.
Morphological studies of HIV-1 virions produced from untreated or G-NH2-treated cells and analyzed with TEM. (a) Wild-type HIV-1 showing characteristic dense, cone-shaped core (left) and round, centrally placed tangential sections (middle and right); (b) HIV-1 treated with 100 μM G-NH2 showing empty, multiple core structures (right); (c) HIV-1 treated with 100 μM G-NH2 showing a double core (left and above) and distorted core structure (right); (d) HIV-1 treated with 100 μM G-NH2 showing an elongated core in an enlarged virus envelope (below), a hollow core (left above), and a double core structure (right). Bars, 100 nm.
DISCUSSION
Since digested proteins and peptides are enzymatically cleaved in the gut to facilitate uptake of dipeptides and free amino acids, we wanted to establish whether any metabolite of GPG-NH2 from such cleavage shows an antiretroviral effect. In the study described in this report, we found that one potential metabolite (G-NH2) from proteolytic cleavage of GPG-NH2 can inhibit the replication of HIV-1 in vitro at approximately the same concentrations as GPG-NH2. Recent data (2) have shown that one enzyme responsible for the conversion of GPG-NH2 to G-NH2 may be dipeptidylpeptidase IV, or CD26. CD26 cleaves dipeptides from the amino terminus of peptides under the condition that the penultimate amino acid is a proline or, with a lesser preference, an alanine or a leucine. CD26 is a membrane-associated peptidase expressed on a variety of cells, but it is also present in a soluble form in plasma and cerebrospinal fluid (for a review, see reference 4). The conversion of GPG-NH2 to G-NH2 should therefore take place in our in vitro cell cultures, since CD26 is present both on the lymphocytes and in the serum component of the growth medium. However, the fact that G-NH2 showed pronounced antiretroviral activity does not exclude the possibility that it may be further metabolized before it exerts its effect.
Further studies showed that G-NH2 inhibited a variety of clinical HIV-1 isolates as well as laboratory strain HIV-1IIIB. The average IC50 of G-NH2 for laboratory strain HIV-1IIIB was twofold lower than the IC50 for the clinical isolates. The reason for this might be that the different cell types and different medium conditions influence the activity of the compound. For clinical isolates, but not the laboratory strain, there was also a twofold increase in the IC50 of G-NH2 compared to that of GPG-NH2, but the reason for this observation is unknown.
The effect of G-NH2 appeared to be restricted to HIV, since no effect on the replication of HSV-1 or a variety of other viruses in cell cultures could be found, including HSV-2, vaccinia virus, and vesicular stomatitis virus in HEL cells; coxsackievirus group B4 and respiratory syncytial virus in HeLa cells; and parainfluenza virus, reovirus type 1, Sindbis virus, and Punta Toro virus in Vero cells (unpublished data). Recent data (2) have also shown that G-NH2 is effective not only against HIV-1 but also against HIV-2 (strains ROD and EHO) in CEM cell cultures.
To ensure that the inhibition of HIV-1 by G-NH2 was not due to any cellular effect, the proliferation and viabilities of G-NH2-treated cells were tested. The same protocol used for culture of PBMCs was used for IC50 calculations, proliferation testing, and viability testing. Even after stimulation with high doses of PHA, the proliferation of the cells could be inhibited with cyclosporine. Concentrations of G-NH2 25 to 50 times higher than the IC50 did not significantly affect proliferation or cell viability, assuring us that the antiretroviral effect found in vitro was not due to any influence on cellular growth.
The TEM studies performed further strengthen the prodrug hypothesis, since the effect of G-NH2 on HIV-1 was similar to that of GPG-NH2 (7). Irregular packing of capsid structures was shown after treatment with either compound, and this mechanism of action still cannot be found among the different classes of antiretroviral drugs.
Enfuvirtide, or T20, is a gp41-derived synthetic peptide that inhibits cell-virus fusion (17). Due to its poor bioavailability, enfuvirtide is administered subcutaneously twice daily (10). The bioavailability of G-NH2 has yet to be established, but since it is a modified amino acid, it is reasonable to predict that after oral administration, active uptake in the gut should be possible. After selective drug pressure in vitro, resistance not only to enfuvirtide but also to all other classes of antiretroviral drugs has been demonstrated (5, 11, 13, 14). Depending on the complexity of mutations needed, the time for selection varies and may be a predictor of how easily resistance will occur in vivo. As for GPG-NH2 (1), resistance to G-NH2 could not be generated even after extensive passaging in vitro. The difficulty with the selection of resistance indicates a favorable situation in vivo, in which treatment with G-NH2 may be beneficial without the rapid development of resistance. However, the possibility that the lack of viral resistance to G-NH2 could imply the direct or indirect involvement of a cellular factor in the antiretroviral mode of action cannot be excluded. Further clinical studies are needed to establish whether this promising new compound can be used for the treatment of HIV infection.
Acknowledgments
We thank Anders Höglund, Ulrika Noborg, and Sung-Oun Stenberg for technical assistance.
This study was supported by Göteborg University, the European Commission (grant HPAW-2002-90001), and Tripep AB.
REFERENCES
- 1.Andersson, E., P. Horal, A. Vahlne, and B. Svennerholm. 2004. No cross-resistance or selection of HIV-1 resistant mutants in vitro to the antiretroviral tripeptide glycyl-prolyl-glycine-amide. Antivir. Res. 61:119-124. [DOI] [PubMed] [Google Scholar]
- 2.Balzarini, J., E. Andersson, D. Schols, P. Proost, J. Van Damme, B. Svennerholm, P. Horal, and A. Vahlne. 2004. Obligatory involvement of CD26/dipeptidyl peptidase IV in the activation of the antiretroviral tripeptide glycylprolylglycinamide (GPG-NH2). Int J. Biochem. Cell Biol. 36:1848-1859. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, A. J., N. D. Rawlings, and J. F. Woessner. 1998. Handbook of proteolytic enzymes. Academic Press, London, United Kingdom.
- 4.De Meester, I., S. Korom, J. Van Damme, and S. Scharpe. 1999. CD26, let it cut or cut it down. Immunol. Today 20:367-375. [DOI] [PubMed] [Google Scholar]
- 5.Dianzani, F., G. Antonelli, O. Turriziani, E. Riva, G. Dong, and D. Bellarosa. 1993. In vitro selection of human immunodeficiency virus type 1 resistant to Ro 31-8959 proteinase inhibitor. Antivir. Chem Chemother. 4:329-333. [Google Scholar]
- 6.European AIDS Clinical Society Euroguidelines Group. 2003. European guidelines for the clinical management and treatment of HIV-infected adults in Europe. AIDS 17(Suppl. 2):S3-S26. [PubMed] [Google Scholar]
- 7.Hoglund, S., J. Su, S. S. Reneby, A. Vegvari, S. Hjerten, I. M. Sintorn, H. Foster, Y. P. Wu, I. Nystrom, and A. Vahlne. 2002. Tripeptide interference with human immunodeficiency virus type 1 morphogenesis. Antimicrob. Agents Chemother. 46:3597-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horal, P., W. W. Hall, B. Svennerholm, J. Lycke, S. Jeansson, L. Rymo, M. H. Kaplan, and A. Vahlne. 1991. Identification of type-specific linear epitopes in the glycoproteins gp46 and gp21 of human T-cell leukemia viruses type I and type II using synthetic peptides. Proc. Natl. Acad. Sci. USA 88:5754-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Japour, A. J., D. L. Mayers, V. A. Johnson, D. R. Kuritzkes, L. A. Beckett, J. M. Arduino, J. Lane, R. J. Black, P. S. Reichelderfer, R. T. D'Aquila, and the RV-43 Study Group, the AIDS Clinical Trials Group Virology Committee Resistance Working Group. 1993. Standardized peripheral blood mononuclear cell culture assay for determination of drug susceptibilities of clinical human immunodeficiency virus type 1 isolates. Antimicrob. Agents Chemother 37:1095-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilby, J. M., J. P. Lalezari, J. J. Eron, M. Carlson, C. Cohen, R. C. Arduino, J. C. Goodgame, J. E. Gallant, P. Volberding, R. L. Murphy, F. Valentine, M. S. Saag, E. L. Nelson, P. R. Sista, and A. Dusek. 2002. The safety, plasma pharmacokinetics, and antiviral activity of subcutaneous enfuvirtide (T-20), a peptide inhibitor of gp41-mediated virus fusion, in HIV-infected adults. AIDS Res. Hum. Retrovir. 18:685-693. [DOI] [PubMed] [Google Scholar]
- 11.Larder, B. A., K. E. Coates, and S. D. Kemp. 1991. Zidovudine-resistant human immunodeficiency virus selected by passage in cell culture. J. Virol. 65:5232-5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariotti, F., J. F. Huneau, S. Mahe, and D. Tome. 2000. Protein metabolism and the gut. Curr. Opin. Clin. Nutr. Metab. Care 3:45-50. [DOI] [PubMed] [Google Scholar]
- 13.Richman, D., C. K. Shih, I. Lowy, J. Rose, P. Prodanovich, S. Goff, and J. Griffin. 1991. Human immunodeficiency virus type 1 mutants resistant to nonnucleoside inhibitors of reverse transcriptase arise in tissue culture. Proc. Natl. Acad. Sci. USA 88:11241-11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rimsky, L. T., D. C. Shugars, and T. J. Matthews. 1998. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J. Virol. 72:986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su, J., E. Andersson, P. Horal, M. H. Naghavi, A. Palm, Y. P. Wu, K. Eriksson, M. Jansson, H. Wigzell, B. Svennerholm, and A. Vahlne. 2001. The nontoxic tripeptide glycyl-prolyl-glycine amide inhibits the replication of human immunodeficiency virus type 1. J. Hum. Virol. 4:1-7. [PubMed] [Google Scholar]
- 16.Wensing, A. M., and C. A. Boucher. 2003. Worldwide transmission of drug-resistant HIV. AIDS Rev. 5:140-155. [PubMed] [Google Scholar]
- 17.Wild, C. T., D. C. Shugars, T. K. Greenwell, C. B. McDanal, and T. J. Matthews. 1994. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]