Abstract
The development of resistance by Plasmodium falciparum to conventional drugs poses a threat to malaria control. There is therefore a need to find new, effective, and affordable remedies for malaria, including those derived from plants. This study demonstrates that crude, reverse-phase high-pressure liquid chromatography (RP-HPLC)-semipurified, and RP-HPLC-purified root extracts of Fagara zanthoxyloides inhibit the growth of P. falciparum in vitro, with 50% inhibitory concentrations (IC50s) of 4.90, 1.00, and 0.13 μg/ml, respectively. Roots of F. zanthoxyloides, known as chewing sticks, are widely used for tooth cleaning in West Africa. Microscopic examination of Giemsa-stained slides showed a virtual absence of schizonts in ring-stage synchronized cultures treated with crude extracts at concentrations of 30 to 60 μg/ml during 36 to 48 h of incubation. These observations suggest that the active constituent in the extract may be cytotoxic for P. falciparum trophozoites, thereby inhibiting their development to the schizont stage. A pure bioreactive fraction was subsequently obtained from the chromatographic separations. When this fraction was mixed with pure fagaronine, the mixture coeluted as a single peak on the analytical RP-HPLC column, suggesting that fagaronine may be the active antimalarial constituent of Fagara root extracts. Additional experiments showed that fagaronine also inhibited P. falciparum growth, with an IC50 of 0.018 μg/ml. The results of this study suggest that the antimalarial activity of fagaronine deserves further investigation.
Malaria represents a major cause of childhood mortality and adult morbidity in many parts of the world. Recent estimates indicate that 300 to 500 million clinical episodes of malaria occur worldwide every year (5, 25). Severe anemia, cerebral malaria, respiratory distress syndrome, and low birth weight are among the complications that contribute to the 25 to 30% malaria-attributable deaths for children under 5 years of age in Africa (13). Studies have also shown that about one million children may die each year from malaria-associated anemia alone and that up to 600,000 children succumb to cerebral malaria annually, with an estimated case fatality rate of about 20% (6, 7, 13). Low birth weight, resulting from maternal placental malaria infections, also contributes about 400,000 deaths annually to malaria mortality (19).
Efforts to reduce the high malaria mortality and morbidity rates have been hampered by the development of resistance, particularly by Plasmodium falciparum, to long-standing inexpensive conventional drugs, such as chloroquine (24). It is noteworthy that quinine and qinghaosu, which are drugs derived from indigenous plants in South America and China, respectively, are relatively unlikely to induce resistance on the part of the malaria parasite. While the major proportion of malaria-attributable deaths occurs in sub-Saharan Africa, neither of these plant-derived drugs are indigenous to the region. We present here the results of a study that examined the antimalaria properties of crude and purified root extracts of Fagara zanthoxyloides, an indigenous plant that is widely used as chewing stick for tooth cleaning in West Africa. The study specifically examined the inhibitory effects of the extracts on the developmental stages of in vitro cultures of P. falciparum.
MATERIALS AND METHODS
Plant extracts and fractionation.
The plant used in the study, F. zanthoxyloides, was obtained from and authenticated at the Arboretum of the Obafemi Awolowo University, Ile-Ife, Nigeria. The roots were washed, air dried, pulverized into powder form, and extracted in cold deionized water (20 g/l00 ml of H2O) for 24 h by mechanical stirring. The aqueous extracts were centrifuged twice at 800 × g for 15 min to remove particulate materials. The supernatants were filter sterilized (0.45-μm-pore-size; Millipore Corp., Bedford, Mass.) and freeze-dried overnight in a Labconco lyophilizer. The dried powder, representing the crude extract, was analyzed and further purified with standard reverse-phase high-pressure liquid chromatography (RP-HPLC), with UV detection set at 280 nm. The column stationary phase was Supelcosil ABZ+Plus, 5 μm (15 cm by 4.6 mm for the analytical column and 25 cm by 10 mm for the semipreparative column). The mobile phase was prepared as a gradient mixture of 0.1% aqueous trifluoroacetic acid (solvent A) and 0.1% trifluoroacetic acid in acetonitrile (solvent B). For the analytical column, a gradient elution (100% solvent A to 70% solvent A:30% solvent B over 30 min, then to 100% solvent B over 15 min, and then back to 100% solvent A over 15 min) was used at a flow rate of 1.5 ml/min. A sample of pure fagaronine was obtained from the National Cancer Institute (Bethesda, Md.) and analyzed with this chromatographic system. The initial semipreparative purification of the Fagara crude extract was performed at a flow rate of 6.0 ml/min by using a gradient elution (100% solvent A to 70% solvent A:30% solvent B over 30 min, then to 100% solvent B over 15 min, and then back to 100% solvent A over 15 min). The peaks obtained from this initial fractionation were pooled into four separate fractions and assayed for antimalarial activity. Fraction 4, the only one showing activity against the in vitro P. falciparum culture, was further purified on the semipreparative column at a flow rate of 3.0 ml/min by using a gradient elution (90% solvent A:10% solvent B to 70% solvent A:30% solvent B for 30 min, then to 100% solvent B over 5 min, and then to 100% solvent A over 5 min). Seven fractions were collected from this elution. These fractions were tested along with a control sample of pure fagaronine for malaria growth-inhibitory properties.
P. falciparum cultures.
P. falciparum strain 3D7 was used in all experiments. Parasites were maintained in cultures of RPMI 1640 medium supplemented with 25 mM HEPES, 23 mM NaHCO3, 10 mM glucose, 10% (vol/vol) heat-inactivated human plasma (O+ or A+), and washed human erythrocytes (A+) at 2% hematocrit (21). The growth medium was replaced daily, and cultures were gassed with a mixture of 90% N2, 5% CO2, and 5% O2. Synchronization to the ring stage was achieved by lysis of cells bearing mature parasites with isosmotic solution containing 300 mM alanine and 10 mM Tris-HCl, pH 7.4 (9). Alanine lyses erythrocytes infected with the trophozoite stage parasite, leaving intact only erythrocytes with ring-stage parasites. The morphological characteristics of the parasites and the level of parasitemia were determined by microscopic examination of Giemsa-stained thin blood smears.
Effects of Fagara extracts.
The effects of the crude Fagara extract on the growth and the stage-specific development of the in vitro parasite cultures were determined as follows. Erythrocyte suspensions with ring-stage synchronized parasites were distributed in triplicate into 24-well plates at 2% parasitemia and 2% hematocrit. Fagara crude extracts were added at final concentrations of 30 and 60 μg/ml. The cell suspensions and the extracts were mixed and incubated at 37°C in a candle jar for 51 h. Aliquots were taken from each well every 3 h for preparation of Giemsa-stained thin smears. The stage distribution of parasite development and the degree of parasitemia were determined by microscopic examination of the smears. For each smear, the number of rings, trophozoites, and schizonts were counted per approximately 1,000 erythrocytes. The total parasitemia was an arithmetic sum of rings, trophozoites, and schizonts at each time point.
IC50 determinations.
The effects of crude extracts of Fagara and of extracts purified to various degrees by RP-HPLC on the growth of ring-stage synchronized malaria cultures were determined by incorporation of [3H]hypoxanthine. The extracts were added at various concentrations in triplicate wells and incubated in a candle jar at 37°C for 24 h. [3H]hypoxanthine was then added to each well at a final activity of 5 μCi/ml. After an additional 24-h incubation period, the cells were harvested onto filters, dried in the oven, transferred to vials with the scintillation fluid fluosafe (Fisher Scientific Co., Norcross, Ga.), and counted on an LS5000CE scintillation counter (Beckman Instruments, Inc., Fullerton, Calif.). Fifty percent inhibitory concentrations (IC50s) of the various extracts were calculated from dose-response curves by using the best sigmoid fits obtained with the program Origin (Microcal Software Inc., Northampton, Mass.). The IC50 was defined as the concentration of a Fagara extract that produced 50% inhibition of parasite growth in comparison with a control culture. Mean IC50s for the extracts were compared by using two-way analysis of variance.
RESULTS
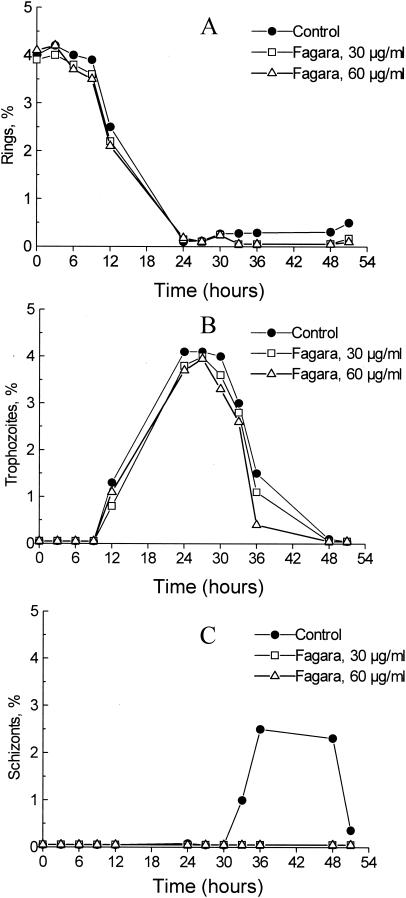
The development of ring-stage synchronized erythrocytic cultures, incubated with Fagara extracts over 51 h, is shown in Fig. 1. The cultures were treated with two concentrations (30 and 60 μg/ml) of crude Fagara extract to ascertain the morphological changes with Fagara treatment and the possible effects on the stage distribution of parasites. In both Fagara-treated and control cultures, ring parasites declined from 4% at 0 h to ≤0.5% between 24 and 30 h, when rings mature into trophozoites. Also in both cultures, ring parasites remained at less than 0.5% for the remainder of the culture period (Fig. 1A). Trophozoites appeared between 12 and 36 h in both control and Fagara-treated cultures, with peaks at 24 h (Fig. 1B). The exposure of erythrocytic parasites to the two concentrations of Fagara resulted in a smaller number of trophozoites at 36 h compared to untreated control cultures. By 48 h, the trophozoites in control and Fagara-treated cultures had almost completely disappeared. Schizonts appeared in control cultures by 33 h and peaked at 36 h of incubation, and this peak was followed by a decline from 48 to 51 h (Fig. 1C). However, schizonts were not seen in any cultures treated with 30 and 60 μg of crude Fagara extract/ml. Examination of the Giemsa-stained slides from Fagara-treated cultures revealed vacuolation and a general decrease in the size of mature trophozoites, indicating growth cessation at this developmental stage of the parasite.
FIG. 1.
Effects of Fagara root extracts on in vitro stage distribution of P. falciparum. The figures represent the distributions of ring (A), trophozoite (B), and schizont (C) stages over time. Each data point represents a mean calculated from 10 microscopic fields with a total of 1,000 red blood cells.
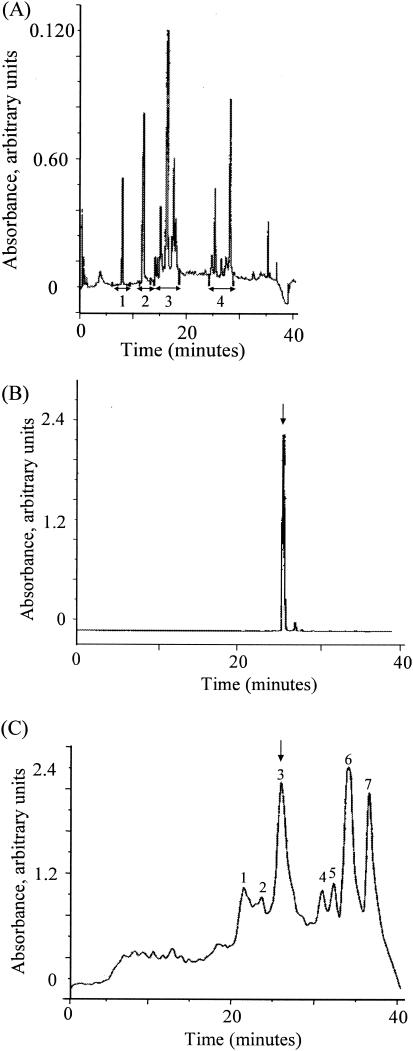
The incorporation of [3H]hypoxanthine into the nucleic acids of parasites was used to determine the IC50s of the crude and the purified extracts of F. zanthoxyloides roots. Table 1 lists the IC50s for the crude and purified Fagara extracts, showing values of 0.13 μg/ml for the semipurified extract and 0.018 μg/ml for the fagaronine sample. The results show a fivefold increase in the bioactivity of fraction 4 from the first RP-HPLC separation compared to the initial freeze-dried crude extract. Concentrations of greater than 30 μg/ml totally inhibited [3H]hypoxanthine incorporation into the nucleic acids of parasites. Figure 2 shows the chromatograms obtained when Fagara root extracts were run through the RP-HPLC columns. The initial crude extract was fractionated into the four fractions shown by using the semipreparative column under the same conditions with a flow rate of 6 ml/min. Fraction 4 was the only one that showed antimalarial growth activity, with an IC50 of 1 μg/ml (Fig. 2A). When pure fagaronine was analyzed under the same analytical chromatographic conditions, a single peak with a retention time of 26 min was obtained (Fig. 2B). Subsequent purification of fraction 4, using semipreparative RP-HPLC, produced seven additional fractions (Fig. 2C), of which fraction 3 was the one with antimalarial activity, with an IC50 of 0.13 μg/ml. When this bioactive fraction was mixed with fagaronine, the mixture coeluted as a single peak on the analytical RP-HPLC column, suggesting that fagaronine may be the active antimalarial constituent of Fagara root extracts. Also, fraction 3 from the second HPLC separation showed an increased activity of about 40-fold over that of the initial crude extract. The fraction also shared the same absorbance profile with fagaronine at 280 nm.
TABLE 1.
IC50s of fagaronine and various Fagara fractions obtained from 48 h in vitro cultures of P. falciparum
| Fractiona | IC50 ± SD (μg/ml) |
|---|---|
| Crude extract | 4.9 ± 0.6 |
| First separation | |
| Fractions 1-3 | No activity |
| Fraction 4 | 1 ± 0.02 |
| Second separation | |
| Fractions 1-2 and 4-7 | No activity |
| Fraction 3 | 0.13 ± 0.03 |
| Pure fagaronine | 0.018 |
FIG. 2.
(A) Analytical RP-HPLC chromatogram of Fagara root extract, using Supelcosil ABZ+Plus, 5 μm, 15 cm by 4.6 mm, 1.5 ml/min, gradient elution of 0.1% trifluoroacetic acid and 0.1% trifluoroacetic acid in acetonitrile, with UV detection at 280 nm. (B) Analytical RP-HPLC chromatogram of pure fagaronine, using Supelcosil ABZ+Plus, 5 μm, 15 cm by 4.6 mm, 1.5 ml/min, gradient elution of 0.1% trifluoroacetic acid and 0.1% trifluoroacetic acid in acetonitrile, with UV detection at 280 nm. (C) Semipreparative RP-HPLC chromatogram of purified fraction 4 of Fagara root extract, using Supelcosil ABZ+Plus, 5 μm, 25 cm by 10 mm, 3 ml/min, gradient elution of 0.1% trifluoroacetic acid and 0.1% trifluoroacetic acid in acetonitrile, with UV detection at 280 nm. The arrow indicates the active fraction which coeluted with pure fagaronine.
DISCUSSION
This study demonstrates that crude, semipurified, and purified extracts of F. zanthoxyloides are effective at inhibiting the in vitro growth and development of the erythrocytic phase of P. falciparum. Microscopic examination of the Giemsa-stained smears showed an absence of schizont development in erythrocyte cultures treated with 30 or 60 μg of Fagara crude extract/ml. Furthermore, the Fagara-treated cultures showed vacuolation and decreased size of mature trophozoites, indicating developmental arrest at this stage of the parasite. These observations suggest that the active constituent may be cytotoxic for P. falciparum trophozoites.
F. zanthoxyloides roots are used as chewing sticks in West Africa (14). They are chewed orally for tooth cleaning by millions of people without any apparent toxicity. It is therefore possible that aqueous extracts of the plant, as used in this study, would not engender human toxicity. Aqueous extracts of several indigenous African plants, as well as fractions extracted with petroleum ether, methanol, and ethanol, have been tested for antiplasmodial activities in various studies (1-3, 10, 22). In almost all of these studies, the aqueous extracts, which would be closest to the traditional medicinal preparations of indigenous plants, have shown the least antiplasmodial activities. Importantly, the Fagara extracts used in this study, including crude, semipurified, and purified extracts, are all water soluble and therefore physiologically compatible for oral ingestion. There has been a long-standing interest in the efficacy of Fagara root extracts as antisickling and anticancer agents (12, 18). Fagaronine is a benzophenanthridine alkaloid that was first isolated from the Fagara root in 1972. Fagaronine inhibits growth and cell differentiation of human erythroleukemia K562 cells and of L1210 murine leukemia cells at an IC50 of 2.22 μg/ml (16). Another study showed that fagaronine also inhibited the growth of HeLa S3 cells at an IC50 of 0.39 μg/ml. These values are about 22- to 123-fold higher than the IC50 of 0.018 μg/ml that has been obtained for fagaronine in this study, indicating that the malaria parasite was very susceptible to the compound. There has also been some concern that the drug may be toxic, leading Takanishi et al. (20) to compare the in vivo activities of fagaronine, nitidine, and other alkaloid derivatives against transplantable murine tumor P-388 cells. They reported that fagaronine showed toxicity only at a high pharmacological dose of 75 mg/kg of body weight. A more recent study used the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay, lactate dehydrogenase leakage, and intracellular glutathione levels to compare the cytotoxic effects of fagaronine and other natural quaternary benzo[c]phenanthridine alkaloids on primary cultures of human and porcine hepatocytes (23). Based on these separate assays, the investigators found no toxicity with fagaronine-treated cells. Finally, another recent study by Ogwal-Okeng et al. (15) examined the acute toxicity of methanolic extract of the root-bark of F. zanthoxyloides in mice. The 50% lethal dose of the extract was found to be 5.0 g/kg of body weight. These three studies clearly demonstrated the nonphysiological toxicity of fagaronine and its semipurified extracts. Other studies have indicated that fagaronine also acts as an inhibitor of DNA topoisomerases I and II and as a DNA intercalating agent (8). The results of our in vitro experiments failed to show that the drug acted on the topoisomerase enzymes of the P. falciparum parasite. It is interesting to put the apparent stage specificity of Fagara extract to the growth of P. falciparum in the context of other plant-derived antimalarials. Quinine, a derivative of the South American plant Cinchona, and qinghaosu, a derivative of the Chinese plant Artemesia, are widely used to treat infections with chloroquine-resistant P. falciparum, the species responsible for the most malaria-associated mortality (4, 11). Like Fagara extract, quinine is active against mature parasite forms, achieving 80 to 100% growth inhibition within 2 to 4 h of drug exposure (17). On the other hand, artemisinin, one of the three drugs derived from Artemisia, affects both rings and schizonts, while dihydroartemisinin inhibits the growth of all parasite stages. Artemether inhibits only ring-stage growth (18).
In conclusion, the results of this study show that extracts derived from Fagara root, which is commonly used as a chewing stick in West Africa, have demonstrable antimalarial activities. The results also show that fagaronine, a derivative of the root extract, inhibited P. falciparum growth at a significantly low IC50 of 0.018 μg/ml. This result suggests that the antimalarial activity of fagaronine deserves further investigation.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) grant no. A144857-04 from the National Institute of Allergy and Infectious Diseases; by NIH research grant no. UHI-HLO3679-05 from the National Heart, Lung and Blood Institute and the Office of Research on Minority Health; and by Howard University General Clinical Research Center grant no. MOI-RR10284.
REFERENCES
- 1.Addae-Kyereme, J., S. L. Croft, H. Kendrick, and C. W. Wright. 2001. Antiplasmodial activities of some Ghanaian plants traditionally used for fever/malaria treatment and of some alkaloids isolated from Pleiocarpa mutica; in vivo antimalarial activity of pleiocarpine. J. Ethnopharmacol. 76:99-103. [DOI] [PubMed] [Google Scholar]
- 2.Agbedahunsi, J. M. 2000. Screening of crude drugs for the treatment of malaria in Nigeria, p. 4-8. In C. O. Adewunmi and S. K. Adesina (ed.), Phytomedicines in malaria and sexually transmitted diseases: challenges for the new millennium. Obafemi Awolowo University, Ile-Ife, Nigeria.
- 3.Ajaiyeoba, E. O., U. I. Abalogu, H. C. Krebs, and A. M. J. Oduola. 1999. In vivo antimalarial activities of Quassia amara and Quassia undulate plant extracts in mice. J. Ethnopharm. 67:321-325. [DOI] [PubMed] [Google Scholar]
- 4.Birku, Y., E. Makonnen, and A. Bjorkman. 1999. Comparison of rectal artemisinin with intravenous quinine in the treatment of severe malaria in Ethiopia. East Afr. Med. J. 76:154-159. [PubMed] [Google Scholar]
- 5.Breman, J. G. 2001. The ears of the hippopotamus: manifestations, determinants and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64(Suppl. 1):1-11. [DOI] [PubMed] [Google Scholar]
- 6.Carme, B., J. C. Bouguenty, and H. Plassart. 1993. Mortality and sequelae due to cerebral malaria in African children in Brazaville, Congo. Am. J. Trop. Med. Hyg. 48:216-221. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt, H. L., and R. W. Snow. 2001. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 64(Suppl. 1):36-44. [DOI] [PubMed] [Google Scholar]
- 8.Larsen, A. K., L. Grondard, J. Couprie, B. Desoize, L. Comor, J. C. Jardillier, and J. F. Riou. 1993. The antileukemic alkaloid fagaronine is an inhibitor of DNA topoisomerases I and II. Biochem. Pharmacol. 46:1403-1412. [DOI] [PubMed] [Google Scholar]
- 9.Loyevsky, M., S. Lytton, B. Mester, J. Libman, A. Shanzer, and Z. I. Cabantchik. 1993. The antimalarial action of desferal involves direct access route to erythrocytic (Plasmodium falciparum) parasites. J. Clin. Investig. 91:218-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masaba, S. C. 2000. The antimalarial activity of Vernonia amygdalina Del (Compositae). Trans. R. Soc. Trop. Med. Hyg. 94:694-695. [DOI] [PubMed] [Google Scholar]
- 11.McGready, R., A. Brockman, T. Cho, D. Cho, M. van Vugt, C. Luxemburger, T. Chongsuphajaisiddhi, N. J. White, and F. Nosten. 2000. Randomized comparison of mefloquine-artesunate versus quinine in the treatment of multidrug-resistant falciparum malaria in pregnancy. Trans. R. Soc. Trop. Med. Hyg. 94:689-693. [DOI] [PubMed] [Google Scholar]
- 12.Messmer, W. M., M. Tin-Wa, H. H. S. Fong, C. Bevelle, N. R. Farnsworth, D. J. Abraham, and J. Trojanek. 1972. Fagaronine, a new tumor inhibitor isolated from Fagara zanthoxyloides Lam. (Rutaceae). J. Pharm. Sci. 61:1858-1859. [DOI] [PubMed] [Google Scholar]
- 13.Murphy, S. C., and J. G. Breman. 2001. Gaps in the childhood malaria burden in Africa: adding cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia and complications of pregnancy to the calculus. Am. J. Trop. Med. Hyg. 64(Suppl. 1):57-67. [DOI] [PubMed] [Google Scholar]
- 14.Odebiyi, O. O., and E. S. Sofowora. 1979. Antimicrobial alkaloids from a Nigerian chewing stick (Fagara zanthoxyloides). Planta Med. 36:204-207. [DOI] [PubMed] [Google Scholar]
- 15.Ogwal-Okeng, J. W., C. Obua, and W. W. Anokbonggo. 2003. Acute toxicity effects of the methanolic extract of Fagara zanthoxyloides (Lam.) root-bark. Afr. Health Sci. 3:124-126. [PMC free article] [PubMed] [Google Scholar]
- 16.Prado, S., S. Michel, F. Tillequin, M. Koch, B. Pffeiffer, A. Pierre, S. Leonce, P. Colson, B. Baldeyrou, A. Lansiaux, and C. Bailly. 2004. Synthesis and cytotoxic activity of benzo[c][1,7] and [1,8]phenanthrolines analogues of nitidine and fagaronine. Bioorg. Med. Chem. 12:3943-3953. [DOI] [PubMed] [Google Scholar]
- 17.Skinner, T. S., L. S. Manning, W. A. Johnston, and T. M. Davis. 1996. In vitro stage-specific sensitivity of Plasmosium falciparum to quinine and artemisinin drugs. Int. J. Parasitol. 26:519-525. [DOI] [PubMed] [Google Scholar]
- 18.Sofowora, E. A., and W. A. Isaacs. 1971. Reversal of sickling and crenation in erythrocytes by the root extract of Fagara zanthoxyloides. Lloydia 34:383-385. [PubMed] [Google Scholar]
- 19.Steketee, R. W., B. L. Nahlen, M. E. Parise, and C. Menendez. 2001. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64(Suppl. 1):28-35. [DOI] [PubMed] [Google Scholar]
- 20.Takanishi, T., M. Suzuki, A. Saiomoto, and T. Kabasawa. 1999. Structural considerations of NK109, an antitumor benzo[c]phenanthridine alkaloid. J. Nat. Prod. (Lloydia) 62:864-867. [DOI] [PubMed] [Google Scholar]
- 21.Trager, W., and J. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 22.Udeinya, I. J. 1993. Anti-malaria activity of Nigerian neem leaves. Trans. R. Soc. Trop. Med. Hyg. 87:471. [DOI] [PubMed] [Google Scholar]
- 23.Ulrichova, J., Z. Dvorak, J. Vicar, J. Lata, J. Smrzova, A. Sedo, and V. Simanek. 2001. Cytotoxicity of natural compounds in hepatocyte cell culture models. The case of quaternary benzo[c]phenanthridine alkaloids. Toxicol. Lett. 125:125-132. [DOI] [PubMed] [Google Scholar]
- 24.Wongsrichanalai, C., A. L. Pickard, W. H. Wernsdorfer, and S. R. Meshnick. 2002. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2:209-218. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 1997. World malaria situation in 1994. Part I. Population at risk. Wkly. Epidemiol. Rec. 72:269-274. [PubMed] [Google Scholar]