Abstract
Incubation in CO2 resulted in higher (≥3 doubling dilution) MICs of telithromycin than those found in ambient air for 31.2% of 346 Streptococcus pneumoniae ermB-positive strains. An increased telithromycin MIC in CO2 was not correlated with loss of its activity in the murine sepsis/peritonitis model.
Incubation in CO2 has been reported to result in higher MICs of macrolides, clindamycin, and telithromycin for Streptococcus pneumoniae than those found in ambient air (6, 9, 10, 11, 15, 16). Conversely, susceptibility testing of other antibiotics, namely β-lactams, evernimicin, and linezolid, does not appear to be affected by the presence or absence of CO2 (3, 5, 7, 8). This study reports the influence of CO2 on the MIC of telithromycin against ermB-positive S. pneumoniae.
MICs of erythromycin and telithromycin in ambient air and CO2.
MICs of erythromycin and telithromycin were determined by using the agar dilution method for 675 clinical strains of S. pneumoniae (13). Plates were incubated for 16 h at 37°C, either in ambient air or in 5% CO2. Susceptibility to erythromycin was also assessed by agar diffusion (15 μg of erythromycin per disk). MICs of telithromycin were also measured by microdilution according to the NCCLS recommendations (13). The MIC breakpoints of erythromycin were defined as the following: susceptible, ≤0.25 mg/liter; resistant, ≥1 mg/liter (13). The MIC breakpoints of telithromycin were defined according to the European Agency for the Evaluation of Medicinal Products (susceptible, ≤0.5 mg/liter; resistant, >2 mg/liter) and according to the proposed NCCLS breakpoints (susceptible, ≤1 mg/liter; resistant, ≥4 mg/liter).
Erythromycin MIC in ambient air was ≥0.25 mg/liter for 345 strains. Ten additional strains for which the erythromycin MICs were ≤0.25 mg/liter had an inhibition zone diameter of <22 mm. These 355 strains were tested for ermB and mefE genes by PCR as previously described (2). Erythromycin resistance genes were distributed as follows: ermB was present in 347 strains, mefE in 4 strains, mefE and ermB in 2 strains, and neither ermB nor mefE in 2 strains. Telithromycin MICs in ambient air (675 strains) were distributed as follows: ≤0.5 mg/liter for 667 strains (98.8%), 1 mg/liter for 4 strains (0.6%), and 2 mg/liter for 4 strains (0.6%). Telithromycin MICs were obtained in CO2 for 659 strains and were ≤0.5 mg/liter for 591 strains (89.7%), 1 mg/liter for 36 strains (5.5%), and 2 mg/liter for 32 strains (4.8%). According to the European breakpoints, the frequency of strains with intermediate susceptibility to telithromycin was 1.2% in ambient air and 10.3% in CO2. These frequencies, according to the NCCLS breakpoints, were 0.6 and 4.9%, respectively.
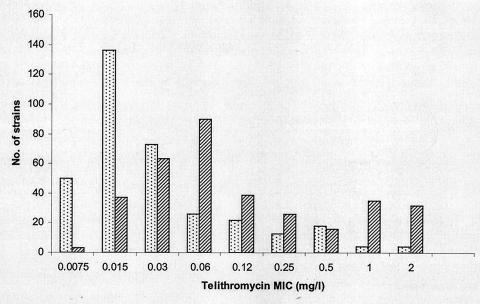
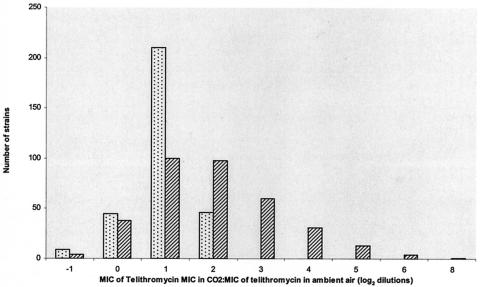
For 346 ermB-positive strains, the telithromycin MIC at which 50% of isolates tested are inhibited (MIC50) in ambient air and in CO2 was 0.015 and 0.06 mg/liter, and the MIC90 was 0.25 and 2 mg/liter, respectively (Fig. 1). The proportion of strains for which the telithromycin MIC was >0.5 mg/liter was 2.3% in ambient air and 21.5% in CO2 (P < 0.001). The proportion of strains for which the telithromycin MIC was >1 mg/liter was 1.2% in ambient air and 11.5% in CO2 (P < 0.001). The distribution of the CO2/ambient air (CO2/AA) ratio is shown for 346 ermB-positive strains and for 310 erythromycin-susceptible or mefE strains in Fig. 2. The median CO2/AA ratio of ermB-positive strains was 2 log2 dilutions (range, −1 to +8). For these 346 strains, the CO2/AA ratio was ≥3 log2 dilutions for 109 strains (31.5%), ≤1 log2 dilutions for 141 strains (40.7%), and 2 log2 dilutions for 96 strains (27.7%). According to the European breakpoints, 71 strains out of 346 (20.5%) were classified in different categories for incubation in ambient air and CO2: 64 were susceptible in ambient air and intermediate in CO2, 6 were intermediate in ambient air and resistant in CO2, and 1 strain was susceptible in ambient air and resistant in CO2. Using the NCCLS breakpoints, 38 strains out of 346 (11.0%) were classified in different categories in ambient air and in CO2: 31 were susceptible in ambient air and intermediate in CO2, 4 were intermediate in ambient air and resistant in CO2, and 3 were susceptible in ambient air and resistant in CO2.
FIG. 1.
Distribution of telithromycin MICs measured in ambient air (light bars) and in 5% CO2 (dark bars) for 346 ermB-positive strains of S. pneumoniae.
FIG. 2.
Distribution of the CO2/AA ratio for 346 ermB-positive strains of S. pneumoniae (dark bars) and for 310 erythromycin-susceptible or mefA-positive strains (light bars).
Reproducibility.
Twenty strains for which the telithromycin MIC in CO2:MIC in ambient air ratios (CO2/AA ratio) was ≤1 log2 dilution were randomly selected from the 346 ermB-positive strains (Group A), as were 20 other strains for which the telithromycin CO2/AA ratio was ≥3 log2 dilutions (Group B). In order to assess its reproducibility, telithromycin MICs were replicated 10 times by using the agar dilution method as described above, both in ambient air and in 5% CO2. MIC reproducibility was expressed as the standard deviation (SD) of log2 MICs for each strain. The SDs for the MICs were calculated after log2 transformation of MICs to express them in doubling dilutions. The SDs of the telithromycin MIC were ≤1 log2 dilution for 31 strains in ambient air (range, 0.0 to 2.0) and for 36 strains in CO2 (range, 0.0 to 1.2). The 10 determinations of the MIC were within ≤3 doubling dilutions for 29 strains in ambient air and for 32 strains in CO2. The mean CO2/AA ratios ranged from 0.5 to 2.0 doubling dilutions in Group A and from 1.9 to 6.2 doubling dilutions in Group B. The SD of the CO2/AA ratio was ≤1 log2 dilutions for 36 out of 40 strains (range, 0.0 to 1.8).
Experimental murine sepsis/peritonitis model.
Among the 40 erythromycin-resistant strains tested in the reproducibility assay, 8 strains were selected for in vivo experiments: 3 strains for which the telithromycin MIC was ≤0.5 mg/liter in ambient air and >0.5 mg/liter in CO2 (strains C05SP06, C08SP13, and C04SP28); 2 strains for which the MICs were ≤0.5 mg/liter in both atmospheres (strains C01SP07 and C22SP03); and 3 strains for which the MICs were >0.5 mg/liter in both atmospheres (C28SP22, C18SP04, and C19SP10). The inoculum was obtained as follows. Bacterial suspensions were prepared from 16-h cultures at 37°C in ambient air on 5% sheep blood agar plates. The inoculum was adjusted to 6 MacFarlands and then was diluted at equal parts with a 10% mucin saline solution (M-2378; Sigma, St. Quentin-Fallavier, France). To quantify the inoculum, 50 μl of the final suspension was plated on sheep blood agar plates after appropriate dilutions, and colonies were counted after a 24-h incubation on sheep blood agar at 37°C in a 5% CO2 atmosphere. Female 28- to 30-g Swiss mice (Elevage Janvier SA, Le Genest St. Isle, France) were kept in cages with free access to food and water. Mice were injected intraperitoneally with 0.7 ml of a fresh suspension. Telithromycin was administered subcutaneously at increasing concentrations (volume, 0.5 ml) immediately and 4 h after inoculation. For each strain, 10 mice were inoculated at each telithromycin dose level, which ranged from 0.01 to 100 mg/kg of body weight, expressed as a single dose. Mice were observed for 6 days and then were sacrificed. This protocol was approved by the University of Nantes experimental therapeutic unit. The median effective dose (ED50) was calculated as previously described by Reed and Muench (14). Mean ED50s were compared using the Mann-Whitney test. The mean inoculum was 107 CFU per mouse (range, 3 × 106 to 5 × 107 CFU per mouse). All nontreated control mice died within 48 h after inoculation. ED50s of the five strains susceptible to telithromycin (geometric mean telithromycin MIC, ≤0.5 mg/liter in ambient air) were almost identical (mean ED50, 0.6 ± 0.2 mg/kg), regardless of whether the MIC determined in CO2 was below or above 0.5 mg/liter (Table 1). Conversely, telithromycin ED50s were higher for the three strains for which the geometric mean telithromycin MIC was >0.5 mg/liter in ambient air (mean ED50, 16.1 ± 3.3 mg/kg; P < 0.05).
TABLE 1.
MICs and ED60s determined in this study
| Strain | Agar dilution MIC
|
CO2/AA ratio | Microdilution MIC | ED50 | |
|---|---|---|---|---|---|
| Ambient air | 5% CO2 | ||||
| C01SP07 | 0.01 ± 0.7 | 0.05 ± 0.5 | 1.5 ± 0.7 | 0.015 | 0.6 |
| C22SP03 | 0.04 ± 0.5 | 0.12 ± 0.7 | 1.2 ± 0.8 | 0.03 | 0.6 |
| C05SP06 | 0.04 ± 1.1 | 0.50 ± 1.2 | 3.5 ± 0.7 | 0.03 | 0.6 |
| C08SP13 | 0.06 ± 1.4 | 0.93 ± 0.8 | 4.0 ± 0.9 | 0.5 | 0.3 |
| C04SP28 | 0.03 ± 0.4 | 0.78 ± 1.2 | 4.4 ± 1.2 | 0.06 | 0.9 |
| C28SP22 | 1.62 ± 0.5 | 3.53 ± 0.0 | 1.3 ± 0.5 | 2 | 17.1 |
| C18SP04 | 0.93 ± 0.3 | 3.31 ± 0.3 | 2.0 ± 0.5 | 1 | 18.8 |
| C19SP10 | 1.00 ± 0.0 | 3.31 ± 0.3 | 1.9 ± 0.3 | 1 | 12.5 |
This study demonstrates that for 346 ermB-positive strains of S. pneumoniae, the telithromycin MICs are higher in CO2 than in ambient air. Although the telithromycin MICs for 2.3% of strains were >0.5 mg/liter in ambient air, this proportion increased to 21.5% in CO2. It should be noted that CO2 does not influence the telithromycin MIC in the same way for all strains of S. pneumoniae, as the MICs for 31.5 and 40.7% of the 346 ermB-positive strains increased to ≥3 and ≤1 doubling dilution, respectively. It therefore seems possible to use the magnitude of the increase in MICs in CO2 to classify strains of ermB-positive pneumococci. Our results with the murine peritonitis/septicemia model suggest that telithromycin remains effective against strains that are susceptible in ambient air (MICs ≤ 0.06 mg/liter), regardless of whether the MIC in CO2 remains low (≤0.12 mg/liter) or increases to intermediate values (≥0.5 mg/liter).
As has already been suggested for macrolides by other studies, these data support the hypothesis that it may be relevant to determine different breakpoints depending on whether the telithromycin susceptibility test is performed in ambient air or in CO2 (6, 9, 15). The British Society for Antimicrobial Chemotherapy, the NCCLS, and the French Society of Microbiology recommend that agar dilution be performed in CO2 for S. pneumoniae (1, 4, 12, 13). The French Society of Microbiology has added to its 2003 recommendations that resistance to telithromycin in CO2 should be confirmed in ambient air (12).
Acknowledgments
We are grateful to Anne-Françoise Miègeville and Virginie Le Mabecque for technical assistance.
This work was supported in part by Aventis Laboratories, Paris, France.
REFERENCES
- 1.Andrews, J. M. 2001. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48(Suppl. S1):5-16. [DOI] [PubMed] [Google Scholar]
- 2.Bemer-Melchior, P., M. E. Juvin, S. Tassin, A. Bryskier, G. C. Schito, and H. B. Drugeon. 2000. In vitro activity of the new ketolide telithromycin compared with those of macrolides against Streptococcus pyogenes: influences of resistance mechanisms and methodological factors. Antimicrob. Agents Chemother. 44:2999-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brueggemann, A. B., K. C. Kugler, and G. V. Doern. 1997. In vitro activity of BAY 12-8039, a novel 8-methoxyquinolone, compared to activities of six fluoroquinolones against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob. Agents Chemother. 41:1594-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.British Society for Antimicrobial Chemotherapy. 4 May 2003, posting date. Disc diffusion method for antimicrobial susceptibility testing. Version 2.1. [Online.] http://www.bsac.org.uk/uploads/may%202003susceptibility1.pdf.
- 5.Chomarat, M., L. Chollet, M. Peyret, and J. P. Flandrois. 1997. Influence of atmospheric conditions during incubation on the susceptibilities of Streptococcus pneumoniae isolates to five β-lactam antibiotics. J. Antimicrob. Chemother. 40:599-601. [DOI] [PubMed] [Google Scholar]
- 6.Fasola, E. L., S. Bajaksouzian, P. C. Appelbaum, and M. R. Jacobs. 1997. Variation in erythromycin and clindamycin susceptibilities of Streptococcus pneumoniae by four test methods. Antimicrob. Agents Chemother. 41:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton-Miller, J. M. T., and S. Shah. 1999. Susceptibility testing of linezolid by two standard methods. Eur. J. Clin. Microbiol. Infect. Dis. 18:225-227. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton-Miller, J. M. T., S. Shah, and D. Loebenberg. 2001. Susceptibility of pneumococci to evernimicin: effect of CO2 and different methodologies. Clin. Microbiol. Infect. 7:339-340. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, J., S. Bouchillon, and D. Pontani. 1999. The effect of carbon dioxide on susceptibility testing of azithromycin, calithromycin and roxithromycin against clinical isolates of Streptococcus pneumoniae and Streptococcus pyogenes by broth microdilution and the Etest: Artemis Project-first-phase study. Clin. Microbiol. Infect. 5:327-330. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, M. M., S. L. Hill, and L. J. V. Piddock. 1999. Effect of carbon dioxide on testing of susceptibilities of respiratory tract pathogens to macrolide and azalide antimicrobial agents. Antimicrob. Agents Chemother. 43:1862-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lonks, J. R., and A. A. Medeiros. 1993. High rate of erythromycin and clarithromycin resistance among Streptococcus pneumoniae isolates from blood cultures from Providence, Rhode Island. Antimicrob. Agents Chemother. 37:1742-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Members of the SFM Antibiogram Committee. 2003. Comité de l'Antibiogramme de la Société Française de Microbiologie report 2003. Int. J. Antimicrob. Agents 21:364-391. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, sixth ed. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 15.Visalli, M. A., M. R. Jacobs, and P. C. Appelbaum. 1997. Susceptibility of penicillin-susceptible and -resistant pneumococci to dirithromycin compared with susceptibilities to erythromycin, azithromycin, clarithromycin, roxithromycin, and clindamycin. Antimicrob. Agents Chemother. 41:1867-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh, F., F. Carnegy, J. Willcock, and S. Amyes. 2004. Comparative in vitro activity of telithromycin against macrolide-resistant and -susceptible Streptococcus pneumoniae, Moraxella catarrhalis and Haemophilus influenzae. J. Antimicrob. Chemother. 53:793-796. [DOI] [PubMed] [Google Scholar]