Abstract
We examined the effect of antibiotic treatment on establishment of intestinal colonization by Candida glabrata in adult mice. Subcutaneous ceftriaxone, piperacillin-tazobactam, clindamycin, and metronidazole promoted increased density of stool colonization, whereas cefepime, levofloxacin, and aztreonam did not. These findings suggest that antibiotics that inhibit intestinal anaerobes promote C. glabrata colonization.
The gastrointestinal tract is a primary site of colonization by Candida species and is frequently a source for hematogenous dissemination in cancer patients (1). Antibiotics play a crucial role in the pathogenesis of Candida infections by inhibiting competing indigenous microflora, thereby facilitating acquisition and overgrowth of Candida species in the gastrointestinal tract and at other sites (1). Several studies suggest that antibiotics that inhibit obligate anaerobes in the intestinal tract may be more likely to promote overgrowth of C. albicans than those that do not (1, 4, 5, 7, 8-11), but little data regarding the effect of antibiotics on colonization with other Candida species are available. We used a mouse model to test the hypothesis that antibiotics with potent activity against intestinal anaerobes (piperacillin-tazobactam, metronidazole, ceftriaxone, and clindamycin) promote overgrowth of Candida glabrata, whereas agents with minimal activity against anaerobes (cefepime, levofloxacin, and aztreonam) do not.
Four C. glabrata strains were used: ATCC 90030, MRL 191, A129, and A239. The fluconazole MICs for the strains were 32, 16, 0.5, and 4 μg/ml, respectively. The experimental protocol was approved by the Cleveland Veterans Affairs Medical Center's Animal Care Committee. Female CF1 mice (Harlan Sprague-Dawley, Indianapolis, Ind.) weighing 25 to 30 g were used. To prevent cross-contamination, mice were housed in individual cages with filter tops. An initial experiment (four mice per group) was performed to examine the ability of the four C. glabrata strains to colonize the intestinal tract. Mice were treated daily with subcutaneous normal saline (0.2 ml) or piperacillin-tazobactam (8 mg in 0.2 ml of normal saline) for 2 days before and 6 days after orogastric inoculation of 108 CFU of C. glabrata with a stainless steel feeding tube (Popper & Sons, New Hyde Park, N.Y.). Piperacillin-tazobactam was chosen for the initial experiment because we have previously demonstrated that it inhibits anaerobes, facultative gram-negative bacilli, and enterococci in the stool of mice (2). Fresh stool samples were collected at baseline and 1, 3, and 6 days after inoculation of C. glabrata. For quantification of C. glabrata, serially diluted stool samples were plated onto Sabouraud dextrose agar (Becton, Dickinson, and Company, Sparks, Md.) containing piperacillin-tazobactam (16 μg/ml) and incubated in room air at 37°C for 48 h. The lower limit of detection was ∼2 log10 CFU/g.
Strains A239 and ATCC 90030 were chosen to evaluate the effect of different antibiotics on establishment of colonization because they had the highest stool densities in the initial experiment. Mice received daily subcutaneous antibiotics or saline for 2 days before and 6 days after orogastric inoculation of 106 CFU of one of the C. glabrata strains. Antibiotics included piperacillin-tazobactam (8 mg/day), ceftriaxone (2.4 mg/day), cefepime (2.0 mg/day), levofloxacin (0.375 mg/day), aztreonam (3.0 mg/day), and metronidazole (4 mg/day); for the experiment with ATCC 90030, clindamycin (1.4 mg/day) was substituted for metronidazole because metronidazole caused significant soft-tissue irritation in the initial experiments. The antibiotic doses were equal to the total daily dose recommended for human adults (milligrams per kilogram of body weight) with the exception of that for metronidazole, which was given at approximately four times the usual dose because increased dosages are required to inhibit the anaerobic microflora of mice (2). Because cefepime, levofloxacin, and aztreonam did not promote overgrowth at the lower doses, higher human equivalent doses calculated by the technique of Freireich et al. (3) (cefepime, 24 mg/day; levofloxacin, 4.7 mg/day; aztreonam, 37.5 mg/day) were also administered for comparison in the experiments with strain A239. The density of C. glabrata was measured as described above. The densities of aerobic and facultative gram-negative bacilli and enterococci were measured by plating the samples onto MacConkey agar (Becton Dickinson and Company) and Enterococcosel agar (Becton Dickinson and Company), respectively. The experiments with strain A239 were performed twice with six mice per group; one experiment was performed with ATCC strain 90030 with four mice per group.
To evaluate antibiotic-associated changes in the stool microflora, denaturing gradient gel electrophoresis (DGGE) of PCR-amplified bacterial rRNA genes was performed as previously described (12) on stool samples obtained from three mice from the above treatment groups. The similarities between the DGGE profiles were assessed by calculating similarity indices based on the Dice similarity coefficient and the unweighted pair group method using arithmetic averages for clustering, and corresponding dendrograms showing relationships among the DGGE profiles were constructed. The clindamycin, aztreonam, and cefepime groups were excluded because we have previously published data demonstrating the effect of these agents on the DGGE patterns of mice (13); clindamycin causes significant disruption of the DGGE patterns, whereas aztreonam and cefepime do not (13).
Data analyses were performed with Stata (College Station, Tex.) software (version 6.0). A one-way analysis of variance was performed to compare the groups, with P values adjusted for multiple comparisons using the Scheffe correction. Student's t test was used to compare DGGE similarity indices among groups.
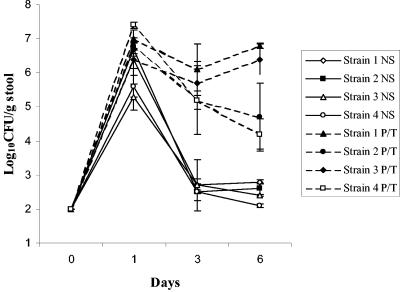
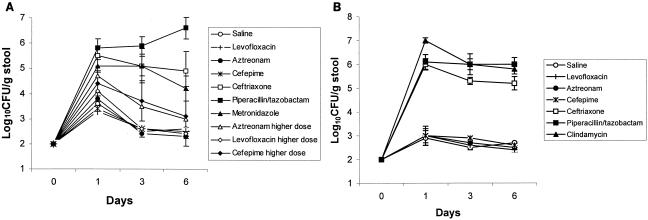
Figure 1 shows the stool densities of the four C. glabrata strains in mice treated with piperacillin-tazobactam or normal saline. The density of colonization was significantly higher in the piperacillin-tazobactam-treated mice than in the saline controls (P < 0.01 for each strain). Figure 2A shows the effect of antibiotic treatment on establishment of colonization by strain A239. In comparison to controls, the ceftriaxone, piperacillin-tazobactam, and metronidazole groups developed increased density of colonization (P ≤ 0.02), whereas the cefepime, levofloxacin, and aztreonam groups did not (P ≥ 0.15). Higher human equivalent doses of cefepime, levofloxacin, and aztreonam calculated by the technique of Freireich et al. (3) also did not promote significant overgrowth of C. glabrata A239, although there was a trend toward higher densities in the aztreonam and cefepime groups. Mice in the ceftriaxone, piperacillin-tazobactam, cefepime, levofloxacin, and aztreonam groups had undetectable levels (limit of detection, ∼2.5 log10 CFU/g) of total aerobic and facultative gram-negative bacilli (P < 0.01 for each group in comparison to controls), whereas the density of these organisms in the metronidazole group (mean ± standard deviation [SD], 5.4 ± 1.2 log10 CFU/g) did not differ from that for the controls (mean ± SD, 4.9 ± 0.61 log10 CFU/g) (P = 0.6). Figure 2B shows the effect of antibiotic treatment on establishment of colonization by ATCC strain 90030. In comparison to controls, the ceftriaxone, piperacillin-tazobactam, and clindamycin groups developed increased density of colonization (P < 0.0001), whereas the cefepime, levofloxacin, and aztreonam groups did not (P ≥ 0.89).
FIG. 1.
Effect of subcutaneous piperacillin-tazobactam administration on the density of stool colonization with four strains of C. glabrata. Mice received subcutaneous piperacillin-tazobactam or normal saline daily from day −2 to day 6 and 108 CFU of the C. glabrata strains on day 0. NS, normal saline; P/T, piperacillin-tazobactam; strain 1, A239; strain 2, A129; strain 3, ATCC 90030; strain 4, MRL 191. Error bars indicate standard errors.
FIG. 2.
Effect of subcutaneous antibiotic administration on the density of stool colonization with C. glabrata strains A239 (A) and ATCC 90030 (B). Mice received subcutaneous antibiotics or normal saline daily from day −2 to day 6 and 106 CFU of C. glabrata on day 0. For strain A239, pooled data from two experiments are shown. Error bars indicate standard errors.
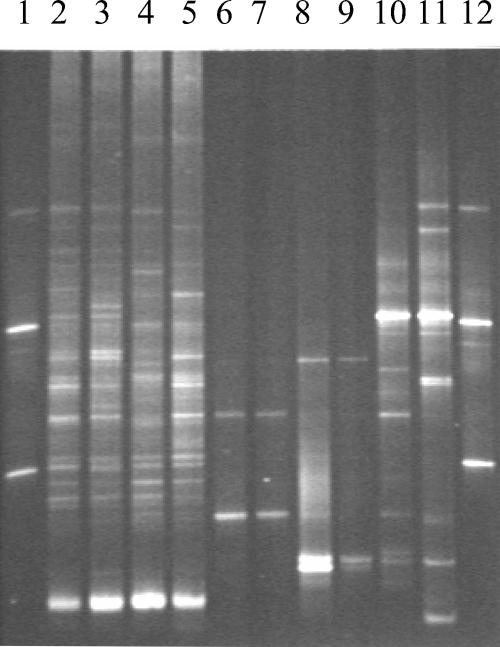
Figure 3 shows a representative DGGE gel. Marked alterations were noted in the DGGE patterns of the ceftriaxone-, piperacillin-tazobactam-, and metronidazole-treated mice (mean similarity indices in comparison to controls, 17, 5, and 31%, respectively), whereas the levofloxacin-treated mice had only minor changes (mean similarity index in comparison to controls, 67%) (P < 0.0001). Preliminary analyses in our laboratory suggest that the bands in the DGGE pattern of the control mice predominantly represent anaerobic bacteria.
FIG. 3.
DGGE patterns of stool samples obtained from mice on day 3 of treatment with subcutaneous saline (lanes 2 and 3), levofloxacin (lanes 4 and 5), piperacillin-tazobactam (lanes 6 and 7), ceftriaxone (lanes 8 and 9), and metronidazole (lanes 10 and 11). A control pattern (lanes 1 and 12) contained PCR products obtained from strains of Escherichia coli, Fusobacterium nucleatum, Bacteroides thetaiotaomicron, and Bacteroides uniformis.
In summary, we found that antibiotics that inhibited intestinal anaerobes based on DGGE analysis (piperacillin-tazobactam, ceftriaxone, metronidazole, and clindamycin) promoted persistent high-density colonization with C. glabrata. Antibiotics that inhibited facultative gram-negative bacilli but not anaerobes (levofloxacin, cefepime, and aztreonam) did not promote C. glabrata colonization; other investigators have demonstrated that such agents either do not promote intestinal colonization with C. albicans or do so only to a modest degree (4, 5, 7, 8, 10, 11). Antibiotic-associated overgrowth of Candida species in the gastrointestinal tract may have important clinical implications. Tomoda et al. (14) found that leukemia patients with ≥105 Candida CFU/g of feces were significantly more likely to develop Candida infections than patients with lower densities of colonization. Louie et al. (6) demonstrated that treatment of febrile neutropenic patients with moxalactam (a potent antianaerobic antibiotic), but not with aztreonam, resulted in inhibition of intestinal anaerobes and an increased frequency of isolation of Candida from clinical cultures. Further studies are needed to evaluate the effect of antibiotic therapy on intestinal overgrowth of C. glabrata in patients.
Acknowledgments
This work was supported by a grant from Ortho-McNeil Pharmaceuticals and by an Advanced Research Career Development Award from the Department of Veterans Affairs to C.J.D.
REFERENCES
- 1.Cole, G. T., A. A. Halawa, and E. J. Anaissie. 1996. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clin. Infect. Dis. 22(Suppl. 2):S73-S88. [DOI] [PubMed] [Google Scholar]
- 2.Donskey, C. J., J. A. Hanrahan, R. A. Hutton, and L. B. Rice. 1999. Effect of parenteral antibiotic administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J. Infect. Dis. 180:384-390. [DOI] [PubMed] [Google Scholar]
- 3.Freireich, E., E. Gehan, D. Rall, L. Schmidt, and H. Skipper. 1966. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey and man. Cancer Chemother. Rep. 50:219-244. [PubMed] [Google Scholar]
- 4.Kennedy, M. J., and P. A. Volz. 1985. Effect of various antibiotics on gastrointestinal colonization and dissemination by Candida albicans. Sabouraudia J. Med. Vet. Mycol. 23:265-273. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy, M. J. 1989. Regulation of Candida albicans populations in the gastrointestinal tract: mechanisms and significance in GI and systemic candidiasis. Curr. Top. Med. Mycol. 3:315-402. [DOI] [PubMed] [Google Scholar]
- 6.Louie, T. J. 1985. Preservation of colonization resistance parameters during empiric therapy with aztreonam in the febrile neutropenic patient. Rev. Infect. Dis. 7(Suppl. 4):S747-S761. [DOI] [PubMed] [Google Scholar]
- 7.Maraki, S., E. Barbounakis, I. Chatzinikolaou, N. Anatoliotakis, M. Plataki, Y. Tselentis, and G. Samonis. 1998. Effects of cefepime, cefixime and ceftibuten on murine gut colonization by Candida albicans.. Chemotherapy 44:405-408. [DOI] [PubMed] [Google Scholar]
- 8.Mavromanolakis, E., S. Maraki, A. Cranidis, Y. Tselentis, D. P. Kontoyiannis, and G. Samonis. 2001. The impact of norfloxacin, ciprofloxacin and ofloxacin on human gut colonization by Candida albicans. Scand. J. Infect. Dis. 33:477-478. [DOI] [PubMed] [Google Scholar]
- 9.Samonis, G., E. J. Anaissie, and G. P. Bodey. 1990. Effects of broad-spectrum antimicrobial agents on yeast colonization of the gastrointestinal tracts of mice. Antimicrob. Agents Chemother. 34:2420-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samonis, G., A. Gikas, E. J. Anaissie, G. Vrenzos, S. Maraki, Y. Tselentis, and G. P. Bodey. 1993. Prospective evaluation of effects of broad-spectrum antibiotics on gastrointestinal yeast colonization of humans. Antimicrob. Agents Chemother. 37:51-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samonis, G., M. Dassiou, and H. Anastassiadou. 1994. Antibiotics affecting gastrointestinal colonization of mice by yeasts. J. Chemother. 6:50-52. [DOI] [PubMed] [Google Scholar]
- 12.Stiefel, U., N. J. Pultz, J. Harmoinen, P. Koski, K. Lindevall, M. S. Helfand, and C. J. Donskey. 2003. Oral administration of β-lactamase preserves colonization resistance of piperacillin-treated mice. J. Infect. Dis. 188:1605-1609. [DOI] [PubMed] [Google Scholar]
- 13.Stiefel, U., N. J. Pultz, M. S. Helfand, and C. J. Donskey. 2004. Increased susceptibility to establishment of vancomycin-resistant Enterococcus intestinal colonization persists after completion of antianaerobic antibiotic treatment in mice. Infect. Control Hosp. Epidemiol. 25:373-379. [DOI] [PubMed] [Google Scholar]
- 14.Tomoda, T., Y. Nakano, and T. Kageyama. 1988. Intestinal Candida overgrowth and Candida infection in patients with leukemia: effect of Bifidobacterium administration. Bifidobacteria Microflora 7:71-74. [Google Scholar]