Abstract
A significant relationship between copper resistance (tcrB), glycopeptide resistance (Tn1546), and macrolide resistance [erm(B)] in Enterococcus faecium isolated from pigs was found. The tcrB gene was located closely upstream of the Tn1546 element. However, the continued use of copper sulfate has not been able to maintain high levels of macrolide and glycopeptide resistance.
Copper sulfate is used as a growth-promoting feed supplement for pig production. In Denmark, piglets receive 175 ppm copper sulfate and slaughter animals receive 35 ppm in the feed. In the past, antibiotic growth promoters, such as avoparcin (a glycopeptide) and tylosin (a macrolide), were also used in animal production. Avoparcin was banned for growth promotion in Denmark in 1995, and tylosin was banned in 1998. However, macrolides are still in use for treatment of sick animals.
In 1998 (6), the percentage of copper-resistant Enterococcus faecium isolates from pigs (76%) was found to be higher than the percentages of resistant isolates from broilers (34%), calves (16%), and sheep (<5%), which receive less or no copper in the feed, and humans (10%). A transferable gene tcrB, which confers resistance to copper in enterococci, was identified in E. faecium from Denmark (6) as well as in isolates from other countries (3). This gene encodes a CPX-type ATPase with similarity to the copper homeostasis ATPase CopB, responsible for efflux of copper in Enterococcus hirae (8).
In Denmark, members of the glycopeptide-resistant E. faecium (GREF) population in pigs are clonally related, as all GREF isolates have the same pulsed-field gel electrophoresis profile (1). Among a large subset of our GREF strains isolated from pigs between 1995 and 2001, we have recently seen that the tcrB gene in all cases examined (n = 55) is located on the same transferable plasmid as the Tn1546 element, as is the erm(B) gene, responsible for the macrolide resistance of these strains (H. Hasman, A. G. Villadsen, and F. M. Aarestrup, submitted for publication). Also, most of the macrolide-resistant E. faecium strains isolated in 1998 were resistant to copper (6).
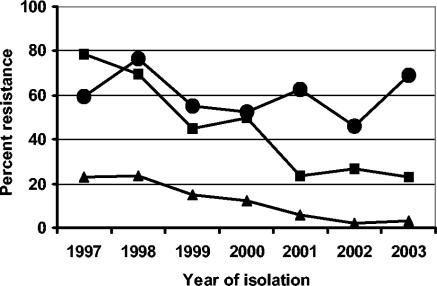
Each year since 1997, epidemiologically independent E. faecium strains have been isolated from Danish pigs at slaughter as part of the DANMAP surveillance program (5). A subset of these (between 40 and 61 isolates) from each of the years between 1997 and 2003 were tested for resistance to copper as previously described (6), and frequencies of resistance to macrolides and glycopeptides were compared with previously determined values (2, 5). The prevalence of copper resistance among E. faecium isolates from pigs (Fig. 1) has remained relatively unchanged, with 60% resistance in 1997, 69% resistance in 2003, and a minimum level of resistance in 2002 (46%). In the same period, macrolide resistance decreased from 79% in 1997 to 23% in 2001 but did not disappear, as it remained relatively constant in the following years (Fig. 1).
FIG. 1.
Development of resistance to copper (circles), macrolides (squares), and glycopeptides (triangles) among E. faecium strains isolated from pigs between 1997 and 2003 in Denmark.
As the unchanged prevalence of copper resistance suggests a selective pressure imposed by the use of copper as a supplement to pigs, it could be argued that this use to some extent coselects for resistance to macrolides and glycopeptides, as genes conferring resistance to them transfer together and thus are genetically linked (6). Indeed, in the years immediately after the ban on macrolides for growth promotion, a significant statistical association (calculated by the chi-square test using EpiInfo, version 6.02, with a two-tailed significance level of 5%) could be observed, as almost all macrolide-resistant isolates were resistant to copper (Table 1). Alternatively, the persistence of macrolide-resistant bacteria could be a result of the continued use of this antibiotic in treatment of sick animals. However, this tenet is not possible to verify, as we do not collect data regarding antibiotic treatment of the animals from where the bacteria were isolated.
TABLE 1.
Number of copper-resistant and copper-sensitive E. faecium isolates from each year between 1997 and 2003 correlated with resistance to macrolides and glycopeptides
| Copper resistancea | Yr | n | Macrolides
|
Glycopeptides
|
||||
|---|---|---|---|---|---|---|---|---|
| No. of isolates:
|
P | No. of isolates:
|
P | |||||
| Eryr | Erys | Vanr | Vans | |||||
| R | 1997 | 52 | 31 | 0 | <0.0001 | 12 | 20 | 0.0016 |
| S | 10 | 11 | 0 | 20 | ||||
| R | 1998 | 59 | 41 | 4 | <0.0001 | 14 | 31 | 0.0260 |
| S | 0 | 14 | 0 | 14 | ||||
| R | 1999 | 40 | 18 | 4 | <0.0001 | 6 | 16 | 0.0243 |
| S | 0 | 18 | 0 | 18 | ||||
| R | 2000 | 40 | 14 | 7 | 0.0574 | 5 | 16 | 0.0486 |
| S | 6 | 13 | 0 | 19 | ||||
| R | 2001 | 51 | 11 | 21 | 0.0200 | 3 | 29 | NSb |
| S | 1 | 18 | 0 | 19 | ||||
| R | 2002 | 52 | 13 | 11 | 0.0004 | 1 | 23 | NS |
| S | 1 | 27 | 0 | 28 | ||||
| R | 2003 | 61 | 11 | 30 | NS | 2 | 40 | NS |
| S | 3 | 17 | 0 | 19 | ||||
R, resistant; S, sensitive.
NS, not significant.
Resistance towards glycopeptides has decreased from 23% in 1997 to 3% in 2002 and 2003 (Fig. 1). We have previously seen a relationship between copper and glycopeptide resistance (6). Therefore, the possibility of a similar relationship was tested for the years 1997 to 2003. Based on the data in Table 1, the relationship were significant only in 1997, 1998, 1999, and 2000, whereas the lack of sufficient glycopeptide-resistant isolates in 2001, 2002, and 2003 weakens the power of the statistical test, thus rendering the results statistically insignificant. Nevertheless, the few glycopeptide-resistant isolates which were found in these three years were in all cases resistant to copper (Table 1). Here, the reduction in prevalence of resistance could be a result of the reduced use of macrolides for treatment compared to the amounts used for growth promotion, as macrolides have previously been shown to coselect for glycopeptide resistance (1, 2). Alternatively, the low level of persistence could be a result of a weak selective effect from the use of copper. But, as the level of glycopeptide resistance has decreased after 1998 and as the level of copper resistance is virtually unchanged throughout the study period, it can be concluded that the use of copper has not been able to maintain the high level of glycopeptide resistance seen prior to the ban on avoparcin and tylosin for growth promotion, regardless of the apparent statistical association between the two determinants.
From the statistical association described, it seemed plausible to assume that the tcrB gene was located in the proximity of the erm(B) gene and the Tn1546 element. To examine if any of the three antibiotic determinants were located close to each other on the plasmid common to our GREF isolates, a plasmid from GREF isolate A17sv1 was purified for further examination as described previously (6). For each of the three antibiotic determinants, a primer set was designed with the Vector NTI software from Invitrogen, in such a way that the primers amplified out of the determinant (Table 2). By combining the three primer sets with each other, a total of 12 different primer set permutations were made and used for long-template PCR with the Expand long-template PCR system (Roche) and amplified according to the manufacturer's recommendations with an annealing temperature of 50°C and an extension time of 10 min for the first 10 cycles and 10 min 20 s for each of the following 20 cycles. This corresponds to amplification of approximately 12 kb of DNA. An amplicon was acquired only with primer tcrB-2 (reading downstream of the tcrB gene) and primer Tn1546-1 (reading upstream of Tn1546). Here, a PCR fragment of approximately 8 kb was amplified. An identical fragment was obtained from eight other of our GREF isolates from pigs.
TABLE 2.
Primers used in this study to amplify the intergenic regions between the erm(B), tcrB, and Tn1546 resistance determinants
| Primer | Sequence (5′-3′) | Position |
|---|---|---|
| erm(B)-1 | TTATTATTTGGTTGAGTACC | Start of erm(B) |
| erm(B)-2 | AGTACCGTTACTTATGAGC | End of erm(B) |
| tcrB-1 | AGCTAGAACCATTATTTGATG | Start of tcrB |
| tcrB-2 | ATCGAGTTGGCTCAGAAGACCAC | End of tcrB |
| Tn1546-1 | CTTACGTGAGATGGGCCGAATAG | Start of Tn1546 (CDS1) |
| Tn1546-2 | AAAGCTTACCTAACACTATAG | Start of Tn1546 (vanZ) |
Five GREF strains from humans isolated in 1995, four from the feces of hospitalized patients with diarrhea and one from a urinary tract infection, have previously been described (4, 7). Examination of these strains showed that all five isolates were resistant to both copper and macrolides, and genes conferring resistance to these two phenotypes transferred together with Tn1546 in conjugation experiments with E. faecium BM4105RF. These strains were tested for a similar link between tcrB and Tn1546 with the same PCR setup as that described above. Here two of the five isolates produced identical bands, showing a close relationship between these two human GREF isolates and the porcine GREF isolates. Plasmid purification indicated that all five strains contained a large plasmid similar to the one found in the porcine GREF clone (data not shown). In conclusion, it seems that both the GREF clone itself and the plasmid have spread from the porcine reservoir to humans and that it still contains the copper resistance gene tcrB.
Coselection caused by copper is not supported by the data presented here, as glycopeptide resistance has been decreasing since the ban, while copper resistance has not. Therefore, there seem to be other factors which contribute more to elimination of the GREF isolates. As we still find GREF 8 years after the ban, the possibility that addition of especially the largest amount of copper to the feed contributes to delaying the elimination of GREF, given the close proximity of the two resistance determinants, cannot be completely excluded. However, significant selection of GREF in pig production seems to have occurred only when the strong selective pressure of glycopeptide or macrolide administration was present.
Acknowledgments
We thank all our technicians for their excellent help.
This work was supported by a grant from the Danish Research Agency—Danish Agricultural and Veterinary Research Council (23-01-0090).
REFERENCES
- 1.Aarestrup, F. M. 2000. Characterization of glycopeptide-resistant Enterococcus faecium (GRE) from broilers and pigs in Denmark: genetic evidence that persistence of GRE in pig herds is associated with coselection by resistance to macrolides. J. Clin. Microbiol. 38:2774-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarestrup, F. M., A. M. Seyfarth, H. D. Emborg, K. Pedersen, R. S. Hendriksen, and F. Bager. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45:2054-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarestrup, F. M., H. Hasman, L. B. Jensen, M. Moreno, I. A. Herrero, L. Dominguez, M. Finn, and A. Franklin. 2002. Antimicrobial resistance among enterococci from pigs in three European countries. Appl. Environ. Microbiol. 68:4127-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aarestrup, F. M., P. Ahrens, M. Madsen, L. V. Pallesen, R. L. Poulsen, and H. Westh. 1996. Glycopeptide susceptibility among Danish Enterococcus faecium and Enterococcus faecalis isolates of animal and human origin and PCR identification of genes within the VanA cluster. Antimicrob. Agents Chemother. 40:1938-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danish Integrated Antimicrobial Resistance Monitoring and Research Programme. 2004. DANMAP 2003—consumption of antimicrobial agents and occurrence of antimicrobial resistance from food animals, food and humans in Denmark (ISSN 1600-2032).
- 6.Hasman, H., and F. M. Aarestrup. 2002. tcrB, a gene conferring transferable copper resistance in Enterococcus faecium: occurrence, transferability, and linkage to macrolide and glycopeptide resistance. Antimicrob. Agents Chemother. 46:1410-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen, L. B., A. M. Hammerum, R. L. Poulsen, and H. Westh. 1999. Vancomycin-resistant Enterococcus faecium strains with highly similar pulsed-field gel electrophoresis patterns containing similar Tn1546-like elements isolated from a hospitalized patient and pigs in Denmark. Antimicrob. Agents Chemother. 43:724-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odermatt, A., H. Suter, R. Krapf, and M. Solioz. 1993. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J. Biol. Chem. 268:12775-12779. [PubMed] [Google Scholar]