Abstract
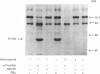
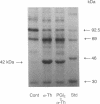
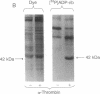
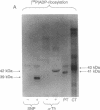
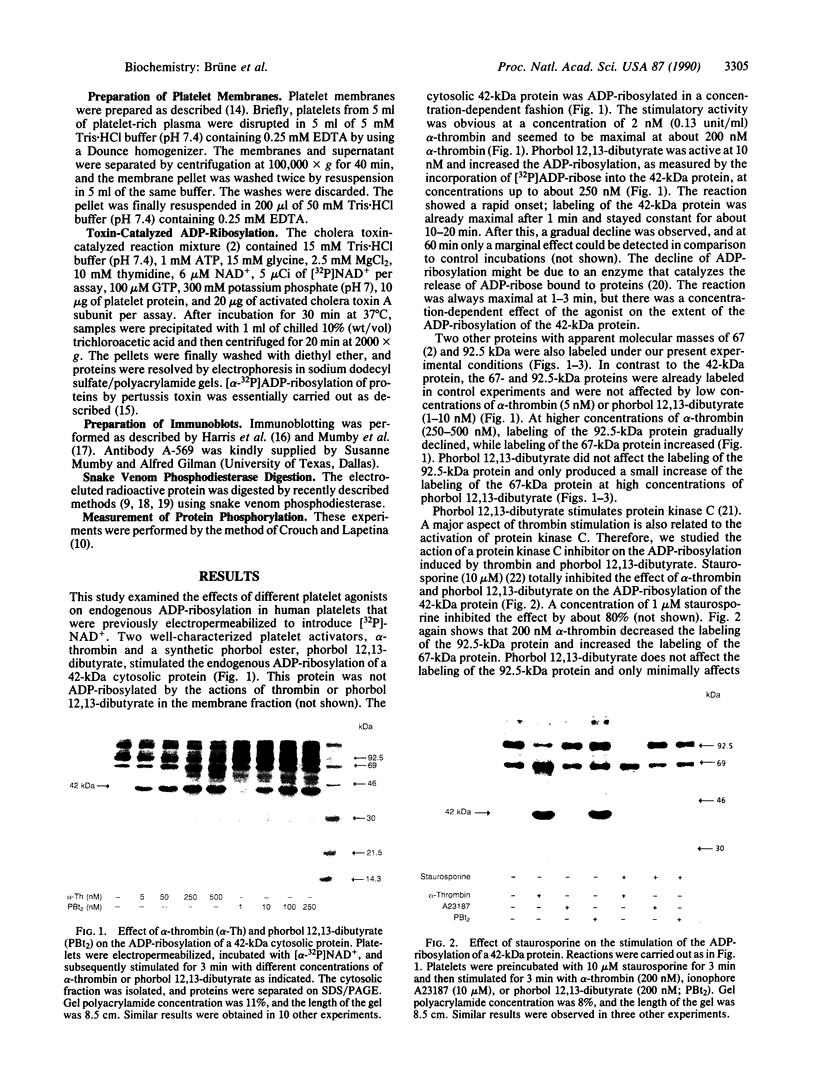
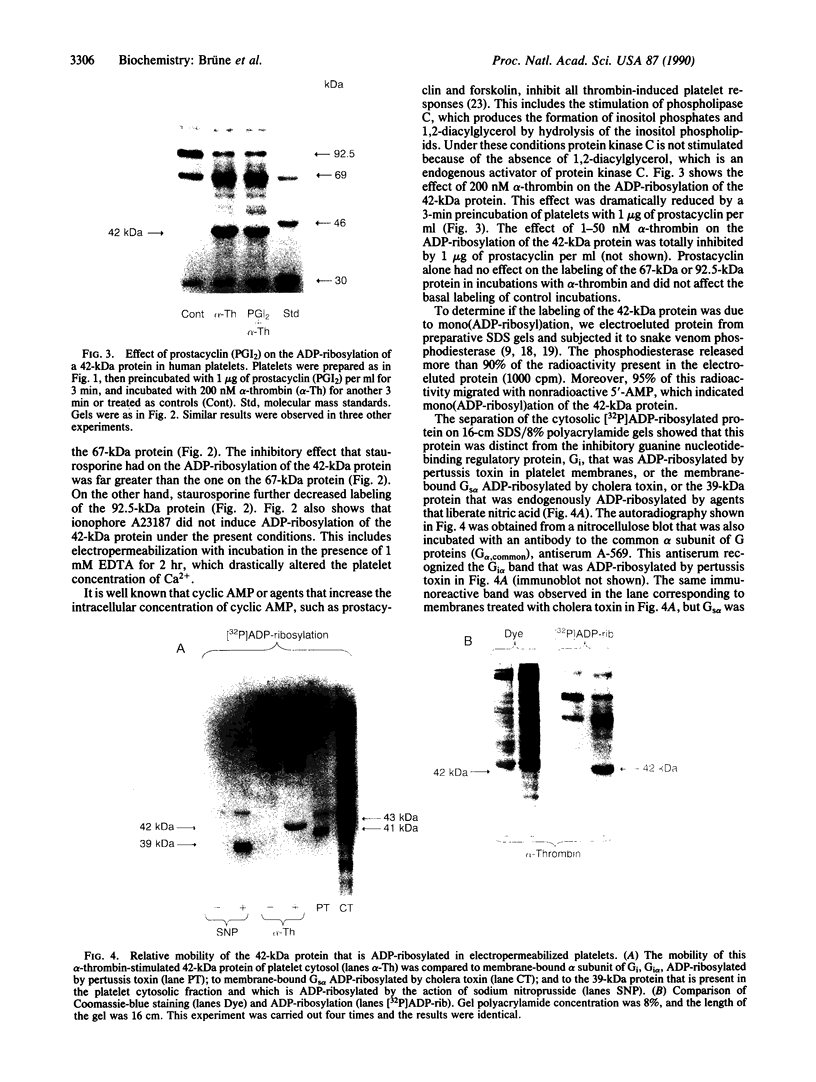
alpha-Thrombin and phorbol 12,13-dibutyrate stimulated the mono(ADP-ribosyl)ation of a 42-kDa cytosolic protein of human platelets. This effect was mediated by protein kinase C activation and was inhibited by protein kinase C inhibitor staurosporine. It also was prevented by prostacyclin, which is known to inhibit the phospholipase C-induced formation of 1,2-diacylglycerol, which is one of the endogenous activators of protein kinase C. On sodium dodecyl sulfate/polyacrylamide gel electrophoresis, the 42-kDa protein that is ADP-ribosylated by alpha-thrombin was clearly distinct from the alpha subunits of membrane-bound inhibitory and stimulatory guanine nucleotide-binding regulatory proteins, respectively Gi alpha and Gs alpha; the 47-kDa protein that is phophorylated by protein kinase C in platelets; and the 39-kDa protein that has been shown to be endogenously ADP-ribosylated by agents that release nitric oxide. This information shows that agonist-induced activation of protein kinase leads to the ADP-ribosylation of a specific protein. This covalent modification might have a functional role in platelet activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Majerus P. W. Isolation of human platelets and platelet surface membranes. Methods Enzymol. 1974;31:149–155. doi: 10.1016/0076-6879(74)31015-4. [DOI] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Chang Y. C., Soman G., Graves D. J. Identification of an enzymatic activity that hydrolyzes protein-bound ADP-ribose in skeletal muscle. Biochem Biophys Res Commun. 1986 Sep 30;139(3):932–939. doi: 10.1016/s0006-291x(86)80267-4. [DOI] [PubMed] [Google Scholar]
- Crouch M. F., Lapetina E. G. A role for Gi in control of thrombin receptor-phospholipase C coupling in human platelets. J Biol Chem. 1988 Mar 5;263(7):3363–3371. [PubMed] [Google Scholar]
- Edmonds C., Griffin G. E., Johnstone A. P. Demonstration and partial characterization of ADP-ribosylation in Pseudomonas maltophilia. Biochem J. 1989 Jul 1;261(1):113–118. doi: 10.1042/bj2610113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. J., MacDermot J., Wilkins A. J. Prostacyclin analogues reduce ADP-ribosylation of the alpha-subunit of the regulatory Gs-protein and diminish adenosine (A2) responsiveness of platelets. Br J Pharmacol. 1987 Mar;90(3):501–510. doi: 10.1111/j.1476-5381.1987.tb11199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris B. A., Robishaw J. D., Mumby S. M., Gilman A. G. Molecular cloning of complementary DNA for the alpha subunit of the G protein that stimulates adenylate cyclase. Science. 1985 Sep 20;229(4719):1274–1277. doi: 10.1126/science.3839937. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Niggli V., Scrutton M. C. Thrombin and activators of protein kinase C modulate secretory responses of permeabilised human platelets induced by Ca2+. Eur J Biochem. 1984 Sep 3;143(2):437–446. doi: 10.1111/j.1432-1033.1984.tb08391.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Shall S., Whish W. J. The relationship between cell growth, macromolecular synthesis and poly ADP-ribose polymerase in lymphoid cells. Exp Cell Res. 1974 Jan;83(1):63–72. doi: 10.1016/0014-4827(74)90688-0. [DOI] [PubMed] [Google Scholar]
- Molina y Vedia L. M., Reep B. R., Lapetina E. G. Platelet cytosolic 44-kDa protein is a substrate of cholera toxin-induced ADP-ribosylation and is not recognized by antisera against the alpha subunit of the stimulatory guanine nucleotide-binding regulatory protein. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5899–5902. doi: 10.1073/pnas.85.16.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina y Vedia L., Nolan R. D., Lapetina E. G. Endogenous ADP-ribosylation in human platelets. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1323–1328. doi: 10.1016/s0006-291x(88)81019-2. [DOI] [PubMed] [Google Scholar]
- Molina y Vedia L., Nolan R. D., Lapetina E. G. The effect of iloprost on the ADP-ribosylation of Gs alpha (the alpha-subunit of Gs). Biochem J. 1989 Aug 1;261(3):841–845. doi: 10.1042/bj2610841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. ADP-ribosylation of guanyl nucleotide-binding regulatory proteins by bacterial toxins. Adv Enzymol Relat Areas Mol Biol. 1988;61:303–379. doi: 10.1002/9780470123072.ch6. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Rosenthal W., Binder T., Schultz G. NADP efficiently inhibits endogenous but not pertussis toxin-catalyzed covalent modification of membrane proteins incubated with NAD. FEBS Lett. 1987 Jan 26;211(2):137–143. doi: 10.1016/0014-5793(87)81424-2. [DOI] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Vitti P., De Wolf M. J., Acquaviva A. M., Epstein M., Kohn L. D. Thyrotropin stimulation of the ADP-ribosyltransferase activity of bovine thyroid membranes. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1525–1529. doi: 10.1073/pnas.79.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]
- Watson S. P., McNally J., Shipman L. J., Godfrey P. P. The action of the protein kinase C inhibitor, staurosporine, on human platelets. Evidence against a regulatory role for protein kinase C in the formation of inositol trisphosphate by thrombin. Biochem J. 1988 Jan 15;249(2):345–350. doi: 10.1042/bj2490345. [DOI] [PMC free article] [PubMed] [Google Scholar]