Abstract
In the present study, Anacker and Ordal agar, marine agar (MA), and Flexibacter maritimus medium (FMM) were compared with the dilute versions of Mueller-Hinton agar (DMHA) medium recommended by the National Committee for Clinical Laboratory Standards (NCCLS) for their use in disk diffusion tests with Tenacibaculum maritimum strains and to calculate the MICs of five drugs by the Etest method. Preliminary growth tests performed with 32 strains of this pathogen on each medium revealed that all strains failed to grow on DMHA, while the remaining media supported good growth of all isolates. In the susceptibility tests, which were carried out with the other three media, all strains were resistant to oxolinic acid and were highly susceptible to amoxicillin and trimethoprim-sulfamethoxazole, showing a good correspondence with the Etest values, which ranged from 0.064 to 0.75 and 0.006 to 1.5 μg/ml, respectively. Enrofloxacin and oxytetracycline produced significantly smaller inhibition zones and MICs on MA than on the other media assayed. However, fast, clear, and well-defined zones of inhibition were displayed for all strains at 24 h of incubation only on FMM by both the disk diffusion assay and Etest. In addition, FMM prepared with commercial sea salts instead of seawater was also suitable for bacterial isolation as well as for susceptibility testing. On the basis of these results, the use of FMM to determine the in vitro susceptibility of T. maritimum and its inclusion in a future revision of the NCCLS M42 report are recommended.
The rapid expansion of the aquaculture industry in the last decade has increased the losses caused by systemic bacterial infections in marine fish farming throughout the world. Although vaccination procedures are used to prevent the majority of bacterial diseases (15, 22, 27), at present a wide range of antimicrobial compounds are still essential for the control of clinical cases of infection in fisheries (29, 30, 31). Several methods of in vitro drug susceptibility testing of fish pathogens have been reported, including the disk diffusion assay, broth and agar dilution procedures (32), and most recently, the Etest method (6; M. Vilariño, J. L. Romalde, C. Ribao, A. E. Toranzo, and J. L. Barja, Abstr. XIX Congr. Nacional Microbiol., abstr. 139, 2003). Of these techniques, the agar disk diffusion method has been used since the 1960s and is the most widely used method in diagnostic laboratories because it is simple to perform, it presents a high degree of reliability in terms of standardization of the drug concentration, and a single bacterial isolate can be tested with a series of antimicrobials in one experiment (19).
The filamentous organism Tenacibaculum maritimum (formerly Flexibacter maritimus) (35) is the causative agent of marine flexibacteriosis, an important disease in fish farms around the world (4, 8, 10, 11, 13, 16, 23, 28, 38). Since the first reports of flexibacteriosis (18, 20), the use of nonselective and/or low-nutrient media, such as marine agar (MA) and Anacker and Ordal agar (AOA) (3) prepared with seawater, has been advocated for the isolation of seawater-dependent, slow-growing T. maritimum isolates from infected fish. However, although both media support the growth of T. maritimum strains, another medium, named Flexibacter maritimus medium (FMM) (24), has been proposed to be the most appropriate for the successful isolation of this species from fish samples due to its ability to allow better growth of T. maritimum in comparison to the growth of heterotrophic halophilic bacteria, such as Vibrio, Pseudomonas, and Alteromonas species, which usually overgrow the plates. In addition, these three media have also been used for the routine drug susceptibility testing of this fastidious pathogen.
Although Alderman and Smith (1) reported a tentative set of antibiotic susceptibility test protocols for use with different bacteria pathogenic for fish, T. maritimum was not included in that guidance document. Recently, the National Committee for Clinical Laboratory Standards (NCCLS) (21) recommended the use of two versions of diluted Mueller-Hinton agar (DMHA), previously proposed for Flavobacterium columnare and Flavobacterium psychrophilum, as the best media for the routine susceptibility testing of T. maritimum. This was probably due to the inclusion of T. maritimum in the guidelines as a member of group III (gliding, flexing, and yellow-pigmented gram-negative bacteria), together with other phenotypically similar fish pathogens of the genera Flavobacterium. Unfortunately, the abilities of the strictly halophilic T. maritimum strains to grow on these Mueller-Hinton agar (MHA) variants have not been tested.
Therefore, the primary objective of the present study was to compare AOA, MA, and FMM with the officially recommended versions of DMHA medium prepared with distilled water or seawater for disk diffusion susceptibility testing with a collection of T. maritimum isolates. The possible replacement of seawater by commercial sea salts was evaluated with all the media used. Finally, the MICs of different drugs in the different media were determined by the Etest method.
MATERIALS AND METHODS
Bacterial strains.
A total of 32 strains of T. maritimum were included in this study (Table 1). This collection comprises 29 strains isolated from seven different marine fish species that belong to the different serotypes and clonal lineages described for this pathogen (4, 5) and three reference strains (NCIMB 2153, NCIMB 2154T, and NCIMB 2158) from the National Collection of Industrial and Marine Bacteria (NCIMB; Aberdeen, United Kingdom). Most of the strains were collected from epizootic outbreaks during the last 10 years and were preserved by freezing them at −70°C in Cryo-bille tubes (AES Laboratory, Combourg, France). These strains were streaked on FMM and incubated at 24°C for 72 h. Before the assay, all bacterial strains were confirmed to be T. maritimum by biochemical testing, serological assays, and species-specific PCR (4, 5). As recommended in NCCLS document M42-R, reference strain Escherichia coli (ATCC 25922) from the American Type Culture Collection (ATCC; Manassas, Va.) was included for quality control in every test run and was grown on MHA plates (Difco Laboratories, Madrid, Spain) at 22 and 35°C for 16 to 20 h. Three F. columnare and two F. psychrophilum isolates were included as growth controls on each version of DMHA medium prepared with distilled water. These strains were routinely grown on tryptic soy agar (Difco) and modified AOA (36), respectively.
TABLE 1.
T. maritimum strains used in this study
| Bacterial isolate | Host species | Origin | Yr of isolation |
|---|---|---|---|
| LR2P | Sole (Solea solea) | Spain | 1995 |
| PC492.1 | Sole (Solea senegalensis) | Spain | 2001 |
| PC503.1 | Sole (S. senegalensis) | Spain | 2001 |
| PC504.1 | Sole (S. senegalensis) | Spain | 2001 |
| PC528.1 | Sole (S. senegalensis) | Spain | 2002 |
| PC529.1 | Sole (S. senegalensis) | Spain | 2002 |
| AZ203.1 | Sole (S. senegalensis) | Spain | 2001 |
| LgH35-O3a | Sole (S. senegalensis) | Spain | 2003 |
| LgV1-04a | Sole (S. senegalensis) | Spain | 2004 |
| ACC8.1 | Sole (S. senegalensis) | Portugal | 2003 |
| PC424.1 | Turbot (Scophthalmus maximus) | Spain | 2000 |
| PC460.1 | Turbot (S. maximus) | Spain | 2001 |
| PC473.1 | Turbot (S. maximus) | Spain | 2001 |
| LD12.1 | Turbot (S. maximus) | Spain | 2001 |
| RM256.1 | Turbot (S. maximus) | Spain | 2002 |
| RI93.1 | Turbot (S. maximus) | Spain | 2002 |
| ACR104.1 | Turbot (S. maximus) | Spain | 2001 |
| RM276.1 | Turbot (S. maximus) | Spain | 2004 |
| JIP 24/99b | Turbot (S. maximus) | Spain | 1999 |
| JIP 46/00b | Turbot (S. maximus) | Spain | 2000 |
| ACC6.1 | Turbot (S. maximus) | Portugal | 2003 |
| PC538.1 | Gilthead sea bream (Sparus aurata) | Spain | 2002 |
| PC560.1 | Gilthead sea bream (S. aurata) | Spain | 2002 |
| DOB102 | Gilthead sea bream (S. aurata) | Spain | 2002 |
| PC868.1 | Gilthead sea bream (S. aurata) | Spain | 2003 |
| DBA4a | Seriola quinqueradiata | Japan | 1986 |
| SSG33 | Salmo salar | Spain | 1993 |
| JIP 32/99b | Sea bass (Dicentrarchus labrax) | France | 1999 |
| LVDH 1577.01b | Sea bass (D. labrax) | France | 2003 |
| NCIMB 2158 | Sole (S. solea) | United Kingdom | 1981 |
| NCIMB 2153 | Blackhead sea bream (Acanthopagrus schlegeli) | Japan | 1976 |
| NCIMB 2154T | Japanese sea bream (Pagrus major) | Japan | 1977 |
Supplied by M. A. Moriñigo, Department of Microbiology, University of Malaga, Malaga, Spain.
Supplied by J. F. Bernardet, Unité de Virologie et Inmunologie Moleculaires, Institut National de la Recherche Agronomique, Jouy-en-Josas Cedex, France.
Test media.
The NCCLS procedure (21) was carefully followed for the preparation of all media used in this study. Dilute 0.3% Mueller-Hinton broth (Difco) with 0.9% agar (Cultimed Panreac Química S.A., Barcelona, Spain) (DMHA) and DMHA supplemented with 5% fetal calf serum (FCS; Cultek S.L., Madrid, Spain) were prepared as described by the NCCLS (21). Due to the halophilic characteristic of this bacterium, the two variants of DMHA were also prepared with seawater. FMM (0.5% peptone [Difco], 0.05% yeast extract [Oxoid Ltd., Basingstoke, England], and 0.001% sodium acetate [Sigma Aldrich Química, S.A., Madrid, Spain] supplemented with 1.5% agar [Cultimed]) and AOA (0.5% tryptone [Becton Dickinson and Co., Le Pont de Claix, France], 0.05% yeast extract [Oxoid], 0.02% sodium acetate, hydrated [Sigma], and 0.02% beef extract [Cultimed] supplemented with 1.5% agar) were prepared with seawater as the diluent, according to the original descriptions (3, 24). MA (Pronadisa, Madrid, Spain) was prepared according to the instructions of the manufacturer. In addition, to avoid dependence on the availability of natural seawater by most laboratories, as well as to facilitate standardization of the protocols, all media (except MA) were also prepared with commercial sea salts (Oxoid) (4%; wt/vol) dissolved in distilled water. All experiments were carried out with three different batches of media.
Antimicrobial disks.
For disk diffusion testing, five chemotherapeutic agents used for the treatment of bacterial diseases in fish were selected. Commercial disks (Oxoid) with oxolinic acid (OA; 2 μg), amoxicillin (AMX; 25 μg), trimethoprim-sulfamethoxazole (SXT [1.25 μg/23.75 μg]; 25 μg), enrofloxacin (ENR; 5 μg), and oxytetracycline (OTC; 30 μg) were used as described for the NCCLS procedures (21).
Preparation of inoculum.
Although all T. maritimum isolates were routinely cultured on FMM agar, the abilities of the strains to grow on all media were also examined by inoculating each strain directly on each medium. To evaluate if the initial medium used in the disk diffusion assays had any influence, three colonies grown on plates with each medium which supported good growth of the strains were used to prepare the starting inocula of all T. maritimum isolates. Bacterial suspensions were prepared in sterile 0.9% saline solution, and just before experimental use the absorbance at 625 nm was measured on a spectrophotometer and was adjusted to 0.08 to 0.10, as indicated in the NCCLS M42 report. Simultaneously, 10-fold dilutions were prepared and 0.1 ml of each of the different dilutions was spread onto each medium to determine the recoverability of the strains (expressed as the number of CFU per milliliter). The plates were incubated at 24°C for 48 to 72 h.
Disk diffusion testing.
The NCCLS recommendations (21) for the disk diffusion assay protocol were strictly followed in the disk diffusion testing methodology used in this study. The diameter of each zone of inhibition was determined to the nearest millimeter after 24, 48, and, if necessary, 72 h of incubation. Reference strain E. coli ATCC 25922 was used for quality control throughout the study, as described above. All tests were carried out in triplicate, and the mean ± standard deviation was calculated.
Etest method to determine MICs.
To determine the MICs, the Etest method (AB Biodisk, Solna, Sweden) was performed according to the instructions in the manufacturer's package insert. Two strains of T. maritimum isolated from sole (strain PC503.1) and turbot (strain PC424.1), representing the two main serotypes described for this pathogen (4), together with all reference strains, were assayed on the same media used for the disk diffusion assay. The following drugs were tested: AMX, SXT, ENR, and tetracycline (an Etest strip of OTC was not commercially available). Antimicrobial agent concentrations ranged from 0.016 to 256 μg/ml for all agents except SXT (1/19), whose concentrations ranged from 0.002 to 32 μg/ml. Three plates per isolate were incubated for up to 48 h at 24°C and examined for the formation of an elliptical zone of growth inhibition. The value printed on the strip edge at the intersection of the growth inhibition zone was recorded as the MIC for T. maritimum. To check the performance of the Etests, E. coli ATCC 25922 was included because the MICs for this strain on MHA are known; it was incubated at 22 and 35°C for 16 to 20 h.
Statistical analysis.
Differences between zone diameters on the different media compared were tested by applying one-way analysis of variance, with a P value of 0.05 indicating statistical significance (33).
RESULTS AND DISCUSSION
The available data on antimicrobial susceptibility testing of some species of fish bacterial pathogens showed that there is no consensus on the basal medium that should be used, giving the impression that an ideal medium for susceptibility testing of fish bacterial pathogens does not exist (12). Alderman and Smith (1) pointed out that there is a pressing need to establish the appropriate medium for some species, although the NCCLS frequently suggests the use of MHA and a modification of that medium for susceptibility testing of new species of fish pathogens; this is the situation with respect to T. maritimum. However, no studies have shown that halophilic bacteria like T. maritimum are able to grow on the proposed medium, DMHA, supplemented or not with FCS.
When preliminary growth tests with the 29 isolates and the 3 reference strains of T. maritimum were performed by inoculating each strain directly onto each medium tested, all T. maritimum strains afforded good growth on MA, AOA, and FMM, as well as the versions of AOA and FMM prepared with commercial sea salts (AOASS and FMMSS, respectively). As we expected, due to the known halophilic nature of this bacterium, DMHA, with and without FCS, prepared with distilled water did not support the growth of any of the T. maritimum strains tested after 7 days of incubation at 24°C. This finding suggests that the two versions of DMHA cannot be recommended for use for the routine disk diffusion susceptibility testing of T. maritimum. In contrast, each batch of DMHA supported the growth of all isolates of F. columnare and F. psychrophilum tested, showing that it is reliable for the in vitro susceptibility testing of both fish bacterial pathogens, as proposed by Hawke and Thune (17) and Bruun et al. (9), respectively. When each version of DMHA prepared with seawater or sea salts was tested, the T. maritimum strains presented no or scant and poorly defined growth.
With the knowledge that MA, AOA, and FMM, as well as the versions prepared with commercial sea salts, allowed the suitable growth of all T. maritimum isolates, only these media were used for comparative testing of the five antibacterial agents commonly used in aquaculture for the treatment of marine flexibacteriosis. In fact, these media have been routinely used for the isolation of T. maritimum strains from the external lesions and internal organs of infected fish (2, 4, 10, 11, 34). Although both variants of FMM and AOA, as well as MA, were capable of providing suitable growth conditions for susceptibility testing, in the first 24 h, 36.36% of the T. maritimum isolates tested on AOA and AOASS grew too poorly to permit the measurement of zone diameters, and 48 h was required before the results could be read (Table 2). These results agree with the values of recoverability of T. maritimum on AOA and AOASS, which were less than those achieved on the other media tested (Table 2). Although the strains grew faster on MA than on AOA or AOASS, poorly defined zones around the disk were produced, leading to inaccuracies in estimations of the inhibition zone sizes. All T. maritimum strains displayed fast, clear, and well-defined zones of inhibition only on FMM and FMMSS after 24 h of incubation, even though the numbers of recoverable cells in the inoculum fell below the concentrations recommended by the NCCLS due to the fastidious growth of this microorganism (Table 2). In addition, these zones of inhibition remained stable during the incubation period. These advantages are convenient for routine aquaculture operations, since a fast decision on the appropriate treatment can be established (14, 29).
TABLE 2.
Comparison of the growth and recoverability of T. maritimum reference strains and isolates on each medium used for disk diffusion assays
| Medium |
T. maritimum reference strains (n = 3)
|
T. maritimum isolates (n = 29)
|
||||
|---|---|---|---|---|---|---|
| Growth by diffusion testa | Recoverability of inoculum (CFU/ml)b | Time (h) to measurement | Growth by diffusion test | Recoverability of inoculum (CFU/ml)b | Time (h) to measurement | |
| MA | ++ | (9.5 ± 4.66) × 107 | 24 | ++ | (2.43 ± 1.02) × 107 | 24 |
| FMM | +++ | (4.54 ± 0.9) × 106 | 24 | +++ | (3.63 ± 3.35) × 106 | 24 |
| FMMSS | +++ | (2.3 ± 0.3) × 106 | 24 | +++ | (3.89 ± 0.39) × 106 | 24 |
| AOA | ++ | (1.25 ± 0.35) × 106 | 48 | + | (1.92 ± 0.61) × 106 | 48 |
| AOASS | ++ | (1.79 ± 0.48) × 106 | 48 | + | (1.68 ± 0.012) × 106 | 48 |
+++, confluent and well-defined growth around the inhibition zones; ++, less well-defined growth and poorly clear inhibition zones; +, very poor growth.
Data are means±standard deviations for all replicates of each strain.
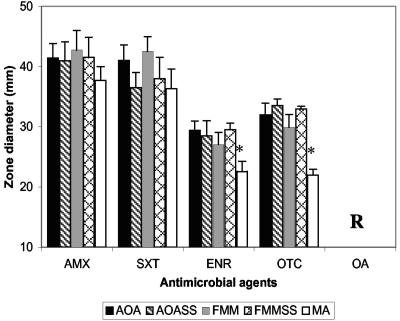
The results of the agar disk diffusion test obtained for all T. maritimum isolates suggested that the nature of the culture medium seems to affect the size of the inhibition zones for two of the five drugs analyzed (Fig. 1). Furthermore, the inhibition zone sizes for the 32 T. maritimum strains grown on MA with ENR and OTC were significantly lower (P < 0.05) than those on the other four media tested. This result is perhaps not surprising due to the qualitative and quantitative differences in the composition of the MA in comparison with those of the oligotrophic media, mainly in the carbon and nitrogen sources, and the presence of excessive amounts of divalent cations, which are known to affect the susceptibility testing results obtained with tetracycline in other culture media, such as tryptone yeast extract salts agar (25, 26).
FIG. 1.
Comparison of in vitro susceptibilities of 29 strains and 3 reference strains of T. maritimum after 48 of incubation on five difference media. All data are means ± standard deviations of three replicates in which the starting inoculum consisted of strains grown on FMM. Asterisks, significant difference (P < 0.05); R, resistance on all media tested.
Regardless of the differences between MA and the remaining media, all T. maritimum strains were totally resistant to OA and showed susceptibility to AMX and SXT, as reported previously (4, 28), with mean inhibition zone sizes ranging from 36 to 43 mm, depending on the medium used. These findings showed a good overall correspondence with the Etest MICs obtained with AMX and SXT, which ranged from 0.064 to 0.75 and 0.006 to 1.5 μg/ml, respectively, as well as with the higher MICs of ENR and tetracycline recorded (Table 3). The MICs were difficult to compare with published results since the tests were done under conditions and with microbial agents different from those used in other studies. However, our MICs are similar to those previously reported for this bacterium by Baxa et al. (7) and Soltani et al. (34), who used agar dilution procedures. As occurred in the disk diffusion tests, FMM and FMMSS provided the best bacterial growth; and consequently, the plates could be read and scored for the MICs after 24 h of incubation, with clear elliptical zones of growth inhibition of T. maritimum detected with the drugs tested.
TABLE 3.
MICs of four antimicrobial agents for two T. maritimum isolates and reference strains determined with five different agars by Etest method
| Antimicrobial agent and medium | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| T. maritimum isolates (n = 2) | T. maritimum NCIMB 2154T | T. maritimum NCIMB 2153 | T. maritimum NCIMB 2158 | E. coli ATCC 25922a | |
| AMX | |||||
| MHA | NAb | NA | NA | NA | 4.0-6.0 |
| AOA | 0.064-0.094 | 0.064-0.094 | 0.064 | 0.064 | 2.0-3.0 |
| AOASS | 0.094-0.125 | 0.064-0.094 | 0.125-0.19 | 0.094-0.19 | 3.0 |
| FMM | 0.094-0.75 | 0.125-0.19 | 0.064-0.094 | 0.125-019 | 1.5-3.0 |
| FMMSS | 0.064-0.125 | 0.064-0.094 | 0.125-0.19 | 0.094-0.125 | 2.0-4.0 |
| MA | 0.25 | 0.25-0.38 | 0.25 | 0.25 | 1.5-2.0 |
| SXTd | |||||
| MHA | NA | NA | NA | NA | 0.047-0.064 |
| AOA | 0.064-1.5 | 0.012-0.016 | 0.008-0.016 | 0.006 | 0.25-0.36 |
| AOASS | 0.023-0.032 | 0.016 | 0.016 | 0.008-0.016 | 0.094-0.38 |
| FMM | 0.094-1.0 | 0.012-0.016 | 0.016 | 0.008-0.016 | 0.125-0.25 |
| FMMSS | 0.012-0.032 | 0.016 | 0.012-0.016 | 0.016-0.023 | 1.5-3.0 |
| MA | 0.023-0.25 | 0.016-0.032 | 0.032-0.064 | 0.023 | 0.19-0.38 |
| ENR | |||||
| MHA | NA | NA | NA | NA | 0.008-0.023 |
| AOA | 1.5-3.0 | 0.75-1.0 | 2.0 | 1.0-1.5 | 3.0-6.0 |
| AOASS | 0.5-1.0 | 0.5-0.75 | 0.75-1.0 | 0.5-0.75 | 0.75-1.0 |
| FMM | 1.0-1.5 | 1.0-1.5 | 0.75-1.0 | 0.75-1.0 | 3.0 |
| FMMSS | 0.5-1.0 | 0.38 | 0.75-1.0 | 0.5-0.75 | 1.0-1.5 |
| MA | 2.0-3.0 | 2.0 | 3.0 | 1.5-2.0 | 4.0-8.0 |
| TCe | |||||
| MHA | NA | NA | NA | NA | 0.75-1.0 |
| AOA | 1.5 | 1.0 | 2.0-3.0 | 1.5-2.0 | Rc |
| AOASS | 0.094-0.125 | 0.25-0.38 | 0.25-0.38 | 0.25-0.38 | R |
| FMM | 0.075-1.0 | 1.5 | 1.0-1.5 | 1.0 | R |
| FMMSS | 0.25-0.38 | 0.0094-0.125 | 0.25-0.38 | 0.25-0.38 | R |
| MA | 3.0-4.0 | 3.0 | 4.0 | 3.0 | R |
Range of MICs obtained after incubation at 22 and 35°C for 16 to 20 h.
NA, not applicable.
R, resistant.
SXT was used with trimethoprim and sulfamethoxazole at a ratio of 1/19.
TC, tetracycline.
On the other hand, the susceptibility of E. coli (ATCC 25922) grown on MHA under standard growth conditions (21) showed acceptable values for all drugs (Table 4). Furthermore, this control organism was also studied with all other media to see whether the pattern of susceptibility to each antimicrobial agent was static or medium dependent. The assays gave consistent results, with significant variations within antimicrobial agents on each type of medium; the E. coli strain was placed in the category of resistance to SXT, ENR, OTC, and OA when it was tested on FMM and AOA, as well as the versions of FMM and AOA prepared with commercial sea salts, and MA (Tables 3 and 4). These findings support the fact that the addition of the seawater or divalent cations to the growth medium reduces the diffusion of some drugs from the disks into the agar (26, 37).
TABLE 4.
Comparison of in vitro susceptibility testing results for quality control strain E. coli ATCC 25922 using MHA and five other media for each antimicrobial agent testeda
| Antimicrobial agent | Inhibition zone diam (mm)
|
|||||
|---|---|---|---|---|---|---|
| MHA | AOA | AOASS | FMM | FMMSS | MA | |
| AMX | 23 ± 1.19 | 24 ± 0 | 23.56 ± 2.73 | 31.33 ± 2.31 | 30.08 ± 2.42 | 27.33 ± 1.15 |
| SXT | 29.12 ± 1.55 | 0 | 0 | 0 | 0 | 0 |
| ENR | 37.87 ± 1.23 | 20 ± 0 | 19.06 ± 2.05 | 20.67 ± 1.15 | 19.23 ± 2.05 | 9.33 ± 1.15 |
| OTC | 23.75 ± 0.45 | 0 | 0 | 0 | 0 | 0 |
| OA | 22.67 ± 1.15 | 9.33 ± 1.15 | 9 ± 1.51 | 0 | 0 | 0 |
E. coli ATCC 25922 was incubated at 35°C for 16 to 20 h. All data are means ± standard deviations.
In addition, it is important that when the starting inocula of T. maritimum were prepared on different media, no influence on the final results of the disk diffusion or Etest assays were detected (data not shown).
In conclusion, we recommend the use of FMM for the susceptibility testing of T. maritimum isolates. To avoid dependence on natural seawater, whose composition can vary among geographical areas, FMM prepared with sea salts is also suitable for bacterial isolation as well as for antibiogram procedures. In addition, we consider that the findings reported here must be taken into account in a future revision of NCCLS report M42.
Acknowledgments
This work was supported in part by grants AGL2003-09307-C02-00 and PETRI95-0657.01.OP from the Ministerio de Ciencia y Tecnología of Spain. R. Avendaño-Herrera also thanks the BID-CONICYT programs of Chile for a research fellowship.
We thank M. Abuín for help with medium preparation. We are grateful to the donors for kindly supplying some of the strains used in this study.
REFERENCES
- 1.Alderman, D. J., and P. Smith. 2001. Development of draft protocols of standard reference methods for antimicrobial agent susceptibility testing of bacteria associated with fish diseases. Aquaculture 196:211-243. [Google Scholar]
- 2.Alsina, M., and A. R. Blanch. 1993. First isolation of Flexibacter maritimus from cultivated turbot (Scophthalmus maximus). Bull. Eur. Assoc. Fish Pathol. 13:157-160. [Google Scholar]
- 3.Anacker, R. L., and E. J. Ordal. 1959. Studies on the myxobacterium Chondrococcus columnaris. I. Serological typing. J. Bacteriol. 78:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avendaño-Herrera, R., B. Magariños, S. López-Romalde, J. L. Romalde, and A. E. Toranzo. 2004. Phenotypic characterization and description of two major O-serotypes in Tenacibaculum maritimum strains isolated from marine fishes. Dis. Aquat. Org. 58:1-8. [DOI] [PubMed] [Google Scholar]
- 5.Avendaño-Herrera, R., J. Rodríguez, B. Magariños, J. L. Romalde, and A. E. Toranzo. 2004. Intraspecific diversity of the marine fish pathogen Tenacibaculum maritimum as determined by randomly amplified polymorphic DNA-PCR. J. Appl. Microbiol. 96:871-877. [DOI] [PubMed] [Google Scholar]
- 6.Barker, G., D. Page, and E. Kehoe. 1995. Comparison of 4 methods to determine MIC's of amoxicillin against Aeromonas salmonicida. Bull. Eur. Assoc. Fish Pathol. 15:100-104. [Google Scholar]
- 7.Baxa, D. V., K. Kawai, and R. Kusuda. 1988. Chemotherapy of Flexibacter maritimus infection. Rep. USA Mar. Biol. Inst. Kochi Univ. 10:9-14. [Google Scholar]
- 8.Bernardet, J. F., B. Kerouault, and C. Michel. 1994. Comparative study on Flexibacter maritimus strains isolated from farmed sea bass (Dicentrarchus labrax) in France. Fish Pathol. 29:105-111. [Google Scholar]
- 9.Bruun, M. S., A. S. Schmidt, L. Madsen, and I. Dalsgaard. 2000. Antimicrobial resistance patterns in Danish isolates of Flavobacterium psychrophilum. Aquaculture 187:201-212. [Google Scholar]
- 10.Cepeda, C., and Y. Santos. 2002. First isolation of Flexibacter maritimus from farmed Senegalese sole (Solea senegalensis, Kaup) in Spain. Bull. Eur. Assoc. Fish Pathol. 22:388-391. [Google Scholar]
- 11.Chen, M. F., D. Henry-Ford, and J. M. Groff. 1995. Isolation and characterization of Flexibacter maritimus from marine fishes of California. J. Aquat. Anim. Health 7:318-326. [Google Scholar]
- 12.Dalsgaard, I. 2001. Selection of media for antimicrobial susceptibility testing of fish pathogenic bacteria. Aquaculture 196:267-275. [Google Scholar]
- 13.Devesa, S., J. L. Barja, and A. E. Toranzo. 1989. Ulcerative skin and fin lesions in reared turbot, Scophthalmus maximus (L.). J. Fish Dis. 12:323-333. [Google Scholar]
- 14.Furones, M. D. 2001. Sampling for antimicrobial sensitivity testing: a practical consideration. Aquaculture 196:303-309. [Google Scholar]
- 15.Gudding, R., A. Lillehaug, P. Midlyng, and F. Brown. 1996. Fish vaccinology. Karger, Basel, Switzerland.
- 16.Handlinger, J., M. Soltani, and S. Percival. 1997. The pathology of Flexibacter maritimus in aquaculture species in Tasmania, Australia. J. Fish Dis. 20:159-168. [Google Scholar]
- 17.Hawke, J. P., and R. L. Thune. 1992. Systemic isolation and antimicrobial susceptibility of Cytophaga columnaris from commercially reared channel catfish. J. Aquat. Anim. Health 4:109-113. [Google Scholar]
- 18.Hikida, M., H. Wayabayashi, H. Egusa, and K. Masumura. 1979. Flexibacter spp. A gliding bacterium pathogenic to some marine fishes in Japan. Bull. Jpn. Soc. Sci. Fish. 45:421-428. [Google Scholar]
- 19.Jorgensen, J. H. 1993. Selection criteria for an antimicrobial susceptibility testing system. J. Clin. Microbiol. 31:2841-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McVicar, A. H., and P. G. White. 1979. Fin and skin necrosis of cultivated Dover sole, Solea solea (L.). J. Fish Dis. 2:557-562. [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 2003. Methods for antimicrobial disk susceptibility testing of bacteria isolated from aquatic animals; a report. NCCLS document M42-R. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.Newman, S. G. 1993. Bacterial vaccines of fish. Annu. Rev. Fish Dis. 3:145-186. [Google Scholar]
- 23.Ostland, V. E., C. LaTrace, D. Morrison, and H. W. Ferguson. 1999. Flexibacter maritimus associated with a bacterial stomatitis in Atlantic salmon smolts reared in net-pens in British Columbia. J. Aquat. Anim. Health 11:35-44. [Google Scholar]
- 24.Pazos, F., Y. Santos, A. R. Macias, S. Núñez, and A. E. Toranzo. 1996. Evaluation of media for the successful culture of Flexibacter maritimus. J. Fish Dis. 19:193-197. [Google Scholar]
- 25.Piddock, L. 1990. Techniques used for the determination of antimicrobial resistance and sensitivity in bacteria. J. Appl. Bacteriol. 68:307-318. [DOI] [PubMed] [Google Scholar]
- 26.Pursell, L., O. B. Samuelsen, and P. Smith. 1995. Reduction in the in-vitro activity of flumequine against Aeromonas salmonicida in the presence of the concentration of Mg2+ and Ca2+ ions found in sea water. Aquaculture 135:245-255. [Google Scholar]
- 27.Romalde, J. L., C. Ravelo, S. López-Romalde, R. Avendaño, B. Magariños, and A. E. Toranzo. Vaccination strategies to prevent important emerging diseases for Spanish aquaculture. In P. J. Midtlyng, T. Wolffrom, and F. Brown (ed.), Progress in fish vaccinology, in press. Karger, Basel, Switzerland.
- 28.Santos, Y., F. Pazos, and J. L. Barja. 1999. Flexibacter maritimus, causal agent of flexibacteriosis in marine fish, p. 1-6. In G. Oliver (ed.), ICES identification leaflets for diseases and parasites of fish and shellfish, no. 55. International Council for the Exploration of the Sea, Copenhagen, Denmark.
- 29.Schnick, R. A. 2001. International harmonization of antimicrobial sensitivity determination for aquaculture drugs. Aquaculture 196:277-288. [Google Scholar]
- 30.Schnick, R. A., D. J. Alderman, R. Armstrong, R. Le Gouvello, S. Ishihara, E. C. Lacierda, S. Percival, and M. Roth. 1997. World wide aquaculture drug and vaccine registration progress. Bull. Eur. Assoc. Fish Pathol. 17:251-260. [Google Scholar]
- 31.Smith, P. 2001. Accuracy, precision and meaning of antimicrobial agent susceptibility testing of bacteria associated with fish diseases. Aquaculture 196:253-266. [Google Scholar]
- 32.Smith, P., M. P. Hiney, and O. B. Samuelsen. 1994. Bacterial resistance to antimicrobial agents used in fish farming: a critical evaluation of method and meaning. Annu. Rev. Fish Dis. 4:273-313. [Google Scholar]
- 33.Sokal, R., and J. Rohlf. 1980. Introducción a la bioestadística. De Reverte S.A., Barcelona, Spain.
- 34.Soltani, M., S. Shanker, and B. L. Munday. 1995. Chemotherapy of Cytophaga/Flexibacter-like bacteria (CFLB) infections in fish: studies validating clinical efficacies of selected antimicrobials. J. Fish Dis. 18:555-565. [Google Scholar]
- 35.Suzuki, M., Y. Nakagawa, S. Harayama, and S. Yamamoto. 2001. Phylogenetic analysis and taxonomic study of marine Cytophaga-like bacteria: proposal for Tenacibaculum gen. nov. with Tenacibaculum maritimum comb. nov. and Tenacibaculum ovolyticum comb. nov., and description of Tenacibaculum mesophilum sp. nov. and Tenacibaculum amylolyticum sp. nov. Int. J. Syst. Evol. Microbiol. 51:1639-1652. [DOI] [PubMed] [Google Scholar]
- 36.Toranzo, A. E., and J. L. Barja. 1993. Fry mortality syndrome (FMS) in Spain. Isolation of the causative bacterium Flexibacter psychrophilus. Bull. Eur. Assoc. Fish Pathol. 13:30-32. [Google Scholar]
- 37.Torkildsen, L., O. Samuelsen, B. Lunestad, and Ø. Bergh. 2000. Minimum inhibitory concentration of chloramphenicol, florfenicol, trimethoprim/sulfadiazine and flumequine in seawater of bacteria associated with scallops (Pecten maximus) larvae. Aquaculture 185:1-12. [Google Scholar]
- 38.Wakabayashi, H., M. Hikida, and K. Masumura. 1986. Flexibacter maritimus sp. nov., a pathogen of marine fishes. Int. J. Syst. Bacteriol. 36:396-398. [Google Scholar]