Abstract
Of 150 clinical isolates of Neisseria gonorrhoeae recovered in 2001, we examined 55 clinical isolates of N. gonorrhoeae for which cefixime MICs were ≥0.125 μg/ml and randomly selected 15 isolates for which cefixime MICs were ≤0.06 μg/ml for analysis of alterations in the penicillin-binding protein 2 (PBP 2) gene. We found insertion of an extra codon (Asp-345a) in the transpeptidase domain of PBP 2, and this insertion occurred alone or in conjunction with other amino acid substitutions. We also found a mosaic PBP 2 that was composed of fragments of the PBP 2 proteins from Neisseria cinera and Neisseria perflava. This mosaic PBP 2 was significantly associated with decreased susceptibilities to penicillin and cephalosporins, especially oral cephalosporins. For most of the isolates with a mosaic PBP 2, the cefixime MICs were ≥0.5 μg/ml and the cefdinir MICs were ≥1 μg/ml. Analysis of chromosomal DNA restriction patterns by pulsed-field gel electrophoresis revealed that most isolates with the mosaic PBP 2 were genetically similar. The recombination events that generated the mosaic PBP 2 would likely have contributed to the decreased sensitivities to cephalosporins. Isolates with the mosaic PBP 2 appear to threaten the efficacy of the currently recommended regimen with cefixime. The emergence of such strains may be the result of the in vivo generation of clones in which interspecies recombination occurred between the penA genes of N. gonorrhoeae and commensal Neisseria species.
Since the emergence of penicillin- and tetracycline-resistant strains of Neisseria gonorrhoeae, selected fluoroquinolones and broad-spectrum cephalosporins have been recommended as the primary therapies for uncomplicated gonococcal infection (5, 15). Fluoroquinolones have been highly effective in curing such infections; however, fluoroquinolone treatment failures associated with the development of resistance to fluoroquinolones and the clinical isolation of N. gonorrhoeae strains with decreased susceptibilities to such agents have been reported since the mid-1990s in Japan (9). As alternatives to fluoroquinolones, cephalosporins are potent antibiotics that are commonly used in the oral form for the treatment of gonococcal infections in Japan. However, decreases in the susceptibilities of present clinical isolates of N. gonorrhoeae to oral cephalosporins, including cefixime, have also been observed (1, 17).
Many studies of the molecular mechanisms that underlie resistance to various classes of antimicrobial agents have been reported. Mutations in the gyrA and parC genes are responsible for resistance to fluoroquinolones in N. gonorrhoeae (7, 8). In addition, alterations in drug permeation and drug efflux can contribute to the level of resistance to fluoroquinolones (18). The latter mechanisms are associated with the development of cross-resistance to structurally unrelated antibiotics (18). The mechanisms for chromosomally mediated resistance to penicillin G and tetracycline in N. gonorrhoeae involve the penA, penB, and mtr mutations. penA causes insertion of a single amino acid into penicillin-binding protein 2 (PBP 2), and this reduces the level of binding of penicillin to PBP 2 (4). The penB mutation, which is a mutation that is linked to the porin gene, reduces porin permeability to hydrophilic antibiotics and plays an important role in the development of resistance to penicillin G, cephalosporins, and tetracycline (11). mtr increases the level of expression of the MtrCDE efflux pump and modulates gonococcal resistance to hydrophobic agents (12). Recently, ponA1 and another resistance locus, termed penC, were shown to be involved in penicillin resistance. ponA1 encodes PBP 1, which contains a single amino alteration of Leu-421 to proline, which decreases the rate of acylation with β-lactam antibiotics. penC is required to transform an intermediate-level penicillin-resistant strain with ponA1 to high-level resistance (20). However, these mechanisms do not appear to affect the susceptibilities of N. gonorrhoeae strains to cephalosporins so much as to threaten the efficacies of currently recommended cephalosporin regimens for the treatment of gonorrhea. Most recently, a mosaic-like structure of the PBP 2 gene was reported in clinical isolates of N. gonorrhoeae with decreased susceptibilities to oral cephalosporins in Japan (2).
We have analyzed the antimicrobial susceptibilities of clinical isolates of N. gonorrhoeae from men with gonorrhea treated at Gifu University Hospital and affiliated hospitals in central Japan (14). In 2001, we observed for the first time isolates with decreased susceptibilities to cefixime (MICs ≥ 0.5 μg/ml) (14). In the present study, we examined those isolates for alterations in the PBP 2 gene and analyzed their genetic similarities by pulsed-field gel electrophoresis (PFGE).
MATERIALS AND METHODS
Clinical isolates of N. gonorrhoeae.
One-hundred fifty strains of N. gonorrhoeae were isolated from patients with gonorrhea who visited the Department of Urology at Gifu University Hospital, Gifu, Japan, and five independent hospitals between January and December 2001. The hospitals were located in the Gifu, Aichi, Shiga, and Shizuoka Prefectures in central Japan. The MICs of penicillin G, cefixime, cefdinir, and ceftriaxone for the 150 isolates were previously determined by the agar dilution method (14). The cefixime MICs for these isolates ranged from ≤0.004 to 1 μg/ml, and cefixime MICs were ≥0.125 μg/ml for 55 isolates. Among the isolates for which cefixime MICs were ≤0.06 μg/ml, five isolates for which the cefixime MIC was 0.015 μg/ml, five isolates for which the cefixime MIC was 0.03 μg/ml, and five isolates for which the cefixime MIC was 0.06 μg/ml were randomly selected for further analysis. These 15 isolates for which cefixime MICs were ≤0.06 μg/ml and the 55 clinical isolates of N. gonorrhoeae for which the cefixime MICs were ≥0.125 μg/ml were included in this study. These 70 isolates were negative for β-lactamase (14).
Sequencing of penA gene.
The nucleotide sequences of the full-length penA genes from the N. gonorrhoeae isolates were determined as reported previously (2). Briefly, genomic DNAs were isolated by sodium dodecyl sulfate lysis, followed by phenol-chloroform extraction. The DNAs were then subjected to PCR. Three sets of primers (primers PenA-A1 and PenA-B1, PenA-A2 and PenA-B2, and PenA-A3 and PenA-B3) were used to amplify three fragments of the penA gene of N. gonorrhoeae (22) (Table 1). The PCR products were purified and then sequenced by the dye terminator method and with an automatic sequencer (model 3100; Applied Biosystems, Inc., Foster City, Calif.). Primer PenA-1, PenA-2, PenA-3, PenA-4, PenB-1, PenB-2, or PenB-3 was used to sequence the corresponding PCR product.
TABLE 1.
Primers used for PCR amplification and sequencing of the penA gene of N. gonorrhoeae
| Primer | Nucleotide sequence (5′ to 3′) | Nucleotide positionsa |
|---|---|---|
| PenA-A1 | CGGGCAATACCTTTATGGTGGAAC | 8-31 |
| PenA-B1 | AACCTTCCTGACCTTTGCCGTC | 655-676 |
| PenA-A2 | AAAACGCCATTACCCGATGGG | 597-617 |
| PenA-B2 | TAATGCCGCGCACATCCAAAG | 1157-1177 |
| PenA-A3 | GCCGTAACCGATATGATCGA | 1003-1022 |
| PenA-B3 | CGTTGATACTCGGATTAAGACG | 1844-1865 |
| PenA-A4 | AATTGAGCCTGCTGCAATTGGC | 1376-1397 |
The nucleotide numbering is derived from the penA gene of a penicillin-susceptible strain of N. gonorrhoeae (GenBank accession no. M32091).
Genomic DNA analysis by PFGE.
The isolates were analyzed for chromosomal polymorphisms by PFGE, as described previously (24). In brief, clinical strains of N. gonorrhoeae were cultured on GCII agar base medium (Becton Dickinson, Franklin Lakes, N.J.) supplemented with 1% IsoVitaleX (Becton Dickinson) and suspended in Pett IV buffer (10 mM Tris-HCl, pH 8.0, 1 M NaCl) to a final concentration of approximately 109 cells/ml. A 0.5-ml aliquot of the suspension was mixed with an equal volume of 1.5% low-melting-temperature agarose, and 0.1 ml of the mixture was distributed into plug molds. Plugs were incubated in a 0.1-mg/ml lysozyme solution, and the DNAs in the plugs were digested with SpeI (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) at 37°C overnight. The restriction fragments were separated by PFGE in 1.0% agarose gels, and electrophoresis was carried out at 6 V/cm for 19.5 h, with pulse times ranging from 5 to 35 s, and at a temperature of 14°C in a CHEF DR III system (Bio-Rad Laboratories, Hercules, Calif.). The gels were stained with ethidium bromide, visualized by UV transillumination, and photographed. The banding patterns were interpreted with BioNumerics software (Applied Maths, Sint-Martenes-Latem, Belgium) with the Dice index and by the unweighted pair group method with arithmetic averages.
Statistical analysis.
MIC distributions were analyzed by the Mann-Whitney U test. All statistical comparisons were two tailed and were performed with significance set at a P value of <0.05.
RESULTS
Alterations of PBP 2.
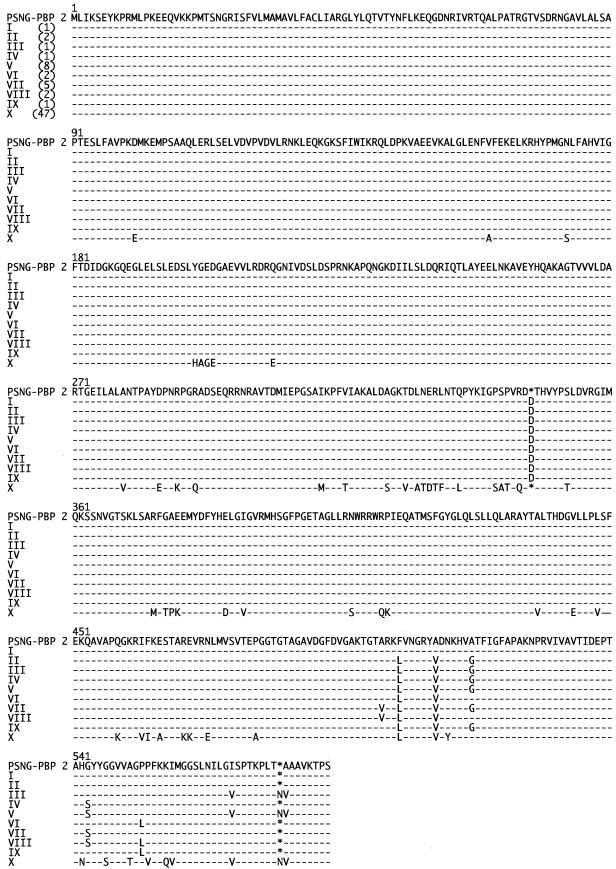
The full-length penA genes from 70 clinical isolates were sequenced. Ten amino acid sequence patterns were found in PBP 2. These sequences were compared to that of penicillin-susceptible strain N. gonorrhoeae LM306 (GenBank accession no. M320921) (Fig. 1). The pattern I to IX sequences were significantly different from the pattern X sequence. In the pattern I sequence, a codon for an extra aspartate (Asp-345a) was inserted, but the remaining amino acid sequence was identical to that of N. gonorrhoeae LM306. This insertion was positioned within the transpeptidase domain of PBP 2 and was found in 23 of 70 isolates. This extra aspartate codon was also present in the pattern II to IX sequences, and the pattern III and V sequences also contained another amino acid insertion (Asn-573a). Three to six of eight substitutions, including Ala-501→Val, Phe-504→Leu, Ala-510→Val, Ala-516→Gly, Gly-542→Ser, Pro-551→Leu, Ile-566→Val, and Ala-574→Val, were observed in the pattern II to IX sequences.
FIG.1.
Amino acid sequences of PBP 2 from N. gonorrhoeae clinical isolates. The amino acid sequence of PBP 2 of penicillin-susceptible N. gonorrhoeae (PSNG-PBP 2) was derived from the nucleic acid sequence of the penA gene of penicillin-susceptible strain LM306 (GenBank accession no. M32091). The amino acid sequences of the PBP 2 proteins of clinical isolates are classified into 10 patterns (patterns I to X) and aligned with that of penicillin-susceptible N. gonorrhoeae (PSNG-PBP 2). The numbers of isolates with each pattern are indicated in parentheses. Dashes indicate amino acid residues identical to those of penicillin-susceptible N. gonorrhoeae (PSNG-PBP 2).
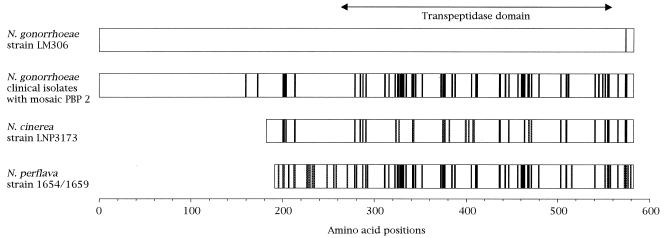
The amino acid sequence of pattern X showed 90% identity with that of PBP 2 of penicillin-susceptible strain N. gonorrhoeae LM306. The pattern X sequence had a mosaic-like structure that was composed of sequence fragments similar to those of portions of PBP 2 from Neisseria cinerea (3) and Neisseria perflava (19) (Fig. 2). In particular, the transpeptidase domain in the pattern X sequence showed 99.3% identity with a mosaic remodel composed of specific fragments of N. cinerea and N. perflava PBP 2. This PBP 2 pattern was found in 47 of 70 isolates.
FIG. 2.
Mosaic-like structure of PBP 2 in N. gonorrhoeae clinical isolates. This mosaic-like structure is composed of the fragments equivalent to those present in the PBP 2 proteins of N. cinerea strain LNP3173 and N. perflava strain 1654/1659. Solid boxes in PBP 2 of N. gonorrhoeae clinical isolates indicate amino acids that differ from the corresponding amino acids in penicillin-susceptible N. gonorrhoeae strain LM306. Solid boxes in the amino acid sequences of PBP 2 of N. cinerea strain LNP3173 and N. perflava strain 1654/1659 indicate amino acids that differ from the corresponding amino acids in N. gonorrhoeae strain LM306 but that are identical to those in N. gonorrhoeae clinical isolates. Shaded boxes in the sequences for N. cinerea and N. perflava indicate amino acids that differ from the corresponding amino acids in N. gonorrhoeae strain LM306 and those in N. gonorrhoeae clinical isolates. Blank boxes indicate amino acids that are identical in all the strains.
Association of alterations in PBP 2 with antimicrobial susceptibility.
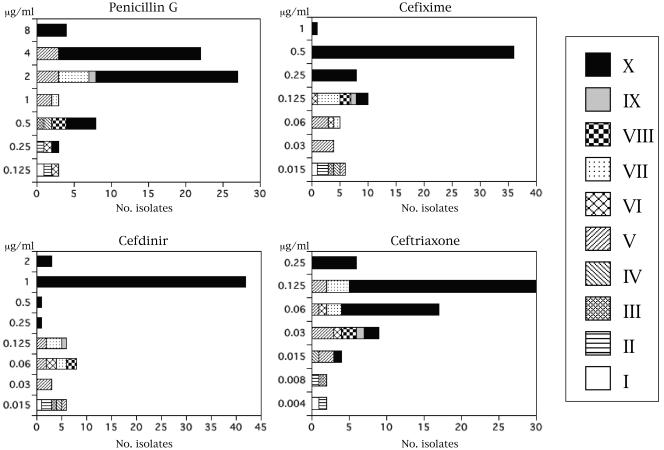
The distributions of MICs of antimicrobial agents for the clinical isolates and the association of alterations in the PBP 2 sequence with the MICs are shown in Fig. 3. The MICs of penicillin G for all 70 isolates in the present study were ≥0.125 μg/ml. For the 23 isolates carrying the Asp-345a insertion in the transpeptidase domain of PBP 2, the penicillin G MICs ranged from 0.125 to 4 μg/ml. The penicillin G MICs for the 47 isolates with the mosaic-like structure of PBP 2 ranged from 0.5 to 8 μg/ml. The distribution of the penicillin G MICs for the isolates with the mosaic-like structure of PBP 2 (pattern X) and that for the isolates with PBP 2 alterations of patterns I to IX overlapped, but the distribution for the former isolates was significantly greater than that for the latter groups of isolates. The cefixime and cefdinir MICs for isolates with the mosaic-like structure of PBP 2 were also significantly higher than those for isolates with PBP 2 alterations of patterns I to IX. Clinical isolates were distinctly divided into two groups on the basis of the distributions of the cefdinir and cefixime MICs (MICs ≥ 0.25 μg/ml and MICs ≤ 0.125 μg/ml). All isolates for which the cefdinir and cefixime MICs were ≥0.25 μg/ml had the mosaic-like structure of PBP 2, and for most isolates the cefdinir MICs (≥1 μg/ml) and the cefixime MICs (≥0.5 μg/ml) were high. For all isolates with the mosaic-like structure of PBP 2, ceftriaxone MICs were ≤0.25 μg/ml, and the isolates were still assigned to the susceptible category. The distribution of ceftriaxone MICs for the isolates with a mosaic-like structure of PBP 2 overlapped somewhat those for isolates with PBP 2 alterations of patterns I to IX. However, the ceftriaxone MICs for isolates with the mosaic-like structure of PBP 2 were significantly higher than those for isolates with PBP 2 alterations of patterns I to IX.
FIG. 3.
Distributions of MICs of penicillin G, cefixime, cefdinir, and ceftriaxone for the clinical isolates of N. gonorrhoeae with various patterns of alterations in PBP 2. The symbols for the patterns of alterations shown in Fig. 1 are indicated in the box on the right.
Genetic relatedness of isolates with the mosaic-like structure of PBP 2.
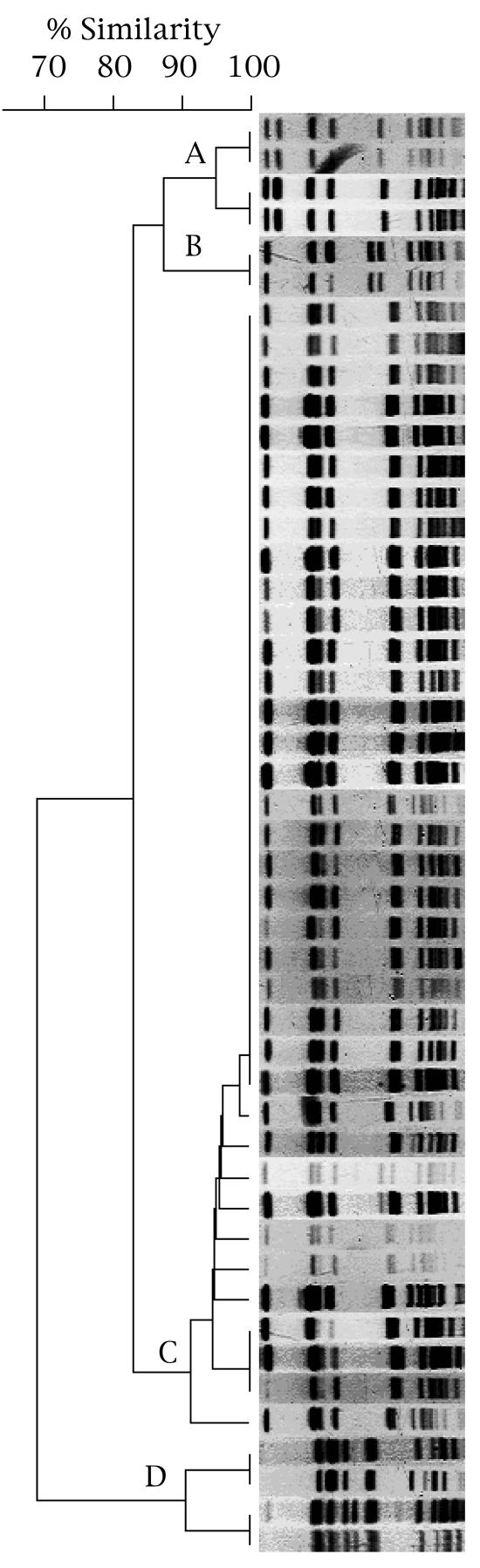
The PFGE profiles of SpeI-digested chromosomal DNAs from 47 isolates with the mosaic-like structure of PBP 2 (pattern X) are shown in Fig. 4. The 47 isolates comprised one major and three minor PFGE profile types. Twenty-six isolates (55.3%) in the major cluster (cluster C) had indistinguishable profiles, and 37 isolates (78.7%), including the 26 cluster C isolates, had profiles with more than 90% similarity. Of the four isolates in the minor cluster (cluster A), two isolates were assigned to each of two groups. The profiles of the isolates in each group were indistinguishable, and the PFGE profiles of the groups were more than 90% similar. However, these profiles showed only 82% similarity to the profiles for the isolates in the major cluster (cluster C). The two isolates in cluster B had indistinguishable profiles, but these profiles also showed only 82% similarity to those of the isolates in the major cluster (cluster C). Of the four isolates in cluster D, two isolates were assigned to each of two groups. The isolates in each group had indistinguishable profiles, and the PFGE profiles of the isolates in these groups were more than 90% similar to each other. However, they showed only 67% similarity to those of the isolates in the major cluster (cluster C). The 26 isolates with indistinguishable PFGE profiles were isolated from patients treated at five independent hospitals.
FIG. 4.
PFGE profiles of SpeI-digested chromosomal DNAs from 47 clinical isolates of N. gonorrhoeae harboring the mosaic-like structure of PBP 2 (pattern X). The dendrogram was created by computer-assisted analysis of the PFGE profiles. The profiles are classified into a major cluster (cluster C) and three minor clusters (clusters A, B, and D).
DISCUSSION
During 2001 we observed a remarkable increase in the frequency of isolation of N. gonorrhoeae strains with decreased susceptibilities to antimicrobial agents, including cephalosporins and fluoroquinolones, in Japan (14). The distribution of cefixime MICs for the clinical isolates had two peaks at 0.03 and 0.5 μg/ml and troughs at 0.125 and 0.25 μg/ml. In the present study, therefore, we examined all 55 clinical isolates of N. gonorrhoeae for which cefixime MICs were ≥0.125 μg/ml and randomly selected 15 isolates for which cefixime MICs were ≤0.06 μg/ml for changes in PBP 2. Insertion of an aspartate (Asp-345a) into the transpeptidase domain of PBP 2 was found, and other amino acid alterations were observed. This insertion of Asp-345a is thought to be an initial step in the development of chromosomally mediated resistance to penicillin (4); however, the contributions of other amino acid substitutions to penicillin resistance are unknown. In the present study, a mosaic-like structure of PBP 2 composed of the fragments similar to those in the PBP 2 proteins of N. cinerea and N. perflava was identified. The interspecies horizontal exchange of the penA genes or parts of them has been observed among penicillin-resistant strains of Neisseria meningitidis (23), N. gonorrhoeae (21, 22), Neisseria lactamica (16), and Neisseria mucosa (16). Commensal species such as Neisseria flavescens and N. cinerea, which are intrinsically more resistant to penicillin than pathogenic species, have been identified as the donors in these interspecies exchanges (3, 21). The elements involved in the interspecies exchanges of the penA genes in N. gonorrhoeae strains isolated in the United States and the United Kingdom that have been reported (21) included a fragment of the N. flavescens penA gene alone or the fragments of the N. flavescens and N. cinera penA genes. These fragments differed from the mosaic-like structure of PBP 2 found in the present study. Recently, however, a mosaic-like structure of PBP 2 was found in clinical isolates of N. gonorrhoeae with reduced susceptibilities to cefixime in Japan (2). This structure was identical, with the exception of one amino acid, to that of the PBP 2 reported here. In the previous study, codon 83 specified valine, whereas in the present study it specified glycine. These mosaic-like structures of PBP 2 did not contain the extra codon (Asp-345a). These mosaic proteins appear to arise not by amino acid substitutions or insertions but through the exchange of regions encoding the penicillin-sensitive transpeptidase domain between closely related species.
The penicillin G, cefdinir, cefixime, and ceftriaxone MICs for isolates with the mosaic-like structure of PBP 2 were significantly higher than those for isolates carrying the extra codon (Asp-345a) with or without additional amino acid substitutions. However, the distributions of the penicillin G MICs for isolates with the mosaic-like structure of PBP 2 and those with the Asp-345a insertion overlapped. Altered PBP 2 and other mechanisms, such as altered penB (11), mtr (12), and ponA1 and penC (20) genes, could ultimately contribute to the level of penicillin resistance in N. gonorrhoeae. The majority of isolates with the mosaic-like structure of PBP 2 showed markedly reduced susceptibilities to oral cephalosporins. The decreased susceptibilities to oral cephalosporins could be associated with the recombination events that form the mosaic-like structure of PBP 2. In the United States, where the cefixime MICs at which 90% of clinical strains of N. gonorrhoeae are inhibited are ≤0.06 μg/ml and resistance to cephalosporins is rare (6), a single dose of 400 mg of cefixime is highly effective for the treatment of uncomplicated gonococcal infection (13). In Japan, where isolates carrying the mosaic-like structure of PBP 2 associated with decreased susceptibility to oral cephalosporins have emerged and spread, a single dose of 400 mg of cefixime can no longer be expected to be effective for the treatment of gonococcal urethritis (10). The MICs of ceftriaxone, which is recommended as an agent for the primary therapy of gonorrhea, for the isolates with the mosaic-like structure of PBP 2 were still within the ceftriaxone-sensitive category, although they were somewhat increased. Therefore, special attention should be given to the emergence of isolates for which ceftriaxone MICs are higher.
Most isolates with the mosaic-like structure of PBP 2 appear to be closely related. The PFGE profiles of SpeI-digested DNAs from 26 of the 47 isolates carrying the mosaic-like structure were indistinguishable. The remaining 11 isolates showed greater than 90% similarity to those with the indistinguishable PFGE profiles. The similarities of these isolates suggest a common ancestor. An outbreak of gonorrhea caused by N. gonorrhoeae strains resistant to oral cephalosporins occurred in Kitakyushu, Japan, in 2001, and PFGE analysis of the isolates revealed that the outbreak was a result of clonal spread (17). In addition, the mosaic-like structure of PBP 2 in clinical isolates of N. gonorrhoeae reported in 2002 in Japan was similar to that found in the present study (12). N. gonorrhoeae strains with the mosaic-like structure of PBP 2 isolated in Japan appear to be homogeneous, and the emergence of such strains could arise from the spread of a limited number of clones. In Japan, longer and multiple dosing regimens with fluoroquinolones and oral cephalosporins, which expose N. gonorrhoeae to low concentrations of these agents, have often been used as the primary treatment for gonorrhea. Such treatment with multiple doses of oral cephalosporins could generate clones in which interspecies recombination between the penA genes of N. gonorrhoeae strains and those of commensal Neisseria species occurs, resulting in reduced susceptibilities to cephalosporins and the spread of such clones.
In conclusion, we identified a mosaic-like structure of PBP 2 that was composed of fragments of the PBP 2 proteins from N. cinerea and N. perflava in clinical isolates of N. gonorrhoeae. Most isolates with the mosaic-like structure of PBP 2 were genetically homogeneous. The mosaic-like alteration of PBP 2 was significantly associated with decreased susceptibilities to penicillin and cephalosporins, especially oral cephalosporins. The cefixime MICs for most of the isolates with the mosaic-like structure of PBP 2 were ≥0.5 μg/ml. These isolates were still susceptible to ceftriaxone; however, the ceftriaxone MICs were increased. To prevent the development and spread of N. gonorrhoeae strains with resistance to ceftriaxone, the agent should not be used at lower-than-recommended doses; or spectinomycin, which is structurally unrelated to ceftriaxone, should be used as the primary therapy for gonorrhea. In addition, the antimicrobial susceptibilities of present gonococcal isolates must be monitored periodically to reevaluate the treatments of choice for the effective control of gonorrhea.
Acknowledgments
We thank Kyoko Hatazaki for technical assistance and laboratory analysis.
REFERENCES
- 1.Akasaka, S., T. Muratani, Y. Yamada, H. Inatomi, K. Takahashi, and T. Matsumoto. 2001. Emergence of cephem- and aztreonam-high-resistant Neisseria gonorrhoeae that does not produce beta-lactamase. J. Infect. Chemother. 7:49-50. [DOI] [PubMed] [Google Scholar]
- 2.Ameyama, S., S. Onodera, M. Takahata, S. Minami, N. Maki, K. Endo, H. Goto, H. Suzuki, and Y. Oishi. 2002. Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob. Agents Chemother. 46:3744-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowler, L. D., Q. Y. Zhang, J. Y. Riou, and B. G. Spratt. 1994. Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis: natural events and laboratory simulation. J. Bacteriol. 176:333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brannigan, J. A., I. A. Tirodimos, Q. Y. Zhang, C. G. Dowson, and B. G. Spratt. 1990. Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 4:913-919. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2002. Guidelines for treatment of sexually transmitted diseases 2002. Morb. Mortal. Wkly. Rep. 51(RR-06):1-80. [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2001. Sexually transmitted disease surveillance 2001 supplement: Gonococcal Isolate Surveillance Project (GISP) annual report 2001. Centers for Disease Control and Prevention, Atlanta, Ga.
- 7.Deguchi, T., M. Yasuda, M. Asano, K. Tada, H. Iwata, H. Komeda, T., Ezaki, I. Saito, and Y. Kawada. 1995. DNA gyrase mutations in quinolone-resistant clinical isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 39:561-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deguchi, T., M. Yasuda, M. Nakano, S. Ozeki, T. Ezaki, I. Saito, and Y. Kawada. 1996. Quinolone-resistant Neisseria gonorrhoeae: correlation of alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV with antimicrobial susceptibility profiles. Antimicrob. Agents Chemother. 40:1020-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deguchi, T., M. Yasuda, I. Saito, and Y. Kawada. 1997. Quinolone-resistant Neisseria gonorrhoeae. J. Infect. Chemother. 3:73-84. [DOI] [PubMed] [Google Scholar]
- 10.Deguchi, T., M. Yasuda, S. Yokoi, K. Ishida, M. Ito, S. Ishihara, K. Minamidate, Y. Harada, K. Tei, K. Kojima, M. Tamaki, and S. Maeda. 2003. Treatment of uncomplicated gonococcal urethritis by double-dosing of 200 mg cefixime at a 6-h interval. J. Infect. Chemother. 9:35-39. [DOI] [PubMed] [Google Scholar]
- 11.Gill, M. J., S. Simjee, K. Al-Hattawi, B. D. Robertson, C. S. Easmon, and C. A. Ison. 1998. Gonococcal resistance to beta-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob. Agents Chemother. 42:2799-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagman, K. E., W. Pan, B. G. Spratt, J. T. Balthazar, R. C. Judd, and W. M. Shafer. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141:611-622. [DOI] [PubMed] [Google Scholar]
- 13.Hook, E. W., III, W. M. McCormack, D. Martin, R. B. Jones, K. Bean, A. N. Maroli, and The STD Study Group. 1997. Comparison of single-dose oral grepafloxacin with cefixime for treatment of uncomplicated gonorrhea in men. Antimicrob. Agents Chemother. 41:1843-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito, M., M. Yasuda, S. Yokoi, S.-I. Ito, Y. Takahashi, S. Ishihara, S.-I. Maeda, and T. Deguchi. 2004. Remarkable increase of Neisseria gonorrhoeae with decreased susceptibility to penicillin, tetracycline, oral cephalosporins, and fluoroquinolones, central Japan, 2001-2002. Antimicrob. Agents Chemother. 48:3185-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joly-Guillou, M. L., and S. Lasry. 1999. Practical recommendations for the drug treatment of bacterial infections of the male genital tract including urethritis, epididymitis and prostatitis. Drugs 57:743-750. [DOI] [PubMed] [Google Scholar]
- 16.Lujan, R., Q. Y. Zhang, J. A. Saez Nieto, D. M. Jones, and B. G. Spratt. 1991. Penicillin-resistant isolates of Neisseria lactamica produce altered forms of penicillin-binding protein 2 that arose by interspecies horizontal gene transfer. Antimicrob. Agents Chemother. 35:300-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muratani, T., S. Akasaka, T. Kobayashi, Y. Yamada, H. Inatomi, K. Takahashi, and T. Matsumoto. 2001. Outbreak of cefozopran (penicillin, oral cephems, and aztreonam)-resistant Neisseria gonorrhoeae in Japan. Antimicrob. Agents Chemother. 45:3603-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-388. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Castillo, A., A. M. Perez-Castillo, and J. A. Saez-Nieto. 1994. Sequence of the penicillin-binding protein 2-encoding gene (penA) of Neisseria perflava/sicca. Gene 146:91-93. [DOI] [PubMed] [Google Scholar]
- 20.Ropp, P. A., M. Hu, M. Olesky, and R. A. Nicholas. 2002. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spratt, B. G., L. D. Bowler, Q. Y. Zhang, J. Zhou, and J. M. Smith. 1992. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J. Mol. Evol. 34:115-125. [DOI] [PubMed] [Google Scholar]
- 22.Spratt, B. G. 1988. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 332:173-176. [DOI] [PubMed] [Google Scholar]
- 23.Spratt, B. G., Q. Y. Zhang, D. M. Jones, A. Hutchison, J. A. Brannigan, and C. G. Dowson. 1989. Recruitment of a penicillin-binding protein gene from Neisseria flavescens during the emergence of penicillin resistance in Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 86:8988-8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unemo, M., T. Berglund, P. Olcen, and H. Fredlund. 2002. Pulsed-field gel electrophoresis as an epidemiologic tool for Neisseria gonorrhoeae: identification of clusters within serovars. Sex. Transm. Dis. 29:25-31. [DOI] [PubMed] [Google Scholar]