Abstract
OBJECTIVES
The diagnosis of chronic pancreatitis in patients with characteristic symptoms but normal pancreatic imaging is challenging. Assessment of pancreatic function through secretin pancreatic function testing (SPFT) has been advocated in this setting, but its diagnostic accuracy is not fully known.
METHODS
This was a retrospective review of patients who received SPFT at our tertiary care institution between January 1995 and December 2008 for suspected chronic pancreatitis. For all patients, medical records were reviewed for evidence of subsequent development of chronic pancreatitis by imaging and/or pathology. Patients were then categorized as “true positive” or “true negative” for chronic pancreatitis based on follow-up imaging or histologic evidence.
RESULTS
In all, 116 patients underwent SPFT. Of the 27 patients who tested positive, 7 were lost to follow-up. Of the remaining 20 SPFT-positive patients, 9 (45 %) developed radiologic or histologic evidence of chronic pancreatitis after a median of 4 years (1–11 years). Of the 89 patients who had negative SPFT testing, 19 were lost to follow-up. Of the remaining 70 patients, 2 were eventually diagnosed with chronic pancreatitis based on subsequent imaging/histology after a median follow-up period of 7 years (3–11 years). The sensitivity of the SPFT in diagnosing chronic pancreatitis was 82 % with a specificity of 86 %. The positive predictive value (PPV) of chronic pancreatitis was 45 % with a negative predictive value (NPV) of 97 %.
CONCLUSIONS
In patients with suspected early chronic pancreatitis and normal pancreatic imaging, SPFT is highly accurate at ruling out early chronic pancreatitis with a NPV of 97 %.
INTRODUCTION
Chronic pancreatitis refers to ongoing inflammation and irreversible destruction of pancreatic tissue (1). This may ultimately manifest with abdominal pain and symptoms of exocrine and endocrine pancreatic insufficiency (2–6).
In its earliest stage, the diagnosis of chronic pancreatitis can be difficult, as morphologic changes may be minimal (1,7,8). Consequently, imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiopancreatography (ERCP) have reduced sensitivity for detecting early or minimal-change disease (9–11). Endoscopic ultrasound (EUS) has emerged as a sensitive and specific test for detecting chronic pancreatitis, particularly advanced disease, although its accuracy in detecting early chronic pancreatitis is still being defined (12). Although histology is the gold standard, it is technically difficult to obtain biopsies of pancreatic tissue safely, with a risk of acute pancreatitis, fistula, pseudocyst, or hemorrhage (11). Indirect tests of pancreatic function include measuring fat content or elastase levels in stool, but these are either nonspecific or positive only in more advanced disease (9,13).
Direct hormone-stimulated tests, using cholcystekinin or secretin, are considered the most sensitive and specific tests of pancreatic insufficiency and have been advocated for use in diagnosing early chronic pancreatitis (1). In these tests, the duodenal aspirate is collected at different time points after stimulation by cholcystekinin or secretin or both. In the case of the cholcystekinin pancreatic function test, the aspirate is examined for pancreatic enzymes to determine acinar-cell reserve. In the secretin pancreatic function test (SPFT), duodenal aspirate volume and bicarbonate concentration are measured to determine ductal-cell reserve. The SPFT was developed over 60 years ago (14,15) and is performed by only a few centers in the United States. Several variations of this test exist, ranging from minor differences in protocol to the use of a purely endoscopic rather than the Dreiling tube-based test (16). The sensitivity of the various forms of the SPFT in detecting chronic pancreatitis ranges from 60 to 94 %, (17–19) with a specificity of 67–95 % (18,19). In these tests, a peak bicarbonate concentration in the duodenal fluid of < 80 mEq/l after weight-based administration of intravenous secretin is considered to be diagnostic of chronic pancreatitis.
The SPFT may be the best alternative gold standard for the diagnosis of chronic pancreatitis (20). It has thus been used to diagnose or exclude early chronic pancreatitis in patients with unexplained abdominal pain of presumed pancreatic origin (21). However, validation of the accuracy of the SPFT in this setting is lacking, with prior studies either limited in follow-up or incorporating small numbers of patients (17,22). It was thus the purpose of this study to determine if patients with suspected chronic pancreatitis and abnormal SPFT, through long-term follow-up, subsequently develop histologic or radiographic changes consistent with chronic pancreatitis compared with those with normal SPFT.
METHODS
We performed a retrospective review of all patients who received SPFT at our tertiary referral center between January 1995 and December 2008. This was approved by the Beth Israel Deaconess Medical Center Institutional Review Board. As per the protocol at our institution, patients are only referred for SPFT if they have a clinical history highly suggestive of chronic pancreatitis. That is, epigastric pain worse with eating, and radiating to the back, and with prior work-up that usually includes a negative esophagastroduodenoscopy, gastric emptying study, abdominal ultrasound, and laboratory testing. Importantly, all of our patients had normal cross-sectional and/or endoscopic pancreatic imaging before referral for SPFT. All patients were evaluated by a Pancreas specialist before performing SPFT.
SPFT
A secretin pancreatic function test was performed as previously described (14). Standard esophagastroduodenoscopy was performed and a guidewire was placed through the endoscope under fluoroscopic guidance beyond the ligament of Treitz. The endoscope was removed keeping the guidewire in place, and a double-lumen gastroduodenal tube or Dreiling tube (manufactured by N. M. Beale Company, Harvard, MA), with gastric and duodenal ports, was placed over the wire with the tip of the tube in the third to fourth portion of the duodenum. The guidewire was then removed after placement of the Dreiling tube was confirmed fluoroscopically. Intravenous synthetic human secretin (acquired from ChiRhoClin, Burtonsville, MD) was administered to stimulate the secretion of bicarbonate. An initial test dose of 0.1 ml was given and if there was no evidence of an adverse or allergic reaction, and the full dose of 0.2 mcg/kg was administered over 2 min. In the recovery room, the gastric port was attached to continuous suction and the gastric aspirate was discarded. The duodenal juice was continuously aspirated from the duodenal port of the Dreiling tube with collections representing 15, 30, 45, and 60 minute intervals after the secretin had been administered. These samples were kept on ice and sent to a standard chemistry lab immediately after the last aliquot was aspirated. Analysis for bicarbonate concentration was performed on all samples using the hospital autoanalyzer. Samples were diluted in a 1:5 ratio with type I H2O before being run on the autoanalyzer. A positive test was defined as a peak bicarbonate level of < 75 mEq/l in any of the duodenal fluid collections following administration of intravenous secretin. A pH of ~ 7 was required to ensure that the aspirated duodenal fluid was not contaminated by gastric contents.
Patient follow-up
Medical records of patients who had undergone SPFT were reviewed for evidence of subsequent development of findings consistent with chronic pancreatitis by imaging and/or pathology from surgical specimens. In addition, records were also reviewed to determine if chronic pancreatitis had been conclusively ruled out, and if an alternative diagnosis had been made. Patients were contacted by telephone if there was insufficient follow-up based on medical record review, including outside records if available.
All subsequent relevant radiology and endoscopic reports were reviewed for documentation of findings consistent with chronic pancreatitis. All images were read by gastrointestinal radiology attendings. Positive findings were reviewed and confirmed by an independent gastrointestinal radiologist with subspecialty in pancreatic imaging (K.J.M.), who was not blinded to the SPFT results/clinical data. Imaging findings consistent with chronic pancreatitis included the following. (i) CT: findings of parenchymal and ductal calcifications, parenchymal atrophy, dilated main pancreatic duct, and dilated side branches (11). In each patient, we evaluated the pancreas for focal or diffuse atrophy and hypertrophy (fullness). Unless mentioned, the size of the pancreas was considered normal. Patients with only fullness of the pancreatic head were considered negative. (ii) ERCP and MRI/MRCP: findings per Cambridge Classification as previously described (11). We also evaluated the pancreas in each patient for abnormal signal intensity on T2, fat-suppressed T1, and postcontrast images (MRI) and attenuation changes on CT. Unless mentioned, the signal intensity and attenuation was normal. No patient had secretin-stimulated MRCP. (iii) EUS: findings based on a nine-point scoring system of pancreatic ductal and parenchymal changes as previously described (23). Patients were considered to have chronic pancreatitis if they had at least five criteria. Otherwise, they were considered to have “negative” imaging for chronic pancreatitis. Pathology slides of surgical specimens were reviewed by a gastrointestinal pathologist (R.N.), not blinded to clinical data, to confirm changes consistent with chronic pancreatitis, including periductal fibrosis, duct dilation, intralobular inflammation, and atrophy. All authors had access to the study data and reviewed and approved the final manuscript.
Statistics
Patients were categorized as “true positive,” or “true negative” for chronic pancreatitis based on follow-up evidence. This allowed for the calculation of sensitivities, specificities, negative predictive value (NPV) and positive predictive value (PPV), and P values based on 2 × 2 tables and paired t-tests.
RESULTS
A total of 116 patients with suspected early chronic pancreatitis underwent SPFT between January 1995 and December 2008. Of these, 89 patients had a negative SPFT, of which 19 were lost to follow-up. Of the 27 patients who tested positive, 7 were lost to follow-up. Table 1 describes the baseline patient demographics. Figure 1a,b shows bicarbonate values at different collection time points.
Table 1.
Patient demographics
| SPFT positive | SPFT negative | |
|---|---|---|
| Number | 20 | 70 |
| Average age (years) | 45.5 ± 13.3 | 45.5 ± 11.1 |
| % Female | 80.0 | 67.1 |
| % White | 70.0 | 77.1 |
| Average peak Bicarb (mEq/l) | 59.4 ± 12.8 | 100.2 ± 13.6 |
| Median peak Bicarb (mEq/l) | 64 (IQR: 49–69.5) | 100.5 (IQR: 90–110) |
| Average follow-up time (years) | 10.4 ± 4.1 | 8.0 ± 4.3 |
Bicarb, bicarbonate; IQR, interquartile range; SPFT, secretin pancreatic function test.
Figure 1.
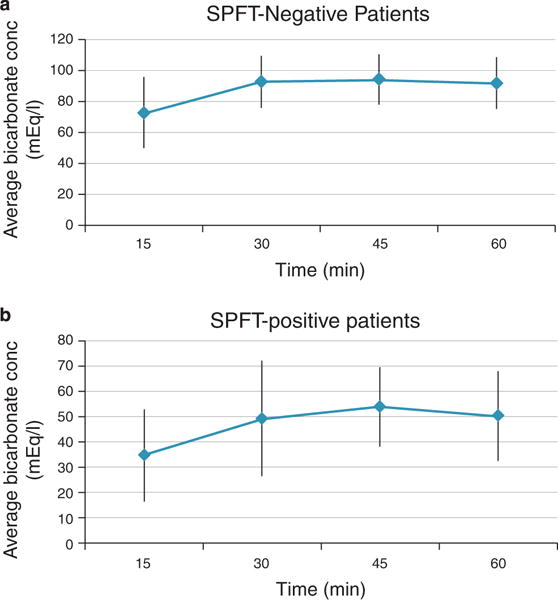
Duodenal bicarbonate values over 60 minutes in patients undergoing SPFT. (a) Average bicarbonate concentration (conc) at each collection time point for secretin pancreatic function test (SPFT)-negative patients. (b) Average bicarbonate concentration at each collection time point for SPFT-positive patients.
Positive SPFT
Among the 20 SPFT-positive patients, 9 (45 %) developed radiologic or histologic evidence of chronic pancreatitis after an average of 4.8 years (range 1–11 years). The overall median follow-up for the 20 SPFT-positive patients was 12 years (range 4–15 years). None had < 4 years of follow-up. Table 2 lists the imaging and pathology findings supporting the diagnosis of chronic pancreatitis in each patient.
Table 2.
Diagnosis of chronic pancreatitis in patients with positive SPFT
| Evidence of chronic pancreatitis in SPFT-positive patients | Time to positive (years) | |
|---|---|---|
| Patient 1 | Changes on MRCP and CT: atrophy of pancreas and mild main pancreatic duct dilatation. | 9 |
| Patient 2 | Pathology of resected distal pancreas with cystic spaces without epithelial lining surrounded by foamy macrophages, foreign body giant cells, and fibroblasts consistent with pseudocystic change, with surrounding fibrosis consistent with chronic pancreatitis. | 1 |
| Patient 3 | Changes on MRCP and CT: atrophy of pancreas. Changes on EUS: pancreas parenchyma with contiguous lobularity, hyperechoic foci, hyperechoic strands, dilated main pancreatic duct, main pancreatic duct with hyperechoic walls. | 9 |
| Patient 4 | Pathology of pancreas from Whipple specimen with dense periductal fibrosis with admixed lymphocytes and eosinophils and complete atrophy of acinar tissue seen adjacent to an intraductal papillary mucinous neoplasm. | 1 |
| Patient 5 | Changes on EUS: pancreas parenchyma with contiguous lobularity, hyperechoic foci, and hyperechoic strands and cysts. Main pancreatic duct with hyperechoic walls, and irregular margins. Dilated side branches. | 5 |
| Patient 6 | Changes on MRCP: pancreas divisum and main-duct irregularities. Changes on EUS: pancreas parenchyma with contiguous lobularity, hyperechoic foci, and hyperechoic strands. Main pancreatic duct with hyperechoic walls. Dilated side branches. | 11 |
| Patient 7 | Pathology of pancreas from Whipple specimen with focal complete atrophy of acinar tissue with isolated, entrapped residual pancreatic ductules embedded within a collagenized, scar-like stroma. | 2 |
| Patient 8 | Changes on MRCP: atrophy of pancreas, main pancreatic duct irregularity and stricture, pseudocyst. | 1 |
| Patient 9 | Changes on MRCP: atrophy of pancreas. Changes on EUS: pancreas parenchyma with contiguous lobularity and cysts. Main pancreatic duct with hyperechoic walls and irregular margins. Atrophic pancreas. | 4 |
CT, computed tomography; EUS, endoscopic ultrasound; MRCP, magnetic resonance cholangiopancreatography; SPFT, secretin pancreatic function test.
In the 11 patients who tested positive but who did not develop supporting evidence of chronic pancreatitis, the mean follow-up time was 11.2 years (range 4–16 years). There was no significant difference in mean or median peak bicarbonate levels between the group of patients who developed imaging or histologic evidence of chronic pancreatitis (n = 9, mean 58.0 mEq/l and median 64 mEq/l) and the group who did not (n = 11, mean 60.6 mEq/l, P = 0.63 and median 64 mEq/l, P = 1.00). Table 3 compares the demographics of positive SPFT patients.
Table 3.
Demographics of patients who had a positive SPFT
| Follow-up imaging/path positive | Follow-up imaging/path negative | P value | |
|---|---|---|---|
| Number | 9 | 11 | |
| % Female | 89 | 73 | 0.369 |
| % White | 78 | 64 | 0.492 |
| Average peak Bicarb (mEq/l) | 58.0 | 60.6 | 0.63 |
| Mean follow-up time (years) | 9.4 | 11.2 | 0.36 |
Bicarb, bicarbonate; SPFT, secretin pancreatic function test.
Among the 9 SPFT-positive patients who developed evidence of chronic pancreatitis in follow-up, the distribution of etiologies of pancreatitis was idiopathic in 8 patients (88.9 %), and related to an anatomic abnormality in 1 patient. Of the eight patients with idiopathic chronic pancreatitis, one patient had a cystic fibrosis transduction receptor (CFTR) gene-related mutation in the absence of a cystic fibrosis phenotype.
Negative SPFT
Of the 70 SPFT-negative patients, only two patients were eventually diagnosed with chronic pancreatitis after an average of 7.9 years. The overall median follow-up of the 70 SPFT-negative patients was 7 years (1–17 years). One patient had follow-up of < 1 year. Another patient had follow-up of < 2 years. The diagnosis was made based on follow-up EUS in one and histology consistent with chronic pancreatitis in the other. Table 4 lists the findings consistent with chronic pancreatitis in these two patients and time interval. The peak bicarbonate values in these patients were 103.5 and 108 mEq/l, respectively.
Table 4.
Evidence of chronic pancreatitis in SPFT-negative patients
| Evidence of chronic pancreatitis in SPFT-negative patients | Time to positive (years) | |
|---|---|---|
| Patient 1 | Changes on EUS: pancreas parenchyma with contiguous lobularity hyperechoic foci, hyperechoic strands, dilated main pancreatic duct, main pancreatic duct with irregular margins and hyperechoic walls. | 3 |
| Patient 2 | Pathology of pancreas from Whipple specimen with patchy atrophy of acinar tissue with interstitial collagenous fibrosis. Ducts with luminal dilation, inspissated pancreatic secretions, and periductal chronic inflammation including plasma cells and lymphocytes. | 4 |
EUS, endoscopic ultrasound; SPFT, secretin pancreatic function test.
Of the remaining 68 patients, the average follow-up period was 8.0 years. In all, 39 patients (57.3 %) were diagnosed with a functional gastrointestinal disorder. Twenty patients (29.4 %) had complete resolution of their symptoms several months later. Two patients (2.9 %) were eventually diagnosed with inflammatory bowel disease. Two (2.9 %) patients were diagnosed with gastroesophageal reflux disease, and two more with a somatization or a psychosomatic disorder. In another two patients, a vascular etiology of their pain was identified. One patient had resolution of her symptoms with altering her diet to exclude gluten, but no diagnosis of celiac disease was made based on further testing. The distribution of alternative diagnoses is shown in Figure 2.
Figure 2.
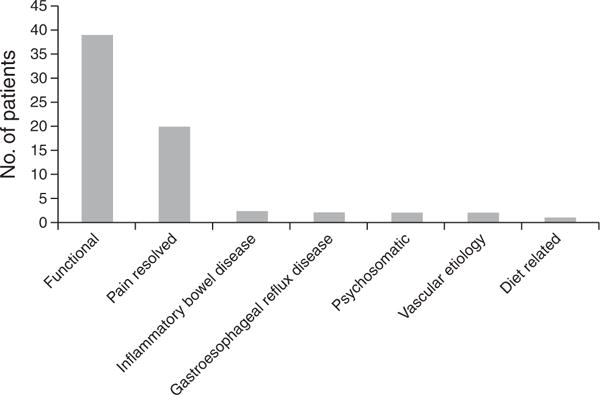
Distribution of alternative diagnoses in patients who were secretin pancreatic function test (SPFT) negative.
Based on the follow-up data in both the SPFT-positive and SPFT-negative groups, the sensitivity of the SPFT in diagnosing subsequent imaging or pathology-proven chronic pancreatitis was 82 %, with a specificity of 86 %. The PPV of the secretin stimulation test in diagnosing subsequent imaging or pathology-proven chronic pancreatitis was 45 %, with a NPV of 97 % (Table 5).
Table 5.
Results of SPFT testing
| Positive follow-up (n) | Negative follow-up (n) | |
|---|---|---|
| SPFT positive (n) | 9 | 11 |
| SPFT negative (n) | 2 | 68 |
SPFT, secretin pancreatic function test.
EUS and SPFT
EUS was performed in 15 of the 90 patients included in the study before SPFT testing. Of the 70 SPFT-negative patients, 9 had a follow-up EUS. Of these, only one EUS demonstrated changes consistent with chronic pancreatitis (patient 1 in Table 4). Of all the 27 SPFT-positive patients, 9 had follow-up EUS. Five were negative and four were positive. The concordance between EUS and SPFT was 0.33 (95 % confidence interval − 0.06 to 0.72).
DISCUSSION
The diagnosis of early chronic pancreatitis in patients with an appropriate clinical history but with normal radiologic or endoscopic imaging is difficult. Several studies suggest that the SPFT is an early predictor of chronic pancreatitis, reflecting fibrosis and inflammation not yet detected by standard imaging modalities (18,22). However, long-term follow-up of patients with suspected early chronic pancreatitis based on SPFT testing is limited. In particular, the outcome of those who are SPFT negative is unclear. This is the first study to analyze the long-term follow-up data of patients who have been investigated for chronic pancreatitis using the SPFT. This is particularly important, given that a positive SPFT is considered by many to be either suggestive of early chronic pancreatitis or a gold standard for diagnosing chronic pancreatitis (1,20).
In our cohort of patients with a positive SPFT, approximately half eventually demonstrated corroborating histologic or radiologic evidence of chronic pancreatitis. Prior studies have suggested that 83–100 % of patients who are SPFT positive will develop subsequent evidence of chronic pancreatitis (22,24). In one study (22), all seven patients with abnormal SPFT/normal ERCP were eventually diagnosed with chronic pancreatitis based on medical history, clinical investigation, and laboratory and morphological examinations. However, the specific findings used to diagnose each patient were not fully described. In the second study (24), five out of six patents were shown to have proven chronic pancreatitis in follow-up examination based on imaging, pathology, or pancreatic function studies. The sixth patient was considered to have a clinical course most consistent with chronic pancreatitis. However, this study was published only in abstract form, and the criteria for diagnosing chronic pancreatitis were not fully described.
In our study, we used a peak bicarbonate concentration of 75 mEq/l as the threshold for a positive SPFT. Previously, a peak bicarbonate concentration of < 80 mEq/l has generally been accepted as consistent with chronic pancreatitis, although it remains unclear how values just below 80 mEq/l should be interpreted (9,18). Using 75 mEq/l as the cutoff resulted in four of our patients being reassigned as having a negative SPFT; these patients would have been considered positive for chronic pancreatitis if 80 mEq/l had been the threshold. On follow-up, none of these patients has developed radiologic or histologic evidence of chronic pancreatitis and remain as “true negatives.” Moreover, recalculating the data using 80 mEq/l as the threshold did not significantly change the NPV (95 % for 80 mEq/l vs. 97 % for 75 mEq/l).
Some authors have suggested that collecting bicarbonate for 45 min after intravenous secretin stimulation is sufficient (25). In our cohort of 70 SPFT-negative patients with adequate follow-up, 2 would have been misclassified as SPFT positive (i.e., peak bicarbonate < 75 mEq/l) if 45 min had been used. One of these patients carries a diagnosis of a functional gastrointestinal disorder, after a follow-up of 6 years. The other patient, whose SPFT was performed in 1996, was eventually diagnosed with irritable bowel syndrome. Thus, 2.9 % of patients would have been misclassified as having chronic pancreatitis if a 45-min end point was used. We thus prefer collection of bicarbonate over 60 min.
With regard to the length of time for the entire procedure, placement of the Dreiling tube takes < 20 min. The subsequent collection of the duodenal aspirate over the next 60 min occurs in the recovery room, by a dedicated pancreas center nurse. Thus, the total length of time for our SPFT is 80 min, of which only 20 min require the presence of a physician, for the endoscopic placement of the Dreiling tube. Our SPFT protocol is thus significantly shorter than the 2–3 h reported by others (26) and remains an efficient procedure.
This study has several limitations. Although it is a long-term study of patients seen at one of the few centers in this country using SPFT, it still remains a single-center review, with associated problems with generalization. For instance, in our cohort of SPFT-positive patients, the percentage of patients with idiopathic chronic pancreatitis was very high and may indicate a referral bias. Furthermore, the basis of minimal change disease or early chronic pancreatitis is dependent on the sensitivity of the relevant pancreatic imaging studies and on the local expertise at a given center.
Ductal-cell secretion of bicarbonate is dependent on an intact CFTR. This raises the possibility of false positive SPFT results due to acquired or inherited CFTR deficiency. Acquired CFTR deficiency may occur in cigarette smokers (27), and two of the nine patients who had positive SPFT tests were current smokers at the time of testing. However, cigarette smoking may also predispose to the development of chronic pancreatitis (28,29). Although our patients were not routinely genotyped, none had been diagnosed with overt cystic fibrosis based on sweat chloride testing. However, only a few patients required sweat chloride testing to evaluate for cystic fibrosis testing based on suggestive clinical history. In addition, it has been previously shown that there is an increased prevalence of CFTR mutations in patients with idiopathic chronic pancreatitis (30), suggesting that rather than confounding the SPFT results, these mutations could be an important part of the pathophysiology of chronic pancreatitis.
All of our patients received sedation for the endoscopic placement of the Dreiling tube. It is believed that moderate sedation with versed and fentanyl does not alter the results of the SPFT (31). Although no patient received sedation with propofol, some may have received higher doses of fentanyl and versed that may potentially confound the response to secretin stimulation. It could also explain why a cutoff of 75 mEq/l is more useful at our institution, rather than the 80 mEq/l previously reported in the literature.
One patient in our positive SPFT cohort (Table 2, patient 4) was eventually diagnosed with main-duct intraductal papillary mucinous neoplasm and surrounding extensive chronic pancreatitis. It is theoretically possible that the presence of mucus within the pancreatic duct could have affected the SPFT result.
In the SPFT-positive cohort, four patients demonstrated pancreatic atrophy on MRCP. Pancreatic atrophy has been used to make the diagnosis of chronic pancreatitis but remains controversial (11,32,33), and may also occur as part of the normal aging process (34). In three of these four patients, follow-up EUS was also positive. Thus, only one patient was diagnosed solely on the basis of atrophy of the pancreas by both CT and MRI.
Although prolonged follow-up adds to the robustness of our study, it also allows for confounding interventions. That is, patients could have developed positive imaging based on interrogations or interventions on the pancreatic duct. Indeed, both patients in the SPFT-negative cohort who subsequently developed histologic or radiographic changes consistent with chronic pancreatitis had multiple ERCPs and pancreatic duct stent placement at a referring hospital because of a presumed diagnosis of chronic pancreatitis. Prior studies have shown that pancreatic duct stenting may lead to pancreatic ductal and parenchymal changes suggestive of chronic pancreatitis (35). If these patients were excluded from the study, then no SPFT-negative patient would have developed radiographic or histologic features of chronic pancreatitis. In the SPFT-positive group, only one patient had known multiple intervening ERCPs before repeat positive imaging.
In our study, patients did not undergo standardized follow-up imaging at a preset interval after the SPFT. However, follow-up imaging was based on clinical symptoms and presentation, and hence the intervals varied. Another limitation of our study is our reliance on patients for some follow-up data. Although we asked for and received permission to acquire outside hospital imaging in patients who underwent repeat imaging that was positive, we did not do so for the imaging in patients who remained negative. Rather, we relied on their own knowledge of their medical history as well as review of medical records, when available. This is a limitation that may be more important in the positive SPFT group, when providers already consider such patients to have chronic pancreatitis, and “ newly ” positive imaging may thus not be emphasized. We would thus have underestimated the PPV and sensitivity of the SPFT.
EUS has been advocated as an alternative to SPFT in diagnosing chronic pancreatitis, with a suggested sensitivity of 72–91 % and specificity of 74–86 %, based on comparisons with ERCP, pancreatic function testing, and histology (12). In studies focusing on early and advanced chronic pancreatitis, there is wide variation in the concordance of the diagnostic accuracy of EUS and SPFT (18,21,36). In the small subset of our patients who had EUS performed after SPFT, there was no clear concordance in diagnosing early chronic pancreatitis. It is worthwhile noting that only 1 of 11 true chronic pancreatitis patients had a negative SPFT or EUS, and hence combined use of both tests may be helpful in excluding chronic pancreatitis. However, larger prospective studies are needed to allow any conclusive recommendations to be made.
In conclusion, we show that < 3 % of patients (2 out of 70) who are SPFT negative and have prior normal endoscopic and cross-sectional imaging are eventually diagnosed with chronic pancreatitis based on follow-up imaging or histology. Thus, SPFT appears to be a highly accurate test for ruling out early chronic pancreatitis with a NPV of 97 %.
The importance of such a diagnostic accuracy in excluding chronic pancreatitis cannot be overstated. Patients suspected of having chronic pancreatitis are oft en subjected to repeated imaging tests and invasive endoscopic procedures in an attempt to confirm the diagnosis. Furthermore, the care of suspected flares typically involves narcotic analgesics, emergency room visits, and hospitalizations with intravenous nutrition. Some will undergo invasive procedures such as celiac plexus blocks, ERCP, and surgeries. Early exclusion of this diagnosis can thus prevent the recurrent utilization of significant medical resources on the inappropriate work-up and management of such patients.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Diagnosing early chronic pancreatitis is difficult.
-
✓
The secretin pancreatic function test is useful at detecting early chronic pancreatitis, but its diagnostic accuracy is not fully known.
WHAT IS NEW HERE
-
✓
The secretin pancreatic function test is highly accurate at ruling out early chronic pancreatitis based on long-term follow-up.
Acknowledgments
Financial support: None.
Footnotes
Guarantor of the article: Sunil Sheth, MD.
Specific author contributions: Gyanprakash Ketwaroo: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis. Alphonso Brown: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis. Benjamin Young: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Rakhi Kheraj: analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Mandeep Sawhney, Koenraad J. Mortele, Robert Najarian, and Sumeet Tewani: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Deborah Dasilva: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content. Steven Freedman: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Sunil Sheth: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content, and study supervision.
Potential competing interests: None.
References
- 1.Toskes PP. Update on diagnosis and management of chronic pancreatitis. Curr Gastroenterol Rep. 1999;1:145–53. doi: 10.1007/s11894-996-0014-8. [DOI] [PubMed] [Google Scholar]
- 2.Warshaw AL. Pain in chronic pancreatitis. Patients, patience, and the impatient surgeon. Gastroenterology. 1984;86:987–9. [PubMed] [Google Scholar]
- 3.Ammann RW, Akovbiantz A, Largiader F, et al. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820–8. [PubMed] [Google Scholar]
- 4.Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med. 1995;332:1482–90. doi: 10.1056/NEJM199506013322206. [DOI] [PubMed] [Google Scholar]
- 5.Layer P, Yamamoto H, Kalthoff L, et al. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107:1481–7. doi: 10.1016/0016-5085(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 6.Lankisch PG, Lohr-Happe A, Otto J, et al. Natural course in chronic pancreatitis. Pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digestion. 1993;54:148–55. doi: 10.1159/000201029. [DOI] [PubMed] [Google Scholar]
- 7.Forsmark CE, Toskes PP. What does an abnormal pancreatogram mean? Gastrointest Endosc Clin N Am. 1995;5:105–23. [PubMed] [Google Scholar]
- 8.Malfertheiner P, Buchler M, Stanescu A, et al. Exocrine pancreatic function in correlation to ductal and parenchymal morphology in chronic pancreatitis. Hepatogastroenterology. 1986;33:110–4. [PubMed] [Google Scholar]
- 9.Lieb JG, II, Draganov PV. Pancreatic function testing: here to stay for the 21st century. World J Gastroenterol. 2008;14:3149–58. doi: 10.3748/wjg.14.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugiyama M, Haradome H, Atomi Y. Magnetic resonance imaging for diagnosing chronic pancreatitis. J Gastroenterol. 2007;42(Suppl 17):108–12. doi: 10.1007/s00535-006-1923-x. [DOI] [PubMed] [Google Scholar]
- 11.Choueiri NE, Balci NC, Alkaade S, et al. Advanced imaging of chronic pancreatitis. Curr Gastroenterol Rep. 2010;12:114–20. doi: 10.1007/s11894-010-0093-4. [DOI] [PubMed] [Google Scholar]
- 12.Stevens T, Parsi MA. Endoscopic ultrasound for the diagnosis of chronic pancreatitis. World J Gastroenterol. 2010;16:2841–50. doi: 10.3748/wjg.v16.i23.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lankisch PG, Schmidt I, Konig H, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mild to moderate exocrine pancreatic insufficiency. Gut. 1998;42:551–4. doi: 10.1136/gut.42.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreiling DA, Hollander F. Studies in pancreatic function; preliminary series of clinical studies with the secretin test. Gastroenterology. 1948;11:714–29. [PubMed] [Google Scholar]
- 15.Diamond JS, Siegal SA. The secretin test in the diagnosis of pancreatic disease with a report of 130 tests. Am J Digest Dis. 1940;7:435. [Google Scholar]
- 16.Conwell DL, Zuccaro G, Jr, Vargo JJ, et al. An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointest Endosc. 2003;57:37–40. doi: 10.1067/mge.2003.14. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa M, Naruse S, Ishiguro H, et al. Evaluating exocrine function tests for diagnosing chronic pancreatitis. Pancreas. 1997;15:402–8. doi: 10.1097/00006676-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Albashir S, Bronner MP, Parsi MA, et al. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol. 2010;105:2498–503. doi: 10.1038/ajg.2010.274. [DOI] [PubMed] [Google Scholar]
- 19.Lieb JG, II, Brensinger CM, Toskes PP. The significance of the volume of pancreatic juice measured at secretin stimulation testing: a single-center evaluation of 224 classical secretin stimulation tests. Pancreas. 2012;41:1073–9. doi: 10.1097/MPA.0b013e318249a271. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury RS, Forsmark CE. Review article: Pancreatic function testing. Aliment Pharmacol Ther. 2003;17:733–50. doi: 10.1046/j.1365-2036.2003.01495.x. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury R, Bhutani MS, Mishra G, et al. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas. 2005;31:63–8. doi: 10.1097/01.mpa.0000164451.69265.80. [DOI] [PubMed] [Google Scholar]
- 22.Lankisch PG, Seidensticker F, Otto J, et al. Secretin-pancreozymin test (SPT) and endoscopic retrograde cholangiopancreatography (ERCP): both are necessary for diagnosing or excluding chronic pancreatitis. Pancreas. 1996;12:149–52. doi: 10.1097/00006676-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18–25. doi: 10.1016/s0016-5107(98)70123-3. [DOI] [PubMed] [Google Scholar]
- 24.Lambiase LFC, Toskes PP. Secretin test diagnoses chronic pancreatitis earlier than ERCP. Gastroenterology. 1993;104:A315. Abstract. [Google Scholar]
- 25.Stevens T, Conwell DL, Zuccaro G, Jr, et al. The efficiency of endoscopic pancreatic function testing is optimized using duodenal aspirates at 30 and 45 minutes after intravenous secretin. Am J Gastroenterol. 2007;102:297–301. doi: 10.1111/j.1572-0241.2006.00949.x. [DOI] [PubMed] [Google Scholar]
- 26.Stevens T, Parsi MA. Update on endoscopic pancreatic function testing. World J Gastroenterol. 2011;17:3957–61. doi: 10.3748/wjg.v17.i35.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantin AM, Hanrahan JW, Bilodeau G, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–44. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 28.Wittel UA, Pandey KK, Andrianifahanana M, et al. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am J Gastroenterol. 2006;101:148–59. doi: 10.1111/j.1572-0241.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 29.Alexandre M, Pandol SJ, Gorelick FS, et al. The emerging role of smoking in the development of pancreatitis. Pancreatology. 2011;11:469–74. doi: 10.1159/000332196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop MD, Freedman SD, Zielenski J, et al. The cystic fibrosis transmembrane conductance regulator gene and ion channel function in patients with idiopathic pancreatitis. Hum Genet. 2005;118:372–81. doi: 10.1007/s00439-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 31.Conwell DL, Zuccaro G, Purich E, et al. The effect of moderate sedation on exocrine pancreas function in normal healthy subjects: a prospective, randomized, cross-over trial using the synthetic porcine secretin stimulated Endoscopic Pancreatic Function Test (ePFT) Am J Gastroenterol. 2005;100:1161–6. doi: 10.1111/j.1572-0241.2005.41386.x. [DOI] [PubMed] [Google Scholar]
- 32.Balci NC, Smith A, Momtahen AJ, et al. MRI and S-MRCP findings in patients with suspected chronic pancreatitis: correlation with endoscopic pancreatic function testing (ePFT) J Magn Reson Imaging. 2010;31:601–6. doi: 10.1002/jmri.22085. [DOI] [PubMed] [Google Scholar]
- 33.Balci C. MRI assessment of chronic pancreatitis. Diagn Interv Radiol. 2011;17:249–54. doi: 10.4261/1305-3825.DIR.3889-10.0. [DOI] [PubMed] [Google Scholar]
- 34.Sato T, Ito K, Tamada T, et al. Age-related changes in normal adult pancreas: MR imaging evaluation. Eur J Radiol. 2012;81:2093–8. doi: 10.1016/j.ejrad.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Sherman S, Hawes RH, Savides TJ, et al. Stent-induced pancreatic ductal and parenchymal changes: correlation of endoscopic ultrasound with ERCP. Gastrointest Endosc. 1996;44:276–82. doi: 10.1016/s0016-5107(96)70164-5. [DOI] [PubMed] [Google Scholar]
- 36.Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretin-stimulated pancreatic function test. Dig Dis Sci. 2007;52:1206–10. doi: 10.1007/s10620-006-9469-6. [DOI] [PubMed] [Google Scholar]


