Abstract
The sexual transmission of human immunodeficiency virus type 1 (HIV-1) is facilitated by inflammation and related epithelial barrier perturbation. Microbicides for vaginal applications are currently being developed to reduce the risk of HIV-1 transmission. However, little is known about their interference with epithelial immune function. In recent clinical trials, nonoxynol-9 (N-9), a virucide with a long history of intravaginal use as a contraceptive, failed to protect against HIV-1 possibly due to mucosal inflammatory damage. Cellulose acetate 1,2-benzenedicarboxylate, also named CAP (for “controls AIDS pandemic”), is an anti-HIV-1 microbicide selected from pharmaceutical excipients that are regarded as safe for oral administration but have not been assessed for potential effects on inflammatory factors in the vaginal environment. Here we use a sensitive human cell culture system to evaluate proinflammatory profiles of soluble CAP in reference to N-9 and known epithelial activators such as tumor necrosis factor alpha (TNF-α) and bacterial lysates. Within 6 h of exposure, TNF-α and N-9 triggered NF-κB and AP-1/cFos activation and upregulated interleukins and an array of chemokines by vaginal and polarized cervical epithelial cells. The induced proinflammatory status continued after removal of stimuli and was confirmed by enhanced transepithelial neutrophil migration. While sustaining stability and anti-HIV-1 activity in the epithelial environment, CAP did not increase the production of proinflammatory mediators during or after exposure, nor did it modify the epithelial resistance to leukocyte traffic. CAP attenuated some TNF-α-induced responses but did not interfere with epithelial cytokine responsiveness to gonococcal determinants. The described system may be useful for predicting proinflammatory side effects of other microbicide candidates for vaginal application.
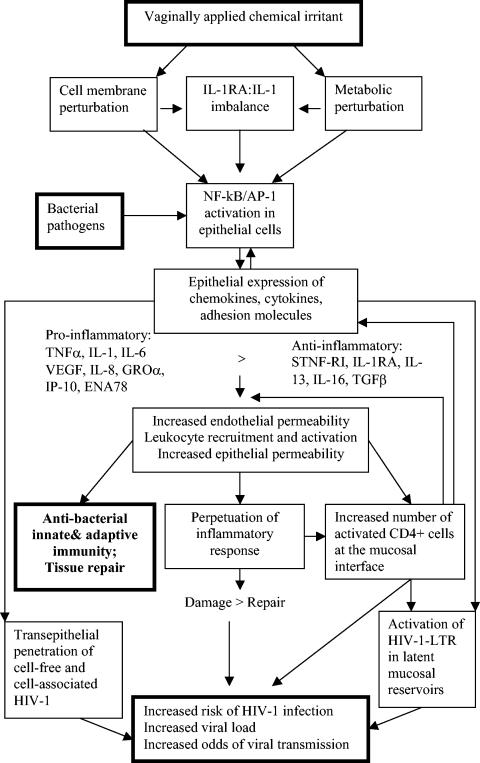
Topical microbicides capable of killing or inactivating virus or blocking viral mucosal entry and at the same time preserving or enhancing the natural mucosal barrier are sought to prevent the sexual transmission of human immunodeficiency virus type 1 (HIV-1) and other viral and bacterial pathogens (61). Currently, about half of the new HIV-1 infections worldwide occur in women who acquire HIV-1 infection during sexual contact, most likely via transepithelial penetration of cell-free virus or HIV-1-infected cells (10, 57). The multilayered stratified epithelium in the vagina and ectocervix and the tight-junction columnar epithelium in the endocervix in unison with the normal vaginal microflora maintain an efficient barrier against HIV-1, which accounts for relatively low odds of acquiring HIV-1 infection by women with a healthy vaginal environment (47). In contrast, women with vaginal inflammatory conditions induced by pathogens or vaginally applied chemical products may be at higher risk resulting from multiple mechanisms of barrier dysfunction (Fig. 1). Previous studies showed that nonoxynol-9 (N-9), which disrupts the viral membrane due to its detergent properties and is widely used in chemical contraceptives and sexual lubricants, can induce a cumulative inflammatory reaction by interleukin-1 (IL-1)-mediated NF-κB activation in cervical and vaginal epithelial cells (21). Consecutive N-9 administrations in healthy subjects were associated with inflammatory sequelae, marked by increased levels of IL-1, IL-8, and macrophage inhibitory protein 1 (MIP-1) in cervicovaginal secretions and recruitment of polymorphonuclear cells and macrophages (21). Inflammatory lesions associated with frequent N-9 use may underlie its failure to protect against HIV-1 in recent phase II and III clinical trials despite N-9's high microbicidal potency in vitro (60, 62). Unfortunately, these effects could not be predicted by conventional toxicology and clinical phase I safety trials, suggesting the need for improved systems for microbicide safety evaluation in the earlier stages of drug development.
FIG. 1.
Association between chemical irritation and proinflammatory pathways that facilitate HIV-1 transmission during sexual intercourse. VEGF, vascular endothelial growth factor; STNF-RI, soluble tumor necrosis factor α receptor I; TGFβ, transforming growth factor β.
Here we apply well-characterized human vaginal and cervical epithelial cell lines (16, 18-20) to model key barrier functions of the cervicovaginal epithelium and to study inflammatory correlates predisposing for HIV-1 infection. Using N-9 as the positive control and the appropriate diluent as the negative control, we characterize the effects of a potent anti-HIV-1 microbicide, cellulose acetate 1,2-benzenedicarboxylate (CAP, for “controls AIDS pandemic”), which belongs to the group of generally regarded as safe pharmaceutical excipients (39, 41, 44). Cellulose acetate 1,2-benzenedicarboxylate has a long history of safe oral use as a matrix ingredient of tablets and capsules and as a coating agent for enteric tablets (35). The soluble CAP polymer used in this study has dual anti-HIV-1 and anti-herpes simplex virus activity in vitro (41, 43). CAP formulated for vaginal application shows potent anti-HIV-1 activity in vitro (42), inhibits vaginal transmission of simian immunodeficiency virus and simian/human immunodeficiency viruses in rhesus monkeys (6, 37), and is currently in clinical phase I trials. This study demonstrates that CAP has no proinflammatory activity and offers an experimental system for preclinical safety evaluation of anti-HIV-1 microbicides that are candidates for vaginal application.
MATERIALS AND METHODS
Reagents.
A soluble form of CAP (polymer mixture with mean molecular mass of 45 to 60 kDa) was supplied by the Lindsley F. Kimball Research Institute of the New York Blood Center (New York, N.Y.) as a 30-mg/ml stock solution in 30 mM sodium acetate buffer (pH 5.9). Fresh stock solutions were prepared every 6 weeks and stored at 4°C to avoid hydrolysis of CAP. The sustained stability of CAP in these stock solutions stored at 4°C was confirmed by high-performance liquid chromatography (HPLC) and a ruthenium red method described elsewhere (40). Working solutions of CAP and acetate buffer controls were freshly prepared for each experiment in appropriate culture media and filtered through a 0.1-μm-pore-size filter (Millipore Corporation, Bedford, Mass.). The stability of CAP in 24-h epithelial cell cultures was confirmed by the ruthenium red method. N-9 and carboxymethyl cellulose were provided by CONRAD, Eastern Virginia Medical School.
The laboratory-adapted subtype B isolate HIV-1IIIB and anti-p24 monoclonal antibody (183-12H-5C) were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (Bethesda, Md.).
Crude water lysates prepared from Neisseria gonorrhoeae laboratory strain F62, as previously described (19), were kindly provided by Caroline Genco at the Department of Infectious Disease, Boston Medical School. Lipopolysaccharide was purchased from Sigma Co. (St. Louis, Mo.). Recombinant human IL-1α, tumor necrosis factor alpha (TNF-α), and soluble CD14 were purchased from R&D Systems (Minneapolis, Minn.).
Sulfasalazine, dimethyl sulfoxide, and fMLP (formyl-methionine leucine phenylalanine) were purchased from Sigma-Aldrich. Monoclonal antibodies (MAbs) against CD11b and CD47 used in the neutrophil transmigration blocking experiments were obtained from Sean Colgan's Laboratory, Brigham and Women's Hospital.
Cells.
Generation of the human papillomavirus type 16/E6E7-immortalized endocervical (End1/E6E7), ectocervical (Ect1/E6E7), and vaginal (Vk2/E6E7) epithelial cell lines from normal human tissues has been previously described (20). Cells were maintained in keratinocyte serum-free medium from GIBCO-Invitrogen (Carlsbad, Calif.) supplemented with bovine pituitary extract, epidermal growth factor (0.1 ng/ml), penicillin-streptomycin, and CaCl2 (0.4 mM) as previously described (20). For some experiments, the cell lines were kept in RPMI 1640 medium (GIBCO-Invitrogen) supplemented with 10% bovine serum (HyClone; Fisher Scientific).
Polarized endocervical epithelial monolayers were generated on polycarbonate membrane inserts with a 6.5-mm diameter and a 3.0-μm pore size (Corning Costar Transwell dual-chamber system; Fisher Scientific). The inserts were coated with 0.05% type IV collagen (Sigma) prepared in 0.2% acetic acid and 7.5% ethanol. For neutrophil transmigration experiments, the inserts were coated with collagen from the bottom site. Endocervical cells (4 × 105) were seeded onto the coated membrane inserts and grown in a 24-well dual-chamber system for 7 to 10 days to achieve polarization. Transepithelial resistance was measured by an EVOM voltmeter (World Precision Instruments, Sarasota, Fla.) to ascertain the physical barrier function (211 ± 7.4 Ω cm2, mean of 24 culture inserts).
Human neutrophils were isolated from peripheral blood as previously described (48) under a protocol approved by the Brigham and Women's Hospital Institutional Review Board for Human Subject Research. Briefly, blood was drawn into syringes with acid citrate dextrose (pH 4.0) and layered over Histopaque and centrifuged for 30 min at 400 × g. The bottom layer was mixed with 2% gelatin in Hanks' buffered salt solution (HBSS) (Ca2+, Mg2+, carbonate, and phenol red free)-10 mM HEPES buffer (pH 7.4; Sigma), and neutrophils were allowed to settle to the bottom for 15 min at 37°C. After an additional centrifugation step, red blood cells were lysed in cold NH4Cl buffer and the cell suspension was immediately centrifuged at 200 × g for 10 min at 4oC. The isolated neutrophils were kept in cold HBSS at 5 × 107 cells/ml until use.
MT-2 cells were obtained from the NIH AIDS Research and Reference Reagent Program and maintained in RPMI medium supplemented with 10% fetal bovine serum, 100 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin (GIBCO Invitrogen)/ml.
Cytotoxicity assays.
Cell viability was assessed by two methods, the mitochondrial function nonradioactive CellTiter96 MTT assay (29) and the CellTiterGlo luminescent ATP assay (Promega, Madison, Wis.), by using a multilabel Victor 2 microplate counter and Wallac 1420 software, version 2.01 (Perkin-Elmer Life Sciences, Boston, Mass.). The epithelial cells were grown in 96-well plates until complete confluence and incubated with twofold dilutions of test agents in keratinocyte medium for 30 min and 6 and 24 h. For the ATP assay, cells were grown in clear-bottom white tissue culture plates (Becton-Dickinson Labware, Franklin Lakes, N.J.) and the plate bottom was covered before reading with white tape to minimize luminescence loss and cross talk. At the end of each treatment period, supernatants were removed for cytokine measurement and replaced with fresh medium. For the MTT assay, the plates were incubated for 4 h in a CO2 incubator with MTT (10 μl of MTT/well) followed by the addition of 100 μl of stop-solubilization reagent (Promega) overnight and reading of optical densities (ODs) at 540 nm and 620 nm. For the ATP assay, plates were incubated for 30 min at room temperature with the luciferase reagent (100 μl/well) on an orbital shaker and luminescence was read at 1 s of integration time/well. Percentages of viable cells in stimulated versus control cultures were calculated based on OD or relative luminescent units.
Inhibition of HIV-1 replication.
The inhibitory activity on infection of MT-2 cells by HIV-1IIIB was determined as previously described (27) in epithelial cell culture supernatants 24 h after conditioning with CAP. Briefly, 50 μl of cell culture sample with CAP (2.5 mg/ml) or without CAP at a twofold dilution was mixed with 50 μl of HIV-1 at 100 50% tissue culture infective doses at 37°C for 30 min before the addition of 100 μl of MT-2 cells (105/ml). After incubation at 37°C overnight, the culture supernatants were removed and fresh medium was added. On the fourth day postinfection, 100 μl of culture supernatants was collected from each well, mixed with an equal volume of 5% Triton X-100, and assayed for p24 antigen, which was quantitated by enzyme-linked immunosorbent assay (ELISA). Briefly, the wells of polystyrene plates (Immulon 1B; Dynex Technology, Chantilly, Va.) were coated with anti-HIV-1 immunoglobulin at 5 μg/ml (prepared from plasma of HIV-seropositive donors with high neutralizing titers against HIV-1IIIB) (51) in 0.085 M carbonate-bicarbonate buffer (pH 9.6) at 4°C overnight, followed by washes with 0.01 M phosphate-buffered saline (PBS) containing 0.05% Tween-20 and blocking with PBS containing 1% dry fat-free milk (Bio-Rad, Inc., Hercules, Calif.). Virus lysates were added to the wells and incubated at 37°C for 1 h. After extensive washes, culture supernatants containing anti-p24 MAb (183-12H-5C) at a 1:100 dilution, biotin-labeled anti-mouse immunoglobulin G1 (Santa Cruz Biotech, Santa Cruz, Calif.), streptavidin-labeled horseradish peroxidase (Zymed, South San Francisco, Calif.), and the substrate 3,3′,5,5′-tetramethylbenzidine (Sigma) were added sequentially. Reactions were terminated by the addition of 1 N H2SO4. Absorbance at 450 nm was recorded by an ELISA reader (Ultra 384; Tecan, Research Triangle Park, N.C.). Recombinant protein p24 (U.S. Biological, Swampscott, Mass.) was included for establishing standard dose-response curves. The percent inhibition of p24 production by a sample was calculated as previously described (27), and the concentration for 50% inhibition was calculated by using the software designated Calcusyn (8). All samples were tested in triplicate.
Cytokine and chemokine assays.
Since cytokine and chemokine mRNA expression is not always translated into protein and therefore the detection of message may not reflect net proinflammatory effects at various incubation times (26), we chose to investigate these effects at the protein level by using traditional ELISA and multiplex proteome analysis. Our preliminary results and previous findings have shown that most chemokines and cytokines, e.g., IL-8 and IL-6, are secreted as they are produced by stimulated vaginal epithelial cells, whereas IL-1 and IL-1 receptor antagonist (IL-1RA) accumulate in the cytoplasm before their release (17). Therefore, we collected culture supernatants for cytokine and chemokine arrays; however, IL-1 and IL-1RA were quantified in both cell supernatants and lysates. Confluent vaginal, ectocervical, and endocervical epithelial monolayers, grown in 96-well plates, were treated with test agents for 6 or 24 h. Supernatants were collected at the end of each treatment period or 18 h after test agent removal. MTT assays that were preformed on the same cultures after supernatant removal ascertained cell viability under each test condition. To determine intracellular IL-1 and IL-1RA concentrations, the cells were thoroughly washed three times in PBS to remove compounds and lysed on ice in 0.5% Triton X-100 in PBS and the cell lysates were assayed by ELISA. Cytokine concentrations were normalized per protein content measured by the Pierce BCA protein assay (Fisher Scientific).
Polarized endocervical monolayers were treated by placing 0.25 ml of test agent on the apical epithelial surface for 6 h and collecting culture medium from the basolateral compartment of the dual-chamber system. Triplicate or quadruplicate cultures were tested for each dose and condition.
Culture supernatants were screened at 1×, 2×, and 10× dilutions in 16-plex SearchLight proteome arrays (Pierce Biotechnology, Inc., Woburn, Mass.). Levels of IL-1α, IL-1RA, IL-6, and IL-8 were quantified in culture supernatants by traditional colorimetric or chemiluminescent ELISA assays (R&D Systems), in a multilabel Victor 2 microplate counter, and Wallac 1420 software, version 2.01 (Perkin Elmer Life Sciences). Possible interference of CAP with cytokine detection in each ELISA was tested by spiking cytokine standards with escalating doses of CAP and equivalent doses of acetate diluent. Curve fit analysis was performed by using Workout, version 1.05, software (DAZDAQ Ltd., Brighton, East Sussex, England).
Transcription factor nuclear translocation assays.
Colorimetric TransFactor ELISA-based assays from BD Biosciences Clontech (Palo Alto, Calif.) were used to determine the effect of test agents on the nuclear translocation of transcription factors from the NF-κB (p50 and cRel) and the AP-1 (cFos) families. Oligonucleotides containing the consensus sequences for each transcription factor were coated onto ELISA wells. Initial stimulations of epithelial cells were performed at 1, 2, and 5 h. The best stimulation was achieved at 5 h. Therefore, this time point was chosen for repeated experiments presented in this study. For each experiment, all compounds were tested in parallel cultures with the same duration of exposure. Nuclear protein was extracted from control and treated cell cultures grown to confluence in T175 tissue culture flasks (approximately 107 cells/sample) by using the TransFactor extraction kit (BD Biosciences Clontech) as per the manufacturer's instruction. Protein content was determined and quantified by the Pierce BCA protein assay (Fisher Scientific). Nuclear extracts were incubated in the wells (30 μg of protein/well) in the absence or presence of competitor oligonucleotides that have the same DNA sequence as the wild-type oligonucleotide coated onto the wells. Bound nuclear transcription factor proteins were detected with specific primary antibody followed by horseradish peroxidase-coupled secondary antibody and 3,3′,5,5′-tetramethylbenzidine substrate supplied by the manufacturer. The reaction was stopped by 0.009 M sodium azide, and the absorbance at 650 nm was read by using the multilabel Victor 2 microplate reader.
Immunocytochemistry.
For immunocytochemistry, epithelial cells were grown in eight chamber tissue culture slides (Fisher Scientific). Confluent monolayers were incubated with test agents for 1 and 6 h, washed in PBS, and fixed with cold absolute ethanol or acetone. The same duration of exposure was used for all compounds in each experiment. In some experiments, we demonstrated NF-κB-dependent activation by using 2 mM sulfasalazine (stock solution in dimethyl sulfoxide) to block IκB phosphorylation and proteasome degradation (65).
Immunocytochemical analysis was performed by using an alkaline phosphatase biotin-streptavidin-amplified system (StrAviGen; BioGenex, San Ramon, Calif.) as previously described (16). A MAb that recognizes an epitope overlapping the nuclear localization sequence of the p65 subunit in NF-κB dimers (Chemicon International, Temecula, Calif.) was used to detect the activated form of NF-κB. The epitope is unmasked following NF-κB activation by its dissociation from the cytoplasmic inhibitor IκB (63).
Transepithelial leukocyte migration.
We adapted a procedure previously established for T84 cells (48, 56) to assess neutrophil migration through the polarized endocervical (End1/E6E7) monolayers in our dual-chamber culture system. After 6 h of treatment, culture inserts were placed in 24-well plate chambers holding 0.5 ml of Ca/Mg-supplemented HBSS-10 mM HEPES. HBSS supplemented with 10 nM fMLP served as a positive control. At least three epithelial monolayers were subjected to each condition. Neutrophil suspensions were placed on the basolateral surface of each monolayer, and the neutrophils were allowed to migrate for 90 min toward the apical epithelial surface facing the bottom chamber of the dual compartment culture system. In some experiments, neutrophils were mixed with anti-CD11b and anti-CD47 MAbs at 10 μg/ml before addition to epithelial culture inserts. At the end of the incubation period, the neutrophils in the bottom migration chambers were lysed with 0.5% Triton X-100 and assessed for myeloperoxidase. Lysates were mixed with equal volumes of ABTS [2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt] substrate (Sigma) in 1 M citrate buffer (pH 4.15) in the presence of 0.03% H2O2, and ODs were read at 405 nm by using the multilabel Victor 2 microplate counter and Wallac 1420 software, version 2.01.
Statistical analysis.
Prism, version 3.0 (GraphPad Software, San Diego, Calif.), was used for analysis of variance and Dunnett's multiple comparison tests. A P value of <0.05 was considered significant.
RESULTS
Soluble CAP at concentrations causing >90% inhibition of HIV-1 replication maintained its stability and anti-HIV-1 activity in the cervicovaginal epithelial cell environment while remaining innocuous to epithelial cell vital metabolic functions.
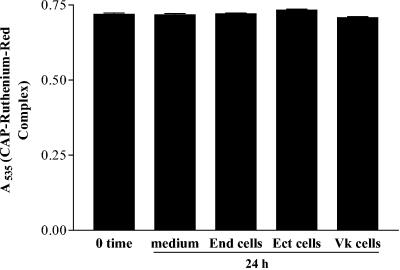
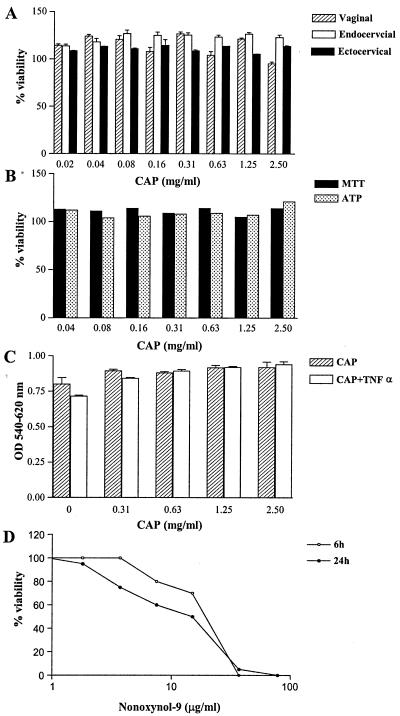
CAP remained stable after continuous culture in conditioned media for 24 h regardless of cell type (Fig. 2 shows 100% recovery of 2.5 mg of CAP/ml from culture supernatants). At the same time, CAP was invariably nontoxic to the vaginal, ectocervical, and endocervical epithelial cells, as demonstrated by the metabolic function MTT and ATP assays (Fig. 3A and B). Similarly, CAP remained nontoxic in the presence of a biologically active dose of the proinflammatory cytokine TNF-α (40 ng/ml), which was used in this study to model a heightened immunoinflammatory state of the vaginal epithelium (Fig. 3C). In comparison, N-9 was toxic to the epithelial cells at about 2-log-lower doses (Fig. 3D).
FIG. 2.
Complete recovery of CAP (2.5 mg/ml) from 24-h supernatants collected from triplicate cultures of endocervical (End), ectocervical (Ect), and vaginal (Vk) epithelial cell lines and cell-free culture media kept at 37°C in a CO2 incubator. The control (0 time) is a freshly prepared CAP solution in culture medium. The vertical axis represents mean OD values ± standard deviations.
FIG. 3.
Epithelial cell viability dose responses assessed by MTT (A to D) and ATP (B) assays. Results represent means ± standard deviations of the results from parallel quadruplicate cultures exposed to CAP in the presence or absence of 40 ng of TNF-α/ml (A to C) for 24 h or to N-9 for 6 and 24 h (D). All viability assessments showed similar results in more than three independent experiments.
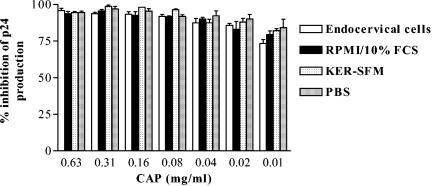
At the same nontoxic dose range, the soluble form of CAP was shown to be effective in inhibiting infection by all of the laboratory-adapted and primary HIV-1 strains tested (28, 41, 43, 44). In this study, we confirmed the sustained anti-HIV-1 inhibitory activity of CAP present in endocervical epithelial cell culture supernatants (Fig. 4). Medium controls (serum-free keratinocyte medium, 10% fetal bovine serum-supplemented RPMI, and PBS) were run in parallel. As shown in Fig. 4, the type of culture medium or the presence of epithelial cells did not affect the inhibitory activity of CAP on HIV-1IIIB infection.
FIG. 4.
Inhibition of HIV-1 replication (p24 production) by 24 h CAP-conditioned culture media in the presence or absence of epithelial (endocervical) cells. Results represent means ± standard deviations of percent inhibition of p24 production measured by ELISA. FCS, fetal calf serum; KER-SFM, keratinocyte serum-free medium.
In contrast to N-9 and TNF-α, CAP did not activate NF-κB and attenuated cFos nuclear binding in cervical and vaginal epithelial cells.
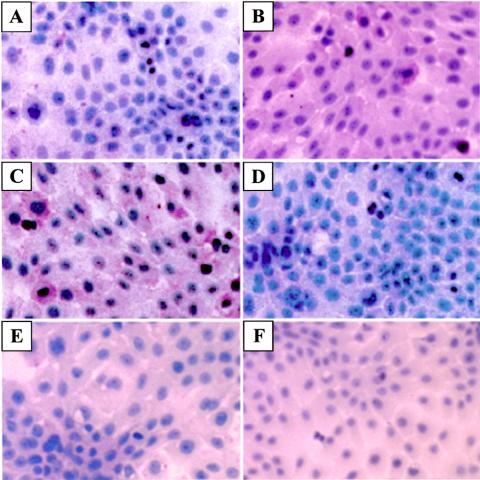
Our laboratory has previously shown that N-9 can induce NF-κB activation in vaginal epithelial cells in vitro and that repeated vaginal use of N-9 is also associated with increased epithelial NF-κB activation (21). In this study, we compared the effects of CAP at its highest concentration (2.5 mg/ml) to those of N-9 used in its nontoxic dose range (1.8 and 3.7 mg/ml for 24- and 6-h incubation times, respectively). TNF-α at nontoxic doses of 20 and 40 ng/ml, as determined by MTT assay (Fig. 3C), served as a positive control. Exposure of epithelial cells to TNF-α and N-9 for 6 h triggered a marked NF-κB activation, as demonstrated by the specific immunocytochemical labeling of the unmasked nuclear localization sequence of NF-κB p65. As shown in Fig. 5, NF-κB activation was observed in the absence of morphological changes in cultures treated for 6 h with nontoxic doses of TNF-α and N-9. At the same time, CAP and a control cellulose derivative remained neutral under the same conditions (Fig. 5E and F).
FIG. 5.
NF-κB (p65) activation in endocervical epithelial cells (End1E6E7) after 6 h of incubation in the presence of medium control (A), N-9 (3.7 μg/ml) (B), TNF-α (25 ng/ml) (C), TNF-α plus NF-κB inhibitor sulfasalazine (2 mM) (D), carboxymethyl cellulose (2.5 mg/ml) (E), and CAP (2.5 mg/ml) (F). The status of activation is indicated by the red labeling of the nuclear localization sequence on the p65 subunit of the NF-κB heterodimer. Original magnification, ×250.
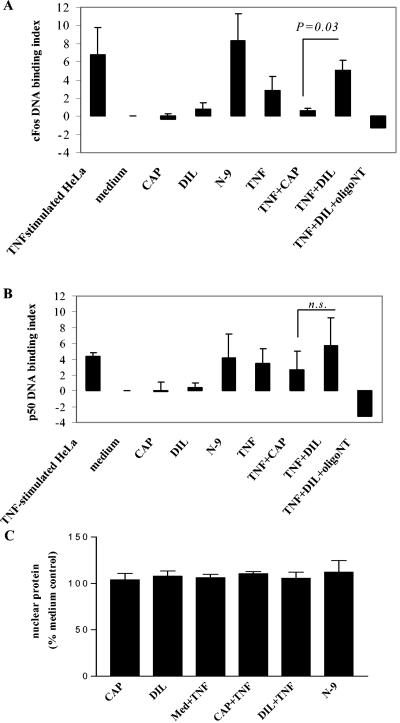
Transfactor assays in parallel cultures demonstrated that TNF-α and N-9 induced 2- to 10-fold-increased DNA binding of NF-κB/p50 and AP-1/cFos proteins recovered from nuclear extracts of stimulated cells compared to those of nonstimulated control cultures. However, CAP at 2.5 mg/ml or its diluent control did not have such an enhancement effect (Fig. 6). Controls with competitor oligonucleotides (500 ng/30 μg of nuclear protein) confirmed the binding specificity between DNA and the transcription factors in these assays. TNF-α-induced nuclear binding of cFos, but not p50, was significantly inhibited (P < 0.05) in cells exposed to CAP (Fig. 6). None of the test compounds had a significant effect on nuclear protein recovery from the cell extracts (Fig. 6).
FIG. 6.
DNA binding of proinflammatory transcription factors recovered from nuclear extracts of epithelial cells exposed for 5 h to nontoxic concentrations of TNF-α (25 ng/ml), N-9 (3.7 μg/ml), and CAP (2.5 mg/ml). Sodium acetate at 2.5 mM served as the diluent control (DIL) for CAP. The effects of TNF-α were tested in the presence or absence of CAP (2.5 mg/ml) and DIL (2.5 mM). The results represent means and standard deviations obtained for at least three independent experiments performed with endocervical, ectocervical, and vaginal cells. The y axes show indexes computed as increases of AP-1/cFos (A) and NF-κB/p50 (B) binding in nuclear extracts of compound-treated cells over those of cells in plain keratinocyte serum-free medium (culture baseline) divided by the blocking buffer background for each factor. Manufacturer-provided nuclear extract of TNF-α-stimulated HeLa cells and competitor oligonucleotides (oligoNT) were used as controls for each assay. (C) Protein recovered from nuclear extracts in five independent experiments. Data are expressed as percentages of untreated (medium [Med]) control and presented as means ± standard deviations. n.s., not significant.
In contrast to TNF-α, CAP did not induce cytokine and chemokine upregulation and basolateral secretion.
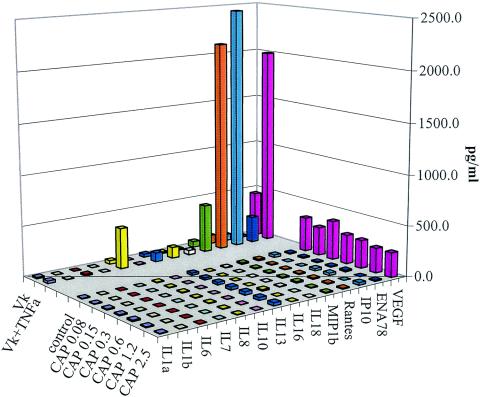
Our laboratory has previously shown that the proinflammatory cytokines TNF-α and IL-1α, like N-9 and gonococcal lysates, trigger a proinflammatory cytokine cascade in cervical and vaginal epithelial cells after their exposure to the above reagents for 24 h (17-19, 21). In this study, we used the proinflammatory cytokine TNF-α as a positive control for comparison with CAP of the potential to trigger the proinflammatory responses in vaginal monolayers and polarized cervical epithelial cells determined by cytokine and chemokine multiplex arrays. After incubation with vaginal cell cultures for 24 h, TNF-α induced the secretion of a plethora of chemokines ([IL-8, MIP-1β, RANTES, IP-10, and ENA78] and cytokines [IL-1α, IL-6, IL-13, IL-16, IL-18, and vascular endothelial growth factor]) in cell culture supernatants. At the same time, the CAP-treated vaginal cells showed low baseline production of all mediators (Fig. 7).
FIG. 7.
Screening by the Pierce Searchlight proteome 16-plex assay shows no change in the cytokine profile of CAP-treated vaginal (Vk) cells (dose range, 0.08 to 2.5 mg/ml) compared to epithelial cells cultured in control medium or medium supplemented with 20 ng of TNF-α/ml (labeled as Vk and Vk + TNF-α, respectively). VEGF, vascular endothelial growth factor.
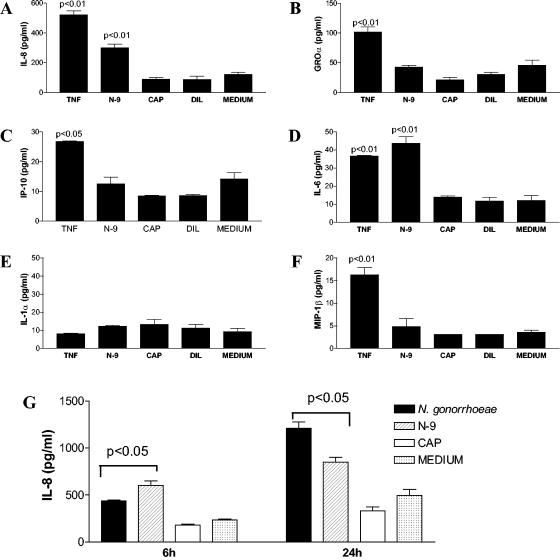
In polarized endocervical epithelial monolayers, apically applied TNF-α significantly increased the basolateral secretion of IL-6, IL-8, growth-related oncogene α (GROα), IP-10, and MIP-1β (P < 0.05) within 6 h of exposure (Fig. 8A to D and F). In parallel cultures, N-9 at a nontoxic concentration (3.7 μg/ml) increased the basolateral secretion of IL-8 and IL-6 (P < 0.05) while CAP (2.5 mg/ml) and the acetate diluent control had no significant effect on any of these mediators (Fig. 8A and D). Similar results were obtained by using CAP at lower concentrations with its diluent (data not shown). There was no difference in IL-1α concentrations (Fig. 8E), which, in accordance with previously established correlations with viable cell counts (17), confirmed the lack of compound-induced cytotoxicity. In comparison to medium control and CAP-treated cultures, gonococcal lysates and N-9 induced significantly increased basolateral secretion of IL-8 not only during the first 6 h of exposure but also 24 h after stimulant removal (Fig. 8G).
FIG. 8.
(A to F) Cytokine and chemokine profiles obtained by Pierce Searchlight proteome arrays from the basolateral culture fluids of polarized endocervical epithelial monolayers after 6 h of incubation in medium supplemented with TNF-α (10 ng/ml), N-9 (3.7 μg/ml), and CAP (2.5 mg/ml) or acetate diluent control (DIL) (2.5 mM). (G) In addition, basolateral IL-8 secretion was measured by ELISA 6 and 24 h after apical treatment of polarized endocervical cells with CAP (2.5 mg/ml), N-9 (3.7 μg/ml), and whole-cell lysate from N. gonorrhoeae (an equivalent of 5 × 105 bacteria/epithelial insert). Data are presented as means and standard deviations of the results from triplicate cultures. Similar results were obtained by using CAP and its diluent at lower concentrations (data not shown). P values indicate concentrations significantly increased compared to the medium control.
CAP had no delayed effects on cytokine and chemokine production by vaginal and cervical epithelial cells.
To further investigate whether the epithelial cell barrier function may be altered in the washout period after removal of microbicide compounds, we evaluated cytokine production as well as resistance of polarized endocervical epithelial monolayers to neutrophils transmigration after 6 h of exposure to CAP and proinflammatory control stimuli.
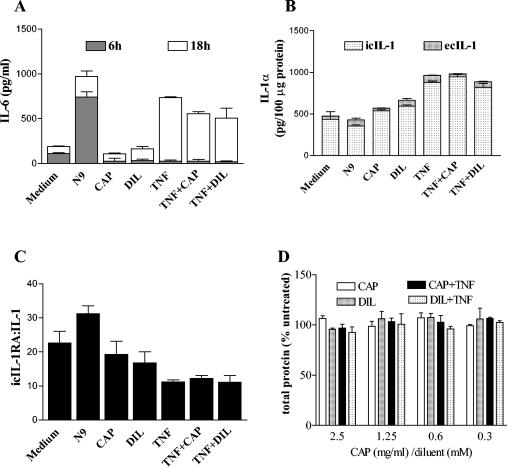
The 6-h stimulation with TNF-α and N-9 induced secretion of IL-6 in epithelial cell culture supernatants that continued in the 18-h washout period after removal of stimulus (Fig. 9A). In contrast, CAP-treated cultures showed no delayed increase of cytokine production within the 18-h follow-up period. CAP-treated TNF-α-stimulated cultures showed a tendency for decreased IL-6 release in the 18-h washout period. However, these effects did not reach significance.
FIG. 9.
Cytokines measured in culture supernatants or cell lysates obtained from vaginal epithelial cells after 6 h of continuous exposure to nontoxic concentrations of N-9 (1.9 μg/ml), TNF-α (25 ng/ml), CAP (2.5 mg/ml), and acetate diluent (DIL) control (2.5 mM) and after a subsequent 18-h washout period. The effects of TNF-α were tested in the presence or absence of CAP (2.5 mg/ml) and DIL (2.5 mM). Similar results were obtained by using CAP and its diluent at lower concentrations (data not shown). (A) IL-6 concentrations in culture supernatants; (B) intracellular (ic) and extracellular (ec) concentrations of IL-1α in culture supernatants and cell lysates, respectively, measured in the 18-h washout period after removal of stimulus; (C) IL-1RA-to-IL-1 ratios computed from concentrations measured in cell lysates obtained at the end of the 18-h washout period; (D) protein concentrations measured in total cell lysates by BCA protein assay. Data are means and standard deviations of the results from duplicate cultures in one of three independent experiments. Results represent means ± standard deviations.
The TNF-α stimulation increased the intracellular IL-1α fraction in the 18-h washout period (Fig. 9B), which was associated with a decreased IL-1RA-to-IL-1α ratio obtained from lysed cells (Fig. 9C). CAP had no significant effects on the intracellular stores of IL-1α per total cellular protein (Fig. 9B) or the IL-1RA-to-IL-1 ratio in resting or TNF-α-treated cultures (Fig. 9C). CAP or control diluent treatment in the presence or absence of TNF-α had no effect on total cellular protein recovery (Fig. 9D).
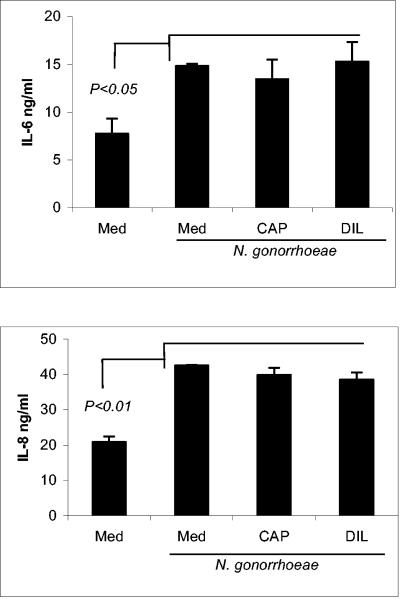
To evaluate possible adverse effects of CAP on epithelial protective responses against bacterial pathogens, we examined whether exposure to CAP may weaken the ability of the epithelial cells to produce IL-6 and IL-8 in response to N. gonorrhoeae challenge. IL-8 plays a critical role in the clearance of bacterial infection by recruitment and activation of polymorphonuclear cells (30). IL-6 is responsible for the production of acute-phase proteins, which function to opsonize bacteria; furthermore, it costimulates T cells and serves as the principal growth factor for activated B cells (13, 24). While amplified epithelial production of IL-8 and IL-6 induced by chemical irritants in the absence of pathogen may lead to mucosal damage (17), the production of these mediators in the acute phase of bacterial infection is part of the normal mucosal defense program (14). In a previously established vaginal and cervical epithelial infection model, both cytokines were induced by bacterial infection and soluble bacterial determinants (18, 19). In this study, endocervical epithelial cells were incubated with escalating doses of CAP or diluent for 24 h, washed, and challenged with a whole N. gonorrhoeae lysate for another 24 h. The supernatants were collected for cytokine measurement by ELISA. The results showed that regardless of preexposure to CAP or diluent and medium controls, N. gonorrhoeae lysates induced a significant increase of IL-6 and IL-8 production by endocervical epithelial cells compared to unstimulated (medium) control (P < 0.05 and P < 0.01, respectively). No significant differences were observed between CAP and diluent treatments (Fig. 10). Similar treatment by CAP did not affect the responses to TNF-α and N-9 (data not shown)
FIG. 10.
Concentrations of IL-6 and IL-8 were measured in endocervical epithelial cell culture supernatants collected after 6 h of preexposure to plain medium (Med), CAP (2.5 mg/ml)m or acetate diluent (DIL) control (2.5 mM), followed by addition of N. gonorrhoeae lysates for 24 h. The results are expressed as means and standard deviations obtained from triplicate cultures and represent one of two independent experiments. P values indicate values significantly different from unstimulated (medium) control.
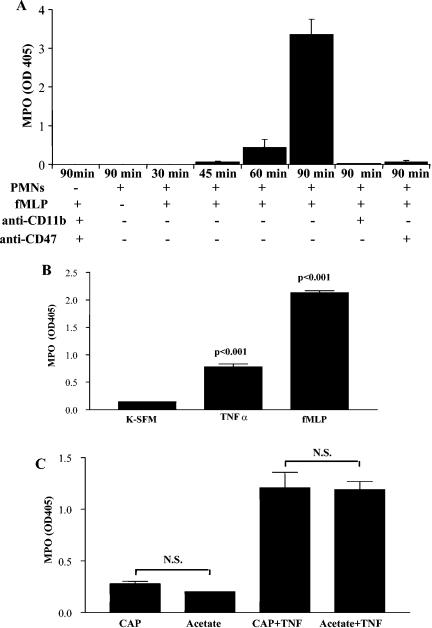
To test the functionality of chemokine responses induced by inflammatory challenge in the presence or absence of CAP, polymorphonuclear neutrophils (PMNs) were allowed to transmigrate across the endocervical epithelial barrier grown on 3-μm-pore-size membranes. In this model system, PMNs moved across the epithelial layer toward the bacterial chemotactic peptide fMLP in a CD11b- and CD47-dependent fashion; however, they could not pass the tight epithelial barrier in the absence of fMLP (Fig. 11A), suggesting that epithelial neutrophil activation was involved in this process (48). TNF-α-induced epithelial activation for 6 h was sufficient to allow basolateral to apical neutrophil passage in the absence of fMLP (Fig. 11B). Exposure to CAP did not modify the epithelial resistance to PMNs and had no significant effect on the TNF-α-induced neutrophil migration (Fig. 11C). No difference was observed between CAP and the diluent control in this bioassay, confirming the inert nature of CAP determined by the chemokine protein arrays and ELISA.
FIG. 11.
Transepithelial migration of human polymorphonuclear cells (PMNs) measured by myeloperoxidase (MPO) activity in the migration chamber of a dual-compartment polarized endocervical cell system. (A) Anti-CD11b and CD47 antibodies were applied to the apical surface, and PMNs were allowed to migrate for 30, 45, 60, and 90 min toward basolaterally applied fMLP (10−6 M); (B) basolateral-to-apical neutrophil migration through TNF-α-preactivated or fMLP-exposed epithelial monolayers; (C) basolateral-to-apical migration of neutrophils through monolayers preexposed to CAP (2.5 mg/ml) or diluent control (DIL, 2 mM) in the presence or absence of TNF-α. Similar results were obtained by using CAP and its diluent at lower concentrations (data not shown). Values represent means and standard deviations of the results from triplicate cultures. N.S., not significant; +, present; −, absent. K = SFM, keratinocyte serum-free medium.
DISCUSSION
The inflammatory reaction within the female reproductive tract may be essential for immune responses to and clearance of bacterial infections. However, in the case of infections by viruses, particularly HIV-1, the proinflammatory mediators may lead to viral replication in latent viral reservoirs and increase the availability of potential host cells for new viral infection (Fig. 1). The strong association that has been established between inflammatory response and HIV-1 acquisition (53, 66) underlines the crucial importance of unraveling the molecular mechanisms of the vaginal mucosal barrier functions in health and disease. Proinflammatory cytokines and the transcription factors governing cytokine expression play a critical role in HIV-1 pathogenesis. Some of them, such as IL-1, IL-8, and IL-6, have been studied in genital fluids as correlates of bacterial infection in vitro and in vivo (5, 7, 15, 25, 54, 64). Moreover, pilot clinical investigations and animal studies have suggested their value as markers of skin irritation, mucosal toxicity, and cervicovaginal inflammation (11, 12, 17, 21, 45). Nevertheless, little is known about their expression and regulation in cervical and vaginal epithelial cells under conditions of chemically induced inflammation.
Inflammatory reactions in the case of chemical irritation can be initiated by membrane damage of the mucosal epithelial cells, which is followed by release of prepackaged cytokines of the IL-1 family and a subsequent cascade of cytokine-mediated inflammatory events (Fig. 1). The membrane damage of epithelial cells leads to immediate release of intracellular stores of IL-1 cytokines and their specific counterpart, IL-1RA. IL-1RA is a structural homologue of IL-1α and -1β, which binds to IL-1RI (the biologically active IL-1 transmembrane receptor) with almost the same avidity as IL-1 but fails to initiate signal transduction due to blocking of the intracytoplasmic association of IL-1RI with the IL-1 receptor accessory protein (3). In different cell systems, it has been estimated that a local ratio of 100 or greater of IL-1RA to IL-1 is needed to inhibit the biologic effects of IL-1 on target cells (2). In the quiescent cervical and vaginal epithelial cells, a ratio of >20 is needed to maintain a low baseline production of most proinflammatory molecules. We found that continuous stimulation of cervical and vaginal epithelial cells with TNF-α and N-9 shifted the intracellular balance due to excess IL-1 production and relative decrease of IL-1RA stores, which agrees with the demonstrated NF-κB (p65 and p50) activation and the increased production of NF-κB-regulated cytokines and chemokines by these cells. These findings correlate with upregulation of both chemokine concentrations and functions measured by increased neutrophil transmigration via the polarized epithelial barrier demonstrated in this study. Some of these chemokines, e.g., IP-10, IL-8, and GROα are not only involved in recruitment of CD4 positive cells and neutrophils but also have been found to directly activate viral replication in HIV-1-infected cells (31-34).
N-9 can disrupt the epithelial cell barrier due to its detergent nature. The nondetergent microbicides that possess low cytotoxicity may still harm the mucosal barrier by inducing or amplifying proinflammatory signals beyond the time when they are present at effective doses to kill or inhibit HIV-1. Proinflammatory cytokines such as IL-1α, IL-6, and TNF-α stimulate viral replication in latently infected cells via NF-κB responsive elements in the long terminal repeat region of HIV-1 and promoter regions of other sexually transmitted viruses such as human cytomegalovirus (50, 52). Latently infected quiescent T cells or monocytes/macrophages may constitute a considerable inducible HIV-1 reservoir in the genital secretions of both men and women (22, 67). Previous studies have shown that cervicovaginal secretions contain factors capable of inducing HIV-1 replication in latently infected cells and that such factors may be enhanced in the washout period after repeated N-9 use (1, 21). Therefore, it is particularly important to rule out perpetuation of inflammatory reactions in the period after microbicide removal. In our model system, nontoxic doses of TNF-α and N-9 induced continuous production of IL-6 and other proinflammatory mediators for 18 h after removal of the stimulus. CAP did not induce an increase of these mediators nor attenuated cytokine production induced by gonococcal determinants. At the same time, CAP decreased while N-9 significantly increased the DNA binding activity of cFos in the vaginal and cervical epithelial cells. The mechanism of CAP-mediated potential anti-inflammatory actions warrants further investigation.
In our study, N-9 caused both NF-κB (p65 and p50) and AP-1 (cFos) activation while CAP remained inert in terms of both factors and even attenuated TNF-induced cFos nuclear binding. Both NF-κB and AP-1 are activated and can potentiate each other in conditions of chronic inflammation and have been implicated in HIV-1 pathogenesis through upregulation of proinflammatory cytokines or binding to viral promoter regions and direct activation of viral replication (4, 36, 49, 59). The NF-κB dimers p65/p50 and p65/p52 can synergize with other transcription factors such as AP-1 to stimulate HIV-1 transcription in infected cells (49). AP-1 is a dimeric factor composed of subunits that bind DNA basic leucine zipper motifs including members of the cFos and cJun oncogene families and the closely related activating factor proteins. AP-1/cFos coregulates the expression of IL-6, IL-8, and other proinflammatory genes together with other transcription factors such as NF-κB and CREB (9). HIV-1 has also been associated with increased AP-1/cFos upregulation in activated T cells and monocytes (23). Thus, the concurrent NF-κB and cFos activation by N-9 may facilitate the synergistic effects of these factors on HIV-1 replication in latently infected mucosal cells. On the other hand, AP-1 is involved in the regulation of the epithelial differentiation program, proliferation control, and carcinogenesis (58). Although the role of cFos, as part of AP-1 or other transcription factor complexes, in the development of benign and malignant genital tumors has not been fully elucidated, its overexpression can promote malignant transformations in vivo and in vitro (38, 46, 55), suggesting that its suppression by CAP in the genital tract may have a beneficial impact on the prevention of cervical cancer. On the other hand, the significantly increased cFos transactivation by N-9, which is still on the market as a contraceptive spermicide, is particularly worrisome and requires further studies.
In conclusion, the proinflammatory transcription factors NF-κB and AP-1 and related proteins emerge as important biomarkers of immunoinflammatory side effects of some vaginal products. The differential effects of N-9 and CAP on the NF-κB and AP-1 proinflammatory pathways observed in our in vitro model system suggest that it can be used for evaluation of vaginal microbicide candidates with various mechanisms of action.
Acknowledgments
This work was supported by NIH/NICHD grant 1P01HD041761.
We thank Sean Colgan from the Center for Experimental Therapeutics and Reperfusion Injury, Brigham and Women's Hospital, for generously sharing reagents and guidance in the performance of the neutrophil migration assay. We also thank Hong Lu at the Viral Immunology Laboratory of the New York Blood Center for determining the inhibitory activity of CAP on HIV-1 replication.
REFERENCES
- 1.Al-Harthi, L. A., G. T. Spear, F. B. Hashemi, A. Landlay, B. E. Sha, and K. A. Roebuck. 1998. A human immunodeficiency virus (HIV)-inducing factor from the female genital tract activates HIV-1 gene expression through the kB enhancer. J Infect. Dis. 178:1343-1351. [DOI] [PubMed] [Google Scholar]
- 2.Arend, W., H. Welgus, R. Thompson, and S. Eisenberg. 1990. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J. Clin. Investig. 85:1694-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arend, W. P. 2002. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 13:323-340. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, P. J., and M. Karin. 1997. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336:1066-1071. [DOI] [PubMed] [Google Scholar]
- 5.Belec, L., R. Gherardi, C. Payan, T. Prazuck, J. E. Malkin, C. T. Benissan, and J. Pillot. 1995. Proinflammatory cytokine expression in cervicovaginal secretions of normal and HIV-infected women. Cytokine 7:568-574. [DOI] [PubMed] [Google Scholar]
- 6.Boadi, T., M. Ratterree, A. Gettie, A. R. Neurath, J. Blanchard, and C. Cheng-Mayer. 2004. Safety and efficacy of cellulose acetate phthalate (CAP) against vaginal transmission of simian/human immunodeficiency viruses in rhesus macaques. Abstr. Microbicides 2004 Conf., abstr. 02414. London, United Kingdom.
- 7.Cauci, S., S. Guaschino, D. de Aloysio, S. Driussi, D. De Santo, P. Penacchioni, and F. Quadrifoglio. 2003. Interrelationships of interleukin-8 with interleukin-1β and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol. Hum. Reprod. 9:53-58. [DOI] [PubMed] [Google Scholar]
- 8.Chou, T. C., and M. P. Hayball. 1991. CalcuSyn: Windows software for dose effect analysis. BIOSOFT, Stapleford, Cambridge, United Kingdom.
- 9.Cloutier, A., T. Ear, O. Borissevitch, P. Larivee, and P. P. McDonald. 2003. Inflammatory cytokine expression is independent of the c-Jun N-terminal kinase/AP-1 signaling cascade in human neutrophils. J. Immunol. 171:3751-3761. [DOI] [PubMed] [Google Scholar]
- 10.Cohn, S. E., and R. A. Clark. 2003. Sexually transmitted diseases, HIV, and AIDS in women. Med. Clin. N. Am. 87:971-995. [DOI] [PubMed] [Google Scholar]
- 11.Coquette, A., N. Berna, A. Vandenbosch, M. Rosdy, and Y. Poumay. 1999. Differential expression and release of cytokines by an in vitro reconstructed human epidermis following exposure to skin irritant and sensitizing chemicals. Toxicol. In Vitro 13:867-877. [DOI] [PubMed] [Google Scholar]
- 12.Corsini, E., A. Primavera, M. Marinovich, and C. L. Galli. 1998. Selective induction of cell-associated interleukin-1a in murine keratinocytes by chemical allergens. Toxicology 129:193-200. [DOI] [PubMed] [Google Scholar]
- 13.Cox, G. 1997. Interleukin-6, p. 81-99. In J. S. Friedland (ed.), Cytokines in health and disease, 2nd ed. Marcel Dekker, New York, N.Y.
- 14.Dinarello, C. A. 2000. Proinflammatory cytokines. Chest 118:503-508. [DOI] [PubMed] [Google Scholar]
- 15.Donders, G. G. G., A. Vereecken, E. Bosmans, and B. Spitz. 2003. Vaginal cytokines in normal pregnancy. Am. J. Obstet. Gynecol. 189:1433-1438. [DOI] [PubMed] [Google Scholar]
- 16.Fichorova, R. N., and D. J. Anderson. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60:508-514. [DOI] [PubMed] [Google Scholar]
- 17.Fichorova, R. N., M. Bajpai, N. Chandra, J. G. Hsiu, M. Spangler, V. Ratnam, and G. F. Doncel. 2004. Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol. Reprod. 71:761-769. [DOI] [PubMed] [Google Scholar]
- 18.Fichorova, R. N., A. Cronin, E. Lien, D. J. Anderson, and R. R. Ingalls. 2002. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of TLR-4-mediated signaling. J. Immunol. 168:2424-2432. [DOI] [PubMed] [Google Scholar]
- 19.Fichorova, R. N., P. J. Desai, F. Gibson, and C. A. Genco. 2001. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect. Immun. 69:5840-5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fichorova, R. N., J. G. Rheinwald, and D. J. Anderson. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847-855. [DOI] [PubMed] [Google Scholar]
- 21.Fichorova, R. N., L. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9 induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Blanco, M., and B. Cullen. 1991. Molecular basis of latency in pathogenic human viruses. Science 254:815-820. [DOI] [PubMed] [Google Scholar]
- 23.Gibellini, D., A. Caputo, S. Capitani, M. La Placa, and G. Zauli. 1997. Upregulation of c-Fos in activated T lymphoid and monocytic cells by human immunodeficiency virus-1 Tat protein. Blood 89:1654-1664. [PubMed] [Google Scholar]
- 24.Henderson, B., S. Poole, and M. Wilson. 1996. Microbial/host interactions in health and disease: who controls the cytokine network? Immunopharmacology 35:1-21. [DOI] [PubMed] [Google Scholar]
- 25.Hitti, J., S. L. Hillier, K. J. Agnew, M. A. Krohn, D. P. Reisner, and D. A. Eschenback. 2001. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet. Gynecol. 97:211-219. [DOI] [PubMed] [Google Scholar]
- 26.House, R. V. 2001. Cytokine measurement techniques for assessing hypersensitivity. Toxicology 158:51-58. [DOI] [PubMed] [Google Scholar]
- 27.Jiang, S., K. Lin, and A. R. Neurath. 1991. Enhancement of human immunodeficiency virus type-1 (HIV-1) infection by antisera to peptides from the envelope glycoproteins gp120/gp41. J. Exp. Med. 174:1557-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang, S., H. Lu, Q. Zhao, G. Wallace, R. J. Shattock, and A. R. Neurath. 2004. Cellulose acetate phthalate inhibits infection by cell-free and cell-associated primary HIV-1 strains. Abstr. Microbicides 2004 Conf., abstr. 02183. London, United Kingdom.
- 29.Korting, H. C., S. Schindler, A. Hartinger, A. Kerschner, T. Angerpointer, and H. I. Maibach. 1994. MTT-assay and neutral red release (NRR)-assay: relative role in the prediction of the irritancy potential of surfactants. Life Sci. 55:533-540. [DOI] [PubMed] [Google Scholar]
- 30.Kunkel, S. L., N. W. Lukacs, S. W. Chensue, and R. M. Strieter. 1997. Chemokines and the inflammatory response, p. 121-131. In J. S. Friedland (ed.), Cytokines in health and disease, 2nd ed. Marcel Dekker, New York, N.Y.
- 31.Lane, B. R., S. R. King, P. J. Bock, R. M. Strieter, M. J. Coffey, and D. M. Markovitz. 2003. The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology 307:122-134. [DOI] [PubMed] [Google Scholar]
- 32.Lane, B. R., J. Liu, P. J. Bock, D. Schols, M. J. Coffey, R. M. Strieter, P. J. Polverini, and D. M. Markovitz. 2002. Interleukin-8 and growth-regulated oncogene alpha mediate angiogenesis in Kaposi's sarcoma. J. Virol. 76:11570-11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane, B. R., K. Lore, P. J. Bock, J. Andersson, M. J. Coffey, R. M. Strieter, and D. M. Markovitz. 2001. Interleukin-8 stimulates human immunodeficiency virus type 1 replication and is a potential new target for antiretroviral therapy. J. Virol. 75:8195-8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane, B. R., R. M. Strieter, M. J. Coffey, and D. M. Markovitz. 2001. Human immunodeficiency virus type 1 (HIV-1)-induced GRO- production stimulates HIV-1 replication in macrophages and T lymphocytes. J. Virol. 75:5812-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, J. C. 1994. Cellulose acetate phthalate, p. 91-93. In P. Weller (ed.), Handbook of pharmaceutical excipients, 2nd ed. American Pharmaceutical Association Publishers, Washington, D.C.
- 36.Mallardo, M., E. Dragonetti, F. Baldassare, C. Ambrosino, G. Scala, and I. Quinto. 1996. An NF-kB site in the 5′-untranslated leader region of human immunodeficiency virus type 1 enhances the viral expression in response to NF-kB-activating stimuli. J. Biol. Chem. 271:20820-20827. [DOI] [PubMed] [Google Scholar]
- 37.Manson, K. H., M. S. Wyand, C. Miller, and A. R. Neurath. 1999. The effect of a cellulose acetate phthalate topical cream on vaginal transmission of simian immunodeficiency virus in rhesus monkeys. Antimicrob. Agents Chemother. 44:3199-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller, A. D., T. Curran, and I. M. Verma. 1984. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell 36:51-60. [DOI] [PubMed] [Google Scholar]
- 39.Neurath, A. R., A. K. Debnath, S. Jiang, N. Strick, and G. J. Dow. 16 November 1999. Method for decreasing the frequency of transmission of viral infections using cellulose acetate phthalate or hydroxypropyl methylcellulose phthalate excipients. U.S. patent 5,985,313.
- 40.Neurath, A. R., and N. Strick. 2001. Quantitation of cellulose acetate phthalate in biological fluids as a complex with ruthenium red. Anal. Biochem. 288:102-104. [DOI] [PubMed] [Google Scholar]
- 41.Neurath, A. R., N. Strick, S. Jiang, Y.-Y. Li, and A. K. Debnath. 2002. Anti-HIV-1 activity of cellulose phthalate: synergy with soluble CD4 and induction of “dead-end” gp41 six-helix bundles. BMC Infect. Dis. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neurath, A. R., N. Strick, and Y.-Y. Li. 2002. Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides. BMC Infect. Dis. 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neurath, A. R., N. Strick, Y.-Y. Li, and A. K. Debnath. 2001. Cellulose acetate phthalate, a common pharmaceutical excipient, inactivates HIV-1 and blocks the coreceptor binding site on the virus envelope glycoprotein gp120. BMC Infect. Dis. 1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neurath, A. R., N. Strick, Y.-Y. Li, K. Lin, and S. Jiang. 1999. Design of a “microbicide” for prevention of sexually transmitted diseases using “inactive” pharmaceutical excipients. Biologicals 27:11-21. [DOI] [PubMed] [Google Scholar]
- 45.Nickoloff, B., and Y. Naidu. 1994. Perturbation of epidermal barrier function correlates with irritation of cytokine cascade in human skin. J. Am. Acad. Dermatol. 30:535-546. [DOI] [PubMed] [Google Scholar]
- 46.Nurnberg, W., M. Artuc, G. Vorbrueggen, F. Kalkbrenner, K. Moelling, B. M. Czarnetzki, and D. Schadendorf. 1995. Nuclear proto-oncogene products transactivate the human papillomavirus type 16 promoter. Br. J. Cancer 71:1018-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padian, N., S. Shiboski, S. Glass, and E. Vittinghoff. 1997. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results of a ten-year study. Am. J. Epidemiol. 146:350-357. [DOI] [PubMed] [Google Scholar]
- 48.Parkos, C. A., S. P. Colgan, and J. L. Madara. 1994. Interactions of neutrophils with epithelial cells: lessons from the intestine. J. Am. Soc. Nephrol. 5:138-152. [DOI] [PubMed] [Google Scholar]
- 49.Perkins, N. D. 1997. Achieving transcriptional specificity with NF-kB. Int. J. Biochem. Cell Biol. 29:1433-1448. [DOI] [PubMed] [Google Scholar]
- 50.Poli, G., A. L. Kinter, and A. S. Fauci. 1994. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proc. Natl. Acad. Sci. USA 91:108-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prince, A. M., B. Horowitz, L. Baker, R. W. Shulman, H. Ralph, J. Valinsky, A. Cundell, B. Brotman, W. Boehle, and F. Rey. 1988. Failure of a human immunodeficiency virus (HIV) immune globulin to protect chimpanzees against experimental challenge with HIV. Proc. Natl. Acad. Sci. USA 85:6944-6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prosch, S., K. Staak, J. Stein, C. Liebenthal, T. Stamminger, H. D. Volk, and D. H. Kruger. 1995. Stimulation of the human cytomegalovirus IE enhancer/promoter in HL-60 cells by TNFalpha is mediated via induction of NF-kappaB. Virology 208:197-206. [DOI] [PubMed] [Google Scholar]
- 53.Quinn, T. C. 1996. Association of sexually transmitted diseases and infection with the human immunodeficiency virus: biological cofactors and markers of behavioral interventions. Int. J. STD AIDS 7(Suppl. 2):17-24. [DOI] [PubMed] [Google Scholar]
- 54.Roos, T., T. R. Martin, J. T. Ruzinski, D. J. Leturcq, S. L. Hillier, D. L. Patton, and D. A. Eschenbach. 1997. Lipopolysaccharide binding protein and soluble CD14 receptor protein in amniotic fluid and cord blood in patients at term. Am. J. Obstet. Gynecol. 177:1230-1237. [DOI] [PubMed] [Google Scholar]
- 55.Saez, E., S. E. Rutberg, E. Mueller, H. Oppenheim, J. Smoluk, S. H. Yuspa, and B. M. Spiegelman. 1995. c-fos is required for malignant progression of skin tumors. Cell 82:721-732. [DOI] [PubMed] [Google Scholar]
- 56.Sanders, S. E., J. L. Madara, D. K. McGuirk, D. S. Gelman, and S. P. Colgan. 1995. Assessment of inflammatory events on epithelial permeability: rapid screening method using fluorescein dextrans. Epithelial Cell Biol. 4:25-34. [PubMed] [Google Scholar]
- 57.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25-34. [DOI] [PubMed] [Google Scholar]
- 58.Shaulian, E., and M. Karin. 2001. AP-1 in cell proliferation and survival. Oncogene 20:2390-2400. [DOI] [PubMed] [Google Scholar]
- 59.Stein, B., A. Baldwin, Jr., D. Ballard, W. Greene, P. Angel, and P. Herrlich. 1993. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 12:3879-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stephenson, J. 2000. Widely used spermicide may increase, not decrease, risk of HIV transmission. JAMA 284:949. [DOI] [PubMed] [Google Scholar]
- 61.Turpin, J. A. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Investig. Drugs 11:1077-1097. [DOI] [PubMed] [Google Scholar]
- 62.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiegne-Traore, C. Uaheowitchai, S. Karim, B. Masse, J. Perriens, M. Laga, and COL-1492 Study Group. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971-977. [DOI] [PubMed] [Google Scholar]
- 63.Van den Brink, G. R., F. J. ten Kate, C. Y. Ponsioen, M. M. Rive, G. N. Tytgat, S. J. H. van Deventer, and M. P. Peppelenbosch. 2000. Expression and activation of NF-κB in the antrum of the human stomach. J. Immunol. 164:3353-3359. [DOI] [PubMed] [Google Scholar]
- 64.Van Voorhis, W., L. Barrett, Y. Sweeney, C. Kuo, and D. Patton. 1997. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect. Immun. 65:2175-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wahl, C., S. Litay, G. Adler, and R. Schmid. 1998. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J. Clin. Investig. 101:1163-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wasserheit, J. N. 1992. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex. Transm. Dis. 19:61-77. [PubMed] [Google Scholar]
- 67.Zhang, H., G. Dornadula, M. Beumont, L. Livornese, B. Van Uitert, K. Henning, and R. J. Pomerantz. 1998. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N. Engl. J. Med. 339:1803-1809. [DOI] [PubMed] [Google Scholar]