Abstract
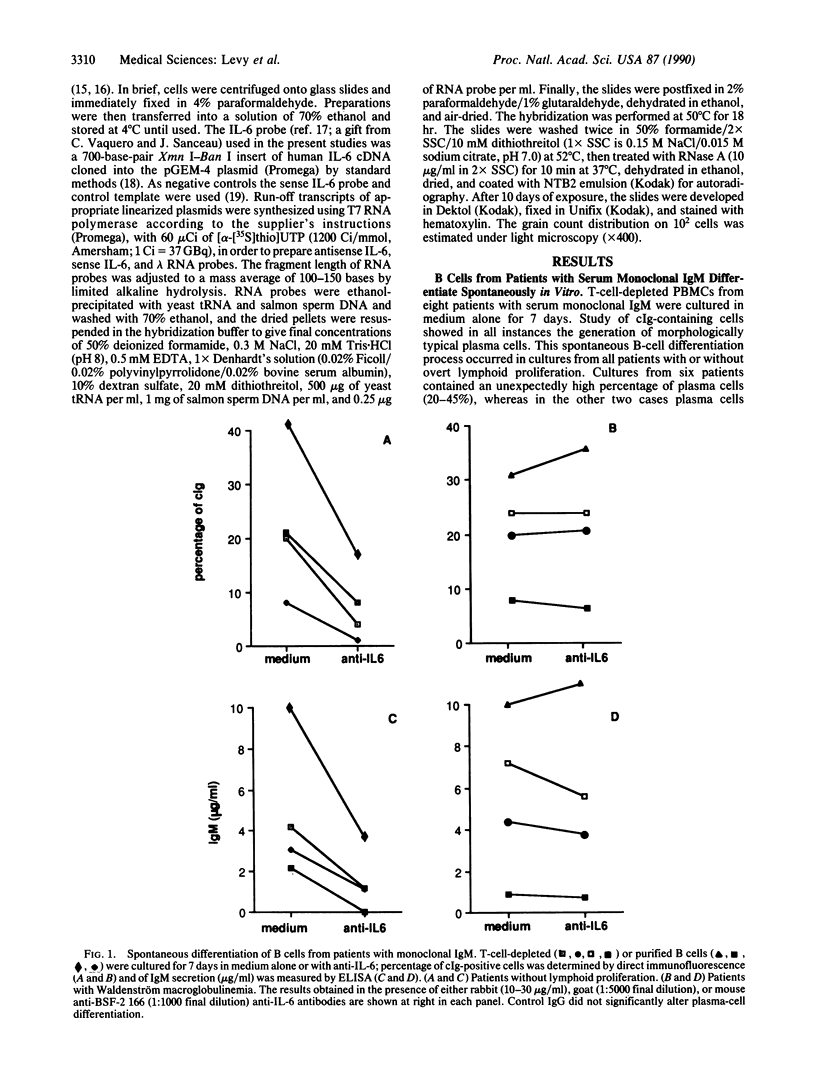
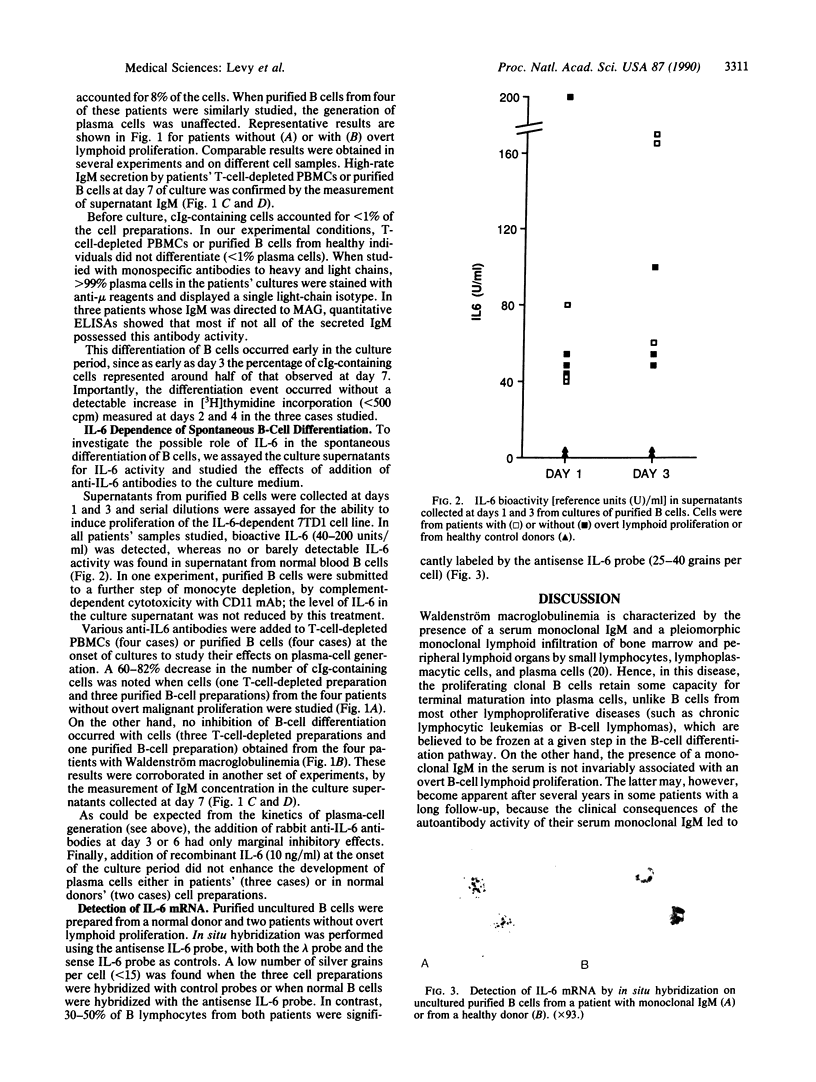
Blood B cells from eight patients with clonal lymphoid disorders characterized by monoclonal IgM secretion (four with malignant plasmacytic proliferation typical of Waldenström macroglobulinemia and four without overt lymphoid neoplasia) were found to spontaneously differentiate in vitro into plasma cells. In all instances, monoclonal plasma cells (8-45% of the cells) were generated from extensively purified B cells or T-cell-depleted peripheral blood mononuclear cells after a 7-day culture period, with a corresponding high rate of IgM secretion into the culture medium. This differentiation occurred in the absence of any cell proliferation process as measured by [3H]thymidine uptake at day 2 or 4. Normal B cells did not differentiate under the same experimental conditions. Detection of interleukin 6 (IL-6) bioactivity in all patients' B-cell culture supernatants as well as of IL-6 mRNA in freshly prepared, uncultured B cells in the two cases studied by in situ hybridization suggested that IL-6 secretion by B cells may play a role in this process. Moreover, in the four patients without overt lymphoid proliferation, B-cell differentiation was significantly inhibited (60-80%) in the presence of anti-IL-6 antibodies. In contrast, anti-IL-6 antibodies did not preclude the differentiation into plasma cells of B cells from the four patients with bona fide Waldenström macroglobulinemia. These results suggest a two-step pathogenesis for such human lymphoplasmacytic clonal proliferations, the initial stage being characterized by an IL-6-dependent autocrine differentiation pathway.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Cooper A. G., Hobbs J. R. Immunoglobulins in chronic cold haemagglutinin disease. Br J Haematol. 1970 Sep;19(3):383–396. doi: 10.1111/j.1365-2141.1970.tb01635.x. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Dellagi K., Dupouey P., Brouet J. C., Billecocq A., Gomez D., Clauvel J. P., Seligmann M. Waldenström's macroglobulinemia and peripheral neuropathy: a clinical and immunologic study of 25 patients. Blood. 1983 Aug;62(2):280–285. [PubMed] [Google Scholar]
- Freeman G. J., Freedman A. S., Rabinowe S. N., Segil J. M., Horowitz J., Rosen K., Whitman J. F., Nadler L. M. Interleukin 6 gene expression in normal and neoplastic B cells. J Clin Invest. 1989 May;83(5):1512–1518. doi: 10.1172/JCI114046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillot-Courvalin C., Labaume S., Brouet J. C. Differentiation by interleukin 2 of a subpopulation of human B cells. Scand J Immunol. 1986 Jun;23(6):679–684. doi: 10.1111/j.1365-3083.1986.tb02004.x. [DOI] [PubMed] [Google Scholar]
- Houssiau F. A., Coulie P. G., Olive D., Van Snick J. Synergistic activation of human T cells by interleukin 1 and interleukin 6. Eur J Immunol. 1988 Apr;18(4):653–656. doi: 10.1002/eji.1830180427. [DOI] [PubMed] [Google Scholar]
- Ikebuchi K., Wong G. G., Clark S. C., Ihle J. N., Hirai Y., Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9035–9039. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- Kucharska-Pulczynska M., Ellegaard J., Hokland P. Analysis of leucocyte differentiation antigens in blood and bone marrow from patients with Waldenström's macroglobulinaemia. Br J Haematol. 1987 Apr;65(4):395–399. doi: 10.1111/j.1365-2141.1987.tb04139.x. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Jirik F., Kabouridis P., Tsoukas C., Hirano T., Kishimoto T., Carson D. A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988 Mar 1;167(3):1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T., Hirano T., Kishimoto T. Establishment of an interleukin 6 (IL 6)/B cell stimulatory factor 2-dependent cell line and preparation of anti-IL 6 monoclonal antibodies. Eur J Immunol. 1988 Jun;18(6):951–956. doi: 10.1002/eji.1830180618. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Hirano T., Tang B., Matsuda T., Horii Y., Nakajima K., Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988 Feb 1;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro S., Debili N., Bernaudin J. F., Vainchenker W., Doly J. Regulation of the expression of IL-6 in human monocytes. J Immunol. 1989 Jun 15;142(12):4339–4345. [PubMed] [Google Scholar]
- Page N., Murray N., Perruisseau G., Steck A. J. A monoclonal anti-idiotypic antibody against a human monoclonal IgM with specificity for myelin-associated glycoprotein. J Immunol. 1985 May;134(5):3094–3099. [PubMed] [Google Scholar]
- Preud'homme J. L., Labaume S. Immunofluorescent staining of human lymphocytes for the detection of surface immunoglobulins. Ann N Y Acad Sci. 1975 Jun 30;254:254–261. doi: 10.1111/j.1749-6632.1975.tb29175.x. [DOI] [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Immunoglobulins on the surface of lymphoid cells in Waldenström's macroglobulinemia. J Clin Invest. 1972 Mar;51(3):701–705. doi: 10.1172/JCI106858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancéau J., Falcoff R., Zilberstein A., Béranger F., Lebeau J., Revel M., Vaquero C. Interferon-beta 2 (BSF-2) mRNA is expressed in human monocytes. J Interferon Res. 1988 Aug;8(4):473–481. doi: 10.1089/jir.1988.8.473. [DOI] [PubMed] [Google Scholar]
- Seligmann M., Brouet J. C. Antibody activity of human myeloma globulins. Semin Hematol. 1973 Apr;10(2):163–177. [PubMed] [Google Scholar]
- Steck A. J., Murray N., Dellagi K., Brouet J. C., Seligmann M. Peripheral neuropathy associated with monoclonal IgM autoantibody. Ann Neurol. 1987 Dec;22(6):764–767. doi: 10.1002/ana.410220614. [DOI] [PubMed] [Google Scholar]
- Takai Y., Wong G. G., Clark S. C., Burakoff S. J., Herrmann S. H. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J Immunol. 1988 Jan 15;140(2):508–512. [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. Modulation of human natural killer cell function by L-leucine methyl ester: monocyte-dependent depletion from human peripheral blood mononuclear cells. J Immunol. 1985 Feb;134(2):786–793. [PubMed] [Google Scholar]
- Van Snick J., Vink A., Cayphas S., Uyttenhove C. Interleukin-HP1, a T cell-derived hybridoma growth factor that supports the in vitro growth of murine plasmacytomas. J Exp Med. 1987 Mar 1;165(3):641–649. doi: 10.1084/jem.165.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Longo D. L., Leonard W. J., Depper J. M., Thompson C. B., Krönke M., Goldman C. K., Sharrow S., Bongiovanni K., Greene W. C. Interleukin 2 receptor (Tac antigen) expression in HTLV-I-associated adult T-cell leukemia. Cancer Res. 1985 Sep;45(9 Suppl):4559s–4562s. [PubMed] [Google Scholar]
- Wernet P., Feizi T., Kunkel H. G. Idiotypic determinants of immunoglobulin M detected on the surface of human lymphocytes by cytotoxicity assays. J Exp Med. 1972 Sep 1;136(3):650–655. doi: 10.1084/jem.136.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodoi J., Uchiyama T. IL-2 receptor dysfunction and adult T-cell leukemia. Immunol Rev. 1986 Aug;92:135–156. doi: 10.1111/j.1600-065x.1986.tb01498.x. [DOI] [PubMed] [Google Scholar]