Abstract
The in vitro activity of parthenolide against Leishmania amazonensis was investigated. Parthenolide is a sesquiterpene lactone purified from the hydroalcoholic extract of aerial parts of Tanacetum parthenium. This isolated compound was identified through spectral analyses by UV, infrared, 1H and 13C nuclear magnetic resonance imaging, DEPT (distortionless enhancement by polarization transfer), COSY (correlated spectroscopy), HMQC (heteronuclear multiple-quantum coherence), and electron spray ionization-mass spectrometry. Parthenolide showed significant activity against the promastigote form of L. amazonensis, with 50% inhibition of cell growth at a concentration of 0.37 μg/ml. For the intracellular amastigote form, parthenolide reduced by 50% the survival index of parasites in macrophages when it was used at 0.81 μg/ml. The purified compound showed no cytotoxic effects against J774G8 macrophages in culture and did not cause lysis in sheep blood when it was used at higher concentrations that inhibited promastigote forms. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis with gelatin as the substrate showed that the enzymatic activity of the enzyme cysteine protease increased following treatment of the promastigotes with the isolated compound. This finding was correlated with marked morphological changes induced by parthenolide, such as the appearance of structures similar to large lysosomes and intense exocytic activity in the region of the flagellar pocket, as seen by electron microscopy. These results provide new perspectives on the development of novel drugs with leishmanicidal activities obtained from natural products.
Leishmaniasis is a group of infectious diseases caused by organisms of the genus Leishmania and is a significant cause of morbidity and mortality in several countries. At present, leishmaniasis threatens 350 million people worldwide, and an estimated 1.5 million to 2 million new cases occur annually (47). The basic treatment for the disease consists of the administration of pentavalent antimonials that were developed more than 50 years ago; however, serious toxic effects and the emergence of resistance are limiting the drugs' usefulness (7, 9, 10). Amphotericin B and pentamidine, the traditional alternatives to antimonials used for the treatment of unresponsive cases, cause serious toxic effects (10, 24, 38). Furthermore, antifungal agents such as imidazole and triazole derivatives inhibit ergosterol biosynthesis and are effective against only some species of Leishmania (5, 6, 7, 22, 44).
The lack of an effective antileishmanial drug has caused a renewed interest in the study of medicinal plants as sources of new chemotherapeutic compounds with better activities and fewer side effects. Many people who live in areas where leishmaniasis is endemic rely on traditional medical systems for treatment. In most cases, the therapy consists of oral administration of plant extracts for the systemic forms of the disease and of topical preparations for the cutaneous forms of infection (25).
Members of the family Asteraceae (also referred to as the family Compositae), which display several kinds of biological activities, have been used for medicinal purposes for many centuries. Plants of the genus Tanacetum are reputed to have excellent medicinal value. The large number of sesquiterpenoids and sesquiterpene lactones that are typical constituents of these plants might be partly or wholly responsible for these effects (1).
The species Tanacetum parthenium, popularly known as feverfew, has been used in folk medicine for the treatment of migraines, tinnitus, giddiness, arthritis, fever, menstrual disorders, difficulty during labor, stomachaches, toothaches, and insect bites (32). Several studies have also reported that feverfew is effective as an herbal remedy for arthritis, pain, and migraine (23, 26, 31, 40, 46).
In the present study we undertook an examination of the potential antileishmanial activity of T. parthenium. A compound obtained from this species was identified and purified by bioassay-guided chemical fractionation. Its antiproliferative effects on Leishmania amazonensis and the ultrastructural changes that it produced in L. amazonensis were evaluated.
MATERIALS AND METHODS
Extraction and purification of the compound.
Parthenolide was isolated and purified as described previously (19, 21, 39), with slight modifications. The aerial parts of T. parthenium were kindly furnished by the Herbarium Laboratório Botānico Ltda (lot 166871; Colombo, Paraná, Brazil). Extraction was done after exhaustive maceration in ethanol-water (90:10) at room temperature in the dark. The extract was filtered, evaporated under vacuum, and lyophilized; and the residue (the hydroalcoholic extract) was directly assayed for its activity against L. amazonensis. Subsequently, the hydroalcoholic extract was chromatographed on a silica gel column with hexane, dichloromethane, ethyl acetate, methanol, and methanol-water (90:10). Each fraction was tested by antiprotozoal activity-guided fractionation. Next, the dichloromethane fraction was chromatographed on a silica gel column with different mixtures of solvents. The hexane-dichloromethane fraction resulted in isolation of a pure compound.
Structure elucidation.
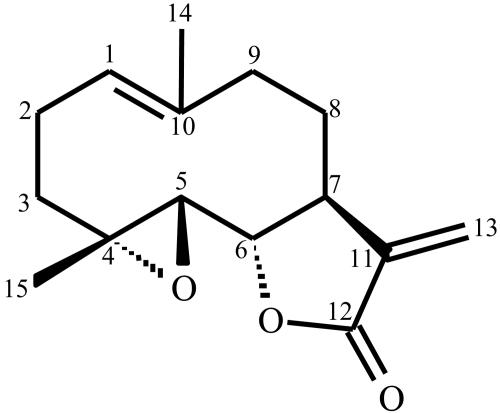
The structure of the isolated compound was identified by chromatography-mass spectrometry (Micromass Quattro LC); nuclear magnetic resonance (NMR; Gemini 2000 BB; Varian), 1H NMR (300 MHz), and 13C NMR (75.5 MHz) analyses in CDCl3; infrared analysis (Bomem-MV 100; Hartmann & Braun-Michelson); and UV analysis (CARY 1E UV-Vis; Varian). A structure corresponding to a sesquiterpene lactone, 4α,5β-epoxy-germacra-1-(10),11(13)-dien-12,6α-olide (parthenolide), was identified (Fig. 1).
FIG. 1.
Chemical structure of the sesquiterpene lactone 4α,5β-epoxy-germacra-1-(10),11-(13)-dien-12,6α-olide (parthenolide) isolated from T. parthenium.
Parasites.
The MHOM/BR/75/Josefa strain of L. amazonensis, originally isolated from a human case of diffuse cutaneous leishmaniasis by C. A. Cuba-Cuba (University of Brasília, Brasília, Distrito Federal, Brazil) was used in the present study. It was maintained at 28°C in Warren's medium (brain heart infusion plus hemin and folic acid) supplemented with 10% heat-inactivated fetal bovine serum in a tissue flask.
Cells.
J774G8 murine macrophages were maintained in tissue flasks in RPMI 1640 medium (Gibco Invitrogen Corporation, Grand Island, N.Y.) with sodium bicarbonate and l-glutamine and supplemented with 10% fetal bovine serum at 37°C in a 5% CO2-air mixture.
Antileishmanial activity.
Promastigote forms of L. amazonensis (106 parasites) were grown in a 24-well plate in Warren's medium supplemented with 10% inactivated fetal bovine serum and different concentrations of the hydroalcoholic extract; hexane, dichloromethane, ethyl acetate, methanol, or methanol-water (90:10) fractions; or pure isolated compound. The cell density for each treatment was determined daily in a hemocytometer (Improved Double Neubauer) with an optical microscope. In all tests, 0.5% dimethyl sulfoxide (DMSO; Sigma Chemical Co., St. Louis, Mo.), a concentration that was used to dissolve the highest dose of the compounds but that had no effect on cell proliferation, and medium alone were used as controls. Each experiment was performed twice on different occasions.
Antiamastigote activity.
In order to evaluate the effect of the isolated compound on intracellular amastigotes, 5 × 105 J774G8 macrophages were plated onto 13-mm coverslips in 24-well plates for 1 h at 37°C in a 5% CO2 atmosphere. Nonadherent cells were removed, and the macrophages were further incubated overnight in RPMI 1640 medium supplemented with 10% fetal bovine serum, as described above. Adherent cells were infected with L. amazonensis promastigotes (logarithmic growth phase) at a parasite/macrophage ratio of 10:1 and incubated for 1 h at 37°C in 5% CO2. Next, free promastigotes were removed by extensive washing with 0.01 M phosphate-buffered saline (pH 7.2). The infected macrophages were treated with the isolated compound at the concentrations indicated below. The cells were incubated; and after 24, 48, and 72 h, the coverslips were washed with 0.01 M phosphate-buffered saline and then fixed in Bouin's solution and stained with Giemsa. The macrophages were also treated with 0.5% DMSO only. At least 200 cells per experiment were inspected by bright-field microscopy. The survival index was calculated by multiplying the percentage of macrophages with internalized parasites and the mean number of internalized parasites per macrophage. These tests were performed in duplicate on separate occasions.
Cytotoxicity assay.
The cytotoxicity assay was carried out in 24-well plates. A suspension of 5 × 105 J774G8 cells was added to each well. The plates were incubated in a 5% CO2-air mixture at 37°C to promote confluent growth of the cells. Different concentrations of purified compound were added to each well containing the cells, and the plates were incubated for 48 h. Next, the cells were homogenized, equal volumes of cell suspension and 0.4% erythrosin B were mixed, and at least 200 cells were counted and evaluated by light microscopy.
A second experiment for evaluation of the cytotoxic effect was the red blood cell lysis assay. Briefly, a 4% suspension of freshly defibrinized sheep blood was prepared in sterile 5% glucose solution. Different concentrations of isolated compound were added to each test tube. The red blood cell suspension was then added, the contents were gently mixed, and the tubes were incubated at 37°C. The minimum lytic concentration is defined as the lowest concentration of a test compound that produces complete or partial lysis of erythrocytes. Amphotericin B was used as the reference drug. These tests were performed in duplicate on separate occasions.
Transmission electron microscopy.
After treatment with the isolated compound, the promastigotes of L. amazonensis were washed in 0.01 M phosphate-buffered saline and fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at 4°C. The cells were postfixed in a solution containing 1% osmium tetroxide and 0.8% potassium ferrocyanide in 0.1 M cacodylate buffer, washed in the same buffer, dehydrated in acetone, and embedded in Epon. Thin sections were stained with uranyl acetate and lead citrate and examined in a Zeiss 900 transmission electron microscope.
Cysteine protease activity.
Control and treated promastigote forms of L. amazonensis lysates were obtained, and cysteine protease activity was characterized by electrophoresis on a sodium dodecyl sulfate (SDS)-15% polyacrylamide gel containing 0.2% gelatin under nonreducing conditions. A protein molecular weight marker (7B; Sigma Chemical Co.) was used. The gels were washed twice for 30 min each time in 0.5 M sodium citrate (pH 5.6) containing 1% Triton X-100, 20 mM 2-mercaptoethanol in the presence or absence of 6 μM E-64 [transepoxysoccinil-l-leucylamido-(4-guanidino)butane; Sigma Chemical Co.] and then incubated for 20 h at 37°C in the same buffer containing 150 mM 2-mercaptoethanol in the presence or absence of 10 μM E-64. The gels were stained with Coomassie brilliant blue R as described previously (14).
RESULTS
Structure elucidation.
The aerial parts of T. parthenium yielded the pure compound parthenolide. 1H NMR (CDCl3, 300 MHz) δ 6.34 (d, J = 3.6 Hz, H-13α), 5.62 (d, J = 3.0 Hz, H-13β), 5.21 (dd, J = 2.7, 12.0 Hz, H-1), 3.86 (t, J = 8.4 Hz, H-6), 2.79 (d, J = 9.0 Hz, H-5), 2.74 to 2.82 (m, H-7), 2.32 to 2.44 (m, H-9β), 2.32 to 2.49 (m, H-2β), 2.11 to 2.21 (m, H-2α, H-3β, H-8α, H-9α), 1.72 (s, H-14), 1.70 to 1.77 (m, H-8β), 1.31 (s, H-15), 1.20 to 1.28 (m, H-3α); 13C NMR (CDCl3, 75.5 MHz) δ 169.3 (C-12), 139.2 (C-11), 134.6 (C-10); 125.3 (C-1), 121.3 (C-13), 82.4 (C-6), 66.4 (C-5), 61.5 (C-4), 47.7 (C-7), 41.2 (C-9), 36.3 (C-3), 30.6 (C-8), 24.1 (C-2), 17.3 (C-15), 16.9 (C-14).
Inhibition of promastigote growth.
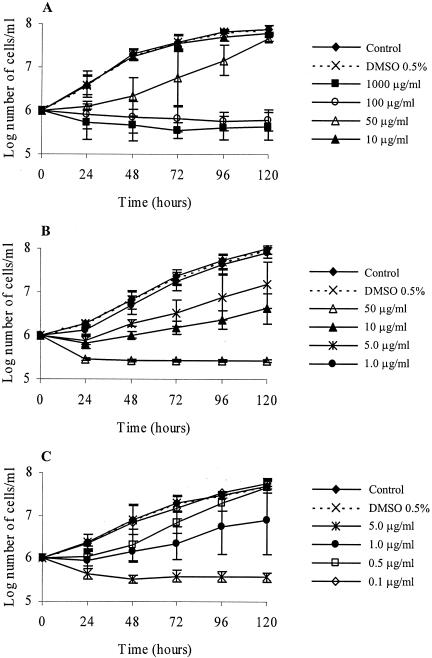
The hydroalcoholic crude extract obtained from aerial parts of T. parthenium was used in order to assay antileishmanial activity. The addition of 100 μg of crude extract per ml to a culture of L. amazonensis promastigote forms induced a rapid lytic effect, i.e., growth arrest and cell lysis after 24 h. The extract inhibited promastigote growth, with a 50% inhibitory concentration (IC50) of 29 μg/ml after 48 h of incubation. Partitioning of the crude extract of T. parthenium with organic solvents yielded five fractions, hexane, dichloromethane, ethyl acetate, methanol, and methanol-water (90:10), the effects of which were tested against the parasite. Of these fractions, the dichloromethane fraction showed the best antileishmanial effect, and cell lysis was observed after 24 h of treatment with a 50-μg/ml concentration of this fraction. The IC50 of this fraction was 3.6 μg/ml after 48 h of incubation. Antipromastigote activity-guided fractionation led to the purification of parthenolide, which induced partial lysis of the promastigotes 24 h after addition at 5 μg/ml. At a concentration of 1 μg/ml, it caused approximately 90% growth inhibition. Parthenolide had an IC50 of 0.37 μg/ml (Fig. 2).
FIG. 2.
Effects of hydroalcoholic extract (A), dichloromethane fraction (B), and parthenolide (C) on the growth of L. amazonensis promastigotes. The parasites were incubated in Warren's medium supplemented with 10% heat-inactivated fetal bovine serum at 28°C for 120 h. The drug was added to the cultures at 0 h, and the cells were counted daily. The bars indicate standard deviations.
Antiamastigote activity.
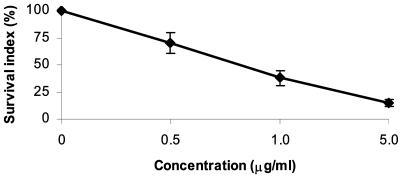
Parthenolide treatment of macrophages infected with amastigote forms showed that the compound influenced the growth of the parasites. After 72 h the percentage of macrophages with internalized parasites was higher for the control than for the macrophages infected and treated with the isolated compound. The mean number of internalized parasites per macrophage treated with parthenolide was markedly lower than that for the control. At 5 μg/ml, parthenolide reduced the internalization of parasites by macrophages by 84.7%, and at 1 μg/ml it reduced the internalization of parasites by 62%. Treatment of cells with 0.5 μg of parthenolide per ml inhibited parasite survival by approximately 29.8%. The survival index indicated that the isolated compound inhibited parasite growth in the macrophages. Thus, parthenolide showed good activity against amastigote forms, with 50% inhibition of cell survival at a concentration of 0.81 μg/ml (Fig. 3).
FIG. 3.
Effect of parthenolide on L. amazonensis-macrophage interaction. J774G8 macrophages were infected with promastigotes of L. amazonensis and then treated with 5, 1, and 0.5 μg of parthenolide per ml. After 72 h the SI (in percent) was calculated by the equation (P2/P1) × 100, where P1 is the survival index for the control and P2 is the survival index for the treated cells. SI was calculated by multiplying the percentage of macrophages with internalized parasites and the mean number of internalized parasites per macrophage. Each bar represents 1 standard deviation.
Cytotoxicity assay.
J774G8 murine macrophages were treated with the pure isolated compound to test the safety of this substance for mammalian cells. After 48 h the viability was checked by the erythrosin B dye exclusion test. When macrophages were treated with parthenolide, the 50% cytotoxic concentration (CC50) was 14 μg/ml. The toxicity for J774G8 macrophages and the activity against the protozoans were compared by using the selectivity index (SI) ratio (CC50 for J774G8 cells/IC50 for protozoans). A value greater than 1 is considered more selective for activity against parasites, and a value less than 1 is considered more selective for activity against cells. The parthenolide was more selective against the parasites than the mammalian cells, with an SI ratio of 37.8.
In the experiment used to evaluate cytotoxicity for red blood cells, amphotericin B showed strong hemolytic activity, with 50% lysis within 60 min of incubation when it was used at 25 μg/ml. Treatment of the positive control with Triton X-114 showed a strong hemolytic effect, with 100% lysis after 60 min, whereas 0.5% DMSO did not cause lysis. The highest concentrations of parthenolide tested (100 μg/ml) caused no hemolysis.
Transmission electron microscopy.
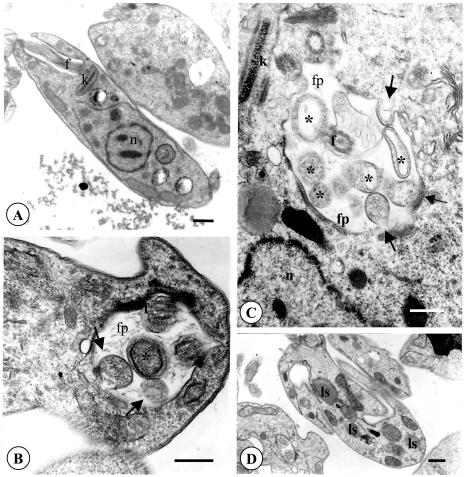
In order to determine the ultrastructural changes to L. amazonensis promastigotes induced by parthenolide, the parasites were treated with 1 μg of purified compound per ml and were processed for routine electron microscopy. Control cells incubated in the presence of 0.05% DMSO, the same concentration used in the final solutions of the test compound, showed no morphological differences compared to the morphologies of the untreated controls (Fig. 4A). Parasites incubated in the presence of parthenolide showed significant morphological alterations. Among these alterations was the appearance of intense exocytic activity in the region of the flagellar pocket, which appeared in the form of protrusions of the cell body toward the flagellar pocket and concentric membranes within the pocket (Fig. 4B and C). Structures similar to large lysosomes were also observed in the cytoplasm (Fig. 4D).
FIG. 4.
Ultrastructural effects of parthenolide on promastigote forms of L. amazonensis. The parasites were incubated with DMSO or medium alone (A) or with 1 μg of parthenolide/ml (the IC90) (B to D) for 72 h. (A) Section showing the normal aspect of the nucleus, the flagellum in the flagellar pocket, and the mitochondrion containing the kinetoplast. (B and C) Promastigote showing intense exocytic activity. The arrows indicate the protusions of the cell body toward the flagellar pocket; the asterisks indicate the vesicles located in the flagellar pocket. (D) The promastigotes also showed some structures similar to large lysosomes in the cytoplasm. fp, flagellar pocket; f, flagellum; k, kinetoplast; n, nucleus; ls, lysosome. Bars, 1 μm.
Effects of parthenolide on cysteine protease activities of promastigote forms.
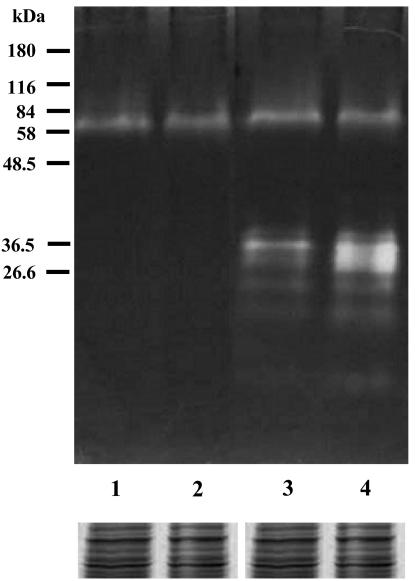
Both the control and the parthenolide-treated promastigotes showed the same protein profile by SDS-polyacrylamide gel electrophoresis (PAGE) (data not shown). To verify the presence of cysteine protease activity, cell homogenates were resolved on polyacrylamide gels containing gelatin under nonreducing conditions. After incubation under conditions suitable for protease activity, staining of the gels with Coomassie blue revealed high levels of enzymatic activity at ∼27 and 45 kDa, and this activity was inhibited by E-64, a well-known inhibitor of cysteine proteases. Protein bands at ∼27 kDa for the control promastigotes of L. amazonensis increased in intensity during treatment of the cells with parthenolide, indicating an increase in enzyme activity due to treatment with this compound (Fig. 5).
FIG. 5.
Cysteine protease activity of L. amazonensis promastigotes in gelatin SDS-polyacrylamide gels. Lanes 1 and 3, untreated cells; lanes 2 and 4, cells treated with 1 μg of parthenolide per ml; lanes 1 and 2, the gel incubated in the presence of E-64, a cysteine protease inhibitor; lanes 3 and 4, the gel incubated in the absence of E-64. The gels were loaded with 30 μg of parasite protein extract per slot. Molecular mass markers are shown at the left. The protein profiles obtained by SDS-PAGE and Coomassie blue staining of control and parthenolide-treated promastigotes are shown as the loading control.
DISCUSSION
Diseases caused by protozoans are responsible for considerable morbidity and mortality throughout the world, but predominantly in the tropics and subtropics. Present treatment regimens for these diseases have severe limitations, and new drugs are urgently required. In this regard, natural products have made and are continuing to make important contributions to this area of therapeutics.
Here we report for the first time a novel pharmacological activity in the extract of T. parthenium, which displayed activity against L. amazonensis in vitro. The hydroalcoholic extract of this plant inhibited promastigote growth with an IC50 of 29 μg/ml, leading us to carry out a bioassay-guided fractionation of the antileishmanial activity. The dichloromethane fraction showed a greater inhibitory effect than the hydroalcoholic extract, with the IC50 of the dichloromethane fraction being 3.6 μg/ml.
An active antileishmanial sesquiterpene lactone was purified by bioactivity-guided fractionation of the aerial parts of T. parthenium, which was identified by chemical analysis as parthenolide. Parthenolide has previously been isolated and characterized from different species of Ambrosia (19, 48), Magnolia (13, 18, 39), and other species of the genus Tanacetum (3, 21). In the study reported here, this compound had an IC50 of 0.37 μg/ml and inhibited parasite growth in macrophages. Sesquiterpene lactones are terpenoid compounds characteristic of the family Asteraceae. A range of biological effects has been ascribed to these compounds, such as cytotoxic, antitumorigenic, antibacterial, antifungal, antiprotozoal, insecticidal, antiulcer, cardiotonic, and antimigraine activities (33, 36).
In preceding studies, Fischer et al. (17) showed that parthenolide has activity against Mycobacterium tuberculosis and M. avium, with MICs of 16 and 64 μg/ml, respectively. Parthenolide also inhibited two tumor cell lines in a concentration-dependent manner, and the effect may have been either cytotoxic or cytostatic (37). Several studies have demonstrated the anti-inflammatory activity of parthenolide (26, 27, 29). It has been demonstrated that parthenolide causes dose-dependent inhibition of the production of tromboxane B2 and leukotriene B4 (40) and binds directly to and inhibits IκB kinase β, the kinase subunit known to play a critical role in cytokine-mediated signaling (29), providing a possible molecular basis for the anti-inflammatory properties. This compound inhibits interleukin-12 production in lipopolysaccharide-stimulated macrophages (27) and could inhibit the expression of intercellular adhesion molecule-1 induced by the cytokines interleukin-1, tumor necrosis factor alpha, and, less strongly, gamma interferon (34). The modulation of molecular expression of these events may be an additional mechanism by which feverfew mediates anti-inflammatory effects.
Recently, plant compounds with widely different chemical structures that show antileishmanial activities have been isolated: ancistrolikokine D from Ancistrocladus likoko (8), coronaridine from Peschiera australis (11), 3-heptadecyl-5-metoxyphenol from Oxalis erythrorhiza (15), canthin-6-one and 5-methoxy-canthin-6-one from Zanthoxylum chiloperone (16), maesabalide from Maesa balansae (20), γ-pirones from Podolepsis hieracioides (28), and 2′,6′-dihydroxy-4′-methoxychalcone from Piper aduncum (41). This demonstrates that medicinal plants hold promise as sources of chemical leads for the development of novel therapeutic agents in the fight against leishmaniasis.
Selectivity assays showed that the action of the isolated compound is specific for the protozoans and is not toxic for J774G8 macrophages. Parthenolide showed no hemolytic effect at a concentration of 100 μg/ml (a concentration 270 times greater than the IC50 for promastigotes). Cytotoxicity tests with natural products are important, given the strong interest in alternative therapies and the therapeutic use of medicinal plants. There are several reasons for the interest in developing drugs of plant origin; the main one is that conventional medical treatments can be inefficient or can result in side effects and ineffective therapy. Given that a large percentage of the world's population has no access to conventional pharmacological treatments, the widespread use of folk medicine and ecological awareness suggest that natural products are harmless (35). However, even though these products have been traditionally used, this is no guarantee of their safety (12), and their efficacy and safety require investigation.
We observed that cysteine protease activity increased in promastigotes treated with 1 μg of parthenolide per ml. It seems that there exists a correlation between the presence of megasomes and higher levels of protease activity in Leishmania (42). It was also reported that the membrane-bounded structures found in promastigotes of L. amazonensis may represent megasome precursors (43). Recent studies have shown that metacyclic promastigotes of L. chagasi have a multivesicular tubule-lysosome structure (2) which contains resident cysteine proteases and which is the site of protein degradation (45). On the other hand, it is known that lysosomal hydrolases are synthesized in the endoplasmic reticulum, and unprocessed proteases accumulate in the Golgi apparatus or the flagellar pocket. Transport of proteins to the lysosomes may occur via two routes: the direct intracellular pathway from the Golgi apparatus to the lysosomes, and the indirect pathway, in which proteins are first transported to the flagellar pocket before they are transported to the lysosome (30). Therefore, we suggest that the intense exocytic activity observed in the region of the flagellar pocket in promastigotes treated with parthenolide may indicate a process of exacerbated protein production by cells as they attempt to survive.
Plants have further potential as sources of therapeutic agents in the search for new and selective agents for the treatment of important tropical diseases caused by protozoans. In recent years, natural products of different biosynthetic origins and of several structural groups have been isolated and have been shown to display activities against different strains of Leishmania (4, 8, 11, 15, 20, 28, 41). In conclusion, the results of the present study show that natural products represent an unparalleled source of molecular diversity in drug discovery and the development of novel antiprotozoal agents. The compound isolated from T. parthenium, which displays leishmanicidal activity in vitro, may be a potential candidate drug for the treatment of this disease.
Acknowledgments
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, Capacitação de Aperfeiçoamento de Pessoal de Nível Superior, Capes, Fundação Araucária, Fundação Ary Frauzino para Pesquisa e Controle do Cāncer, and Programa de Pós-graduação em Ciências Farmacêuticas, Universidade Estadual de Maringá.
REFERENCES
- 1.Abad, M. J., P. Bermejo, and A. Villar. 1995. An approach to the genus Tanacetum L. (Compositae): phytochemical and pharmacological review. Phytother. Res. 9:79-92. [Google Scholar]
- 2.Alberio, S. O., S. S. Dias, F. P. Faria, R. A. Mortara, C. L. Barbiéri, and E. F. Haapalainen. 2004. Ultrastructural and cytochemical identification of megasome in Leishmania (Leishmania) chagasi. Parasitol. Res. 92:246-254. [DOI] [PubMed] [Google Scholar]
- 3.Aljancic, I., V. Vajs, V. Bulatovic, N. Menkovic, and S. Milosavljevic. 2001. Parthenolide from aerial parts of Tanacetum larvatum. Biochem. Syst. Ecol. 29:655-657. [DOI] [PubMed] [Google Scholar]
- 4.Araújo, C. A. C., L. V. Alegrio, and L. L. Leon. 1998. Antileishmanial activity of compounds extracted and characterized from Centrolobium sclerophyllum. Phytochemistry 49:751-754. [Google Scholar]
- 5.Beach, D. H., L. J. Goad, and G. G. Holz, Jr. 1988. Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Mol. Biochem. Parasitol. 31:149-162. [DOI] [PubMed] [Google Scholar]
- 6.Berman, J. D. 1996. Treatment of New World cutaneous and mucosal leishmaniasis. Clin. Dermatol. 14:519-522. [DOI] [PubMed] [Google Scholar]
- 7.Berman, J. D. 1997. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 24:684-703. [DOI] [PubMed] [Google Scholar]
- 8.Bringmann, G., W. Saeb, M. Rückert, J. Mies, M. Michel, V. Mudogo, and R. Brun. 2003. Ancistrolikokine D, a 5,8′-coupled naphthylisoquinoline alkaloid, and related natural products from Ancistrocladus likoko. Phytochemisty 62:631-636. [DOI] [PubMed] [Google Scholar]
- 9.Croft, S. L. 1988. Recent developments in the chemotherapy of leishmaniasis. Trends Pharmacol. Sci. 9:376-381. [DOI] [PubMed] [Google Scholar]
- 10.Croft, S. L., and G. H. Coombs. 2003. Leishmaniasis—current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19:502-508. [DOI] [PubMed] [Google Scholar]
- 11.Delorenzi, J. C., M. Attias, C. R. Gattass, M. Andrade, C. Rezende, A. C. Pinto, A. T. Henriques, D. C. Bou-Habib, and E. M. B. Saraiva. 2001. Antileishmanial activity of indole alkaloid from Peschiera australis. Antimicrob. Agents Chemother. 45:1349-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edzard, E. 1998. Harmless herbs? A review of the recent literature. Am. J. Med. 104:170-178. [DOI] [PubMed] [Google Scholar]
- 13.El-Feraly, F. S., Y. M. Chan, and G. A. Capiton. 1979. Isolation and characterization of peroxycostunolide (Verlotorin) and peroxyparthenolide from Magnolia grandiflora. Carbon-13 nuclear magnetic resonance spectroscopy of costunolide and related compounds. J. Org. Chem. 44:3952-3955. [Google Scholar]
- 14.Fairbanks, G., T. L. Steck, and D. F. H. Wallach. 1971. Eletrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10:2606-2617. [DOI] [PubMed] [Google Scholar]
- 15.Feresin, G. E., A. Tapia, M. Sortino, S. Zacchino, A. R. Arias, A. Inchausti, G. Yaluff, J. Rodriguez, C. Theoduloz, and G. Schmeda-Hirschmann. 2003. Bioactive alkyl phenols and embelin from Oxalis erythrorhiza. J. Ethnopharmacol. 88:241-247. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira, M. E., A. R. Arias, S. T. Ortiz, A. Inchausti, H. Nakayama, C. Thouvenel, R. Hocquemiller, and A. Fournet. 2002. Leishmanicidal activity of two canthin-6-one alkaloids, two major constituents of Zanthoxylum chiloperone var. angustifolium. J. Ethnopharmacol. 80:199-202. [DOI] [PubMed] [Google Scholar]
- 17.Fischer, N. H., J. D. Weidenhamer, J. L. Riopel, L. Quijano, and M. A. Menelaou. 1990. Stimulation of witchweed germination by sesquiterpene lactones: a structure-activity study. Phytochemistry 29:2479-2483. [Google Scholar]
- 18.Fischer, N. H., T. Lu, C. L. Cantrell, J. Castañeda-Acosta, L. Quijano, and S. G. Franzblau. 1998. Antimycobacterial evaluation of germacrolides. Phytochemisty 49:559-564. [DOI] [PubMed] [Google Scholar]
- 19.Geissman, T. A., and S. Matsueda. 1968. Sesquiterpene lactones. Constituents of diploid and polyploid Ambrosia dumosa Gray. Phytochemistry 7:1613-1621. [Google Scholar]
- 20.Germonprez, N., L. V. Puyvelde, L. Maes, M. V. Tri, and N. D. Kimpe. 2004. New pentacyclic triterpene saponins with strong anti-leishmanial activity from the leaves of Maesa balansae. Tetrahedron 60:219-228. [Google Scholar]
- 21.Gören, N., and E. Tahtasakal. 1997. Sesquiterpenoids from Tanacetum argenteum subsp. canum var. canum. Phytochemistry 45:107-109. [DOI] [PubMed] [Google Scholar]
- 22.Hart, D. T., W. J. Lauwers, G. Willemsens, H. V. Bossche, and F. R. Opperdoes. 1989. Perturbation of sterol biosynthesis by itraconazole and ketoconazole in Leishmania mexicana mexicana infected macrophages. Mol. Biochem. Parasitol. 33:123-134. [DOI] [PubMed] [Google Scholar]
- 23.Heptinstall, S., L. Williamson, A. White, and J. R. A. Mitchell. 1985. Extracts of feverfew inhibit granule secretion in blood platelets and polymorphonuclear leucocytes. Lancet N. Am. Ed. 325:1071-1074. [DOI] [PubMed] [Google Scholar]
- 24.Herwaldt, B. L. 1999. Leishmaniasis. Lancet N. Am. Ed. 354:1191-1199. [DOI] [PubMed] [Google Scholar]
- 25.Iwu, M. M., J. E. Jackson, and B. G. Schuster. 1994. Medicinal plants in the fight against leishmaniasis. Parasitol. Today 10:65-68. [DOI] [PubMed] [Google Scholar]
- 26.Jain, N. K., and S. K. Kulkarni. 1999. Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J. Ethnopharmacol. 68:251-259. [DOI] [PubMed] [Google Scholar]
- 27.Kang, B. Y., S. W. Chung, and T. S. Kim. 2001. Inhibition of interleukin-12 production in lipopolysaccharide-activated mouse macrophages by parthenolide, a predominant sesquiterpene lactone in Tanacetum parthenium: involvement of nuclear factor-κB. Immunol. Lett. 77:159-163. [DOI] [PubMed] [Google Scholar]
- 28.Kayser, O., A. F. Kiderlen, and S. L. Croft. 2003. Antileishmanial activity of two γ-pyrones from Podolepsis hieracioides (Asteraceae). Acta Trop. 86:105-107. [DOI] [PubMed] [Google Scholar]
- 29.Kwok, B. H. B., B. Koh, M. I. Ndubuisi, M. Elofsson, and C. M. Crews. 2001. The anti-inflammatory natural product parthenolide from the medicinal herb feverfew directly binds to and inhibits IκB kinase. Chem. Biol. 8:759-766. [DOI] [PubMed] [Google Scholar]
- 30.McConville, M. J., K. A. Mullin, S. C. Ilgoutz, and R. D. Teasdale. 2002. Secretory pathway of trypanosomatid parasites. Microbiol. Mol. Biol. Rev. 66:122-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy, J. J., S. Heptinstall, and J. R. S. Mittchel. 1988. Randomised double-bind placebo-controlled trial of feverfew in migraine prevention. Lancet N. Am. Ed. 332:189-192. [DOI] [PubMed] [Google Scholar]
- 32.Newall, C. A., L. A. Anderson, and J. D. Phillipson. 2002. Matricária, p. 191-193. In Plantas medicinais: guia para profissional de saúde. Premier, São Paulo, Brazil.
- 33.Picman, A. K. 1986. Biological activities of sesquiterpene lactones. Biochem. Syst. Ecol. 14:255-281. [Google Scholar]
- 34.Piela-Smith, T. H., and X. Liu. 2001. Feverfew and the sesquiterpene lactone parthenolide inhibit intercellular adhesion molecule-1 expression in human synovial fibroblasts. Cell. Immunol. 209:89-96. [DOI] [PubMed] [Google Scholar]
- 35.Rates, S. M. K. 2001. Plants as source of drugs. Toxicon 39:603-613. [DOI] [PubMed] [Google Scholar]
- 36.Robles, M., M. Aregullin, J. West, and E. Rodriguez. 1995. Recent studies on the zoopharmacognosy, pharmacology and neurotoxicology of sesquiterpene lactones. Planta Med. 61:199-203. [DOI] [PubMed] [Google Scholar]
- 37.Ross, J. J., J. T. Arnason, and H. C. Birnboim. 1999. Low concentration of the feverfew component parthenolide inhibit in vitro growth of tumor lines in a cytostatic fashion. Planta Med. 65:126-129. [DOI] [PubMed] [Google Scholar]
- 38.Sereno, D., P. Holzmuller, and J. L. Lemesre. 2000. Efficacy of second line drugs on antimonyl-resistant amastigotes of Leishmania infantum. Acta Trop. 74:25-31. [DOI] [PubMed] [Google Scholar]
- 39.Song, Q., M. L. Gomez-Barrios, F. R. Fronczek, D. Vargas, L. B. Thien, and N. H. Fischer. 1998. Sesquiterpenes from southern Magnolia virginiana. Phytochemistry 47:221-226. [Google Scholar]
- 40.Sumner, H., U. Salan, D. W. Knight, and J. R. S. Hoult. 1992. Inhibition of 5-lipoxigenase and cyclo-oxigenase in leukocytes by feverfew. Involvement of sesquiterpene lactones and other components. Biochem. Pharmacol. 43:2313-2320. [DOI] [PubMed] [Google Scholar]
- 41.Torres-Santos, E. C., D. L. Moreira, M. A. C. Kaplan, M. N. Meirelles, and B. Rossi-Bergmann. 1999. Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob. Agents Chemother. 43:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Traub-Cseko, Y. M., R. W. Almeida, L. K. Boukai, D. Costa-Pinto, S. M. Duboise, and D. McMahon-Prati. 1993. Cysteine proteinases of Leishmania. Cien. Cult. 45:339-342. [Google Scholar]
- 43.Ueda-Nakamura, T., M. Attias, and W. Souza. 2001. Megasome biogenesis in Leishmania amazonensis: a morphometric and cytochemical study. Parasitol. Res. 87:89-97. [DOI] [PubMed] [Google Scholar]
- 44.Vannier-Santos, M. A., J. A. Urbina, A. Martiny, A. Neves, and W. Souza. 1995. Alterations induced by the antifungal conpounds ketoconazole and terbinafine in Leishmania. J. Eukaryot. Microbiol. 42:337-346. [DOI] [PubMed] [Google Scholar]
- 45.Waller, R. F., and M. J. McConville. 2002. Developmental changes in lysosome morphology and function of Leishmania parasites. Int. J. Parasitol. 32:1435-1445. [DOI] [PubMed] [Google Scholar]
- 46.Williams, C. A., J. B. Harborne, H. Geiger, and J. R. S. Hoult. 1999. The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflamatory properties. Phytochemistry 51:417-423. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. 2001. Leishmaniasis: the disease and its impact. [Online.] http://www.who.int/emc/diseases/leish/leisdis1.html. Accessed 14 April 2003.
- 48.Yoshioka, H., W. Renold, N. H. Fischer, A. Higo, and T. J. Mabry. 1970. Sesquiterpene lactones from Ambrosia confertiflora (Compositae). Phytochemistry 9:823-832. [Google Scholar]