Abstract
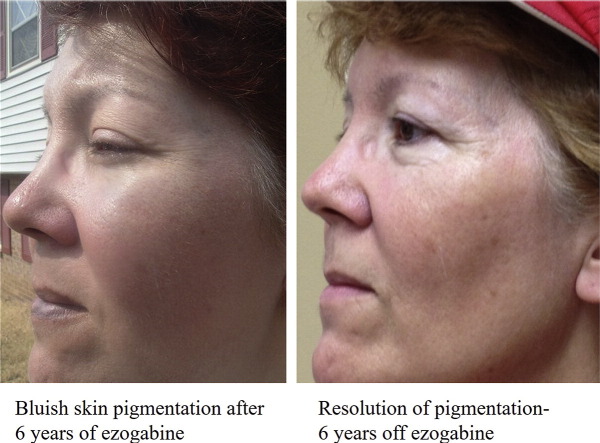
There is concern that bluish skin discoloration associated with ezogabine treatment could be permanent. We present a case of ezogabine-induced skin discoloration that resolved completely after discontinuation. A 55-year-old woman started ezogabine 400 mg three times a day at age 41. Bluish pigmentation over the toe nails, finger nails, around eyes and over and around lips was first noted after 5 years of treatment. Ezogabine was discontinued eight years after initiation. Skin discoloration improved within 6 months and completely resolved within 6 years of discontinuation. This case suggests that ezogabine-induced discoloration is reversible after discontinuation of treatment.
Keywords: Ezogabine, Retigabine, Anti-seizure drug, Side effect, Skin discoloration, Reversibility
Graphical abstract
1. Introduction
Ezogabine (EZG; North American adopted name), also known as retigabine (RTG; international non-proprietary name), is an anti-seizure drug (ASD) that was approved as adjunctive treatment for focal-onset seizures in March 2011 in Europe, and in June 2011 in the United States. EZG/RTG is a neuronal potassium channel opener with a primary mechanism of action as a positive allosteric modulator of KCNQ2-5 (Kv7.2–7.5) ion channels thereby causing hyperpolarization and reduced firing of high-frequency action potentials [1]. In vitro studies have also suggested that EZG/RTG enhanced inhibitory effects of gamma-aminobutyric acid (GABA) by modulating GABA A receptors [1]. The efficacy of EZG/RTG (600, 900 and 1200 mg/day) as adjunctive therapy in patients with focal-onset seizures has been demonstrated in three randomized, double-blind, placebo-controlled trials [2], [3], [4].
The most frequently reported drug-related adverse reactions, reported by more than 10% of patients, in the regulatory placebo-controlled trials were nonspecific dizziness, somnolence, and fatigue at 600 and 900 mg per day [2], as well as confusion, dysarthria, ataxia, blurred vision, tremor and nausea at 1200 mg per day [3]. EZG/RTG also had relatively unique adverse effects, such as urinary retention, presumed related to an effect on potassium channels in bladder smooth muscle [5], and abnormal cutaneous pigmentation. The latter was reported in an FDA-issued warning in 2013, stating that EZG/RTG can cause retinal pigment changes and blue discoloration of the skin [6]. The warning did not provide information on the natural course of this pigmentation after discontinuation of EZG/RTG, and little is known about the reversibility of skin pigmentation.
We present a case of EZG/RTG induced skin discoloration that resolved almost completely after discontinuation of EZG/RTG.
2. Case report
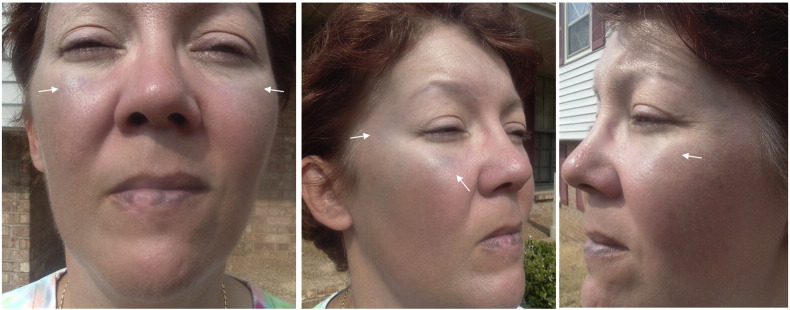
A 55-year-old female developed focal seizures at 21 years of age. Early seizures consisted of a blank stare, confusion, then wandering about. They recurred approximately four times per week for one year, remitted for 6 years, and then relapsed. She described an aura of warmth in the chest which could progress to partial loss of awareness, blank stare, drooling, lip smacking, and loss of posture with falling. She averaged three seizures per month despite taking carbamazepine and pregabalin. At age 41 she was enrolled into a blinded EZG/RTG clinical trial and the study drug was started in June 2001. She had significant improvement in seizure frequency decreasing seizures to one focal seizure without impaired consciousness every 8 weeks. Open label treatment with EZG/RTG 400 mg three times daily was started in August 2001. Seizures became gradually more frequent, and the dose of EZG/RTG was increased by 200 mg in April 2002, to 400 mg in the am, 400 mg at noon, and 600 mg in the evening. She reported peak adverse effects of drowsiness and dizziness one hour after her doses. In January 2004 she asked to reduce her dose back to 400 mg three times daily due to these adverse effects. During her clinic visit in September 2006, she reported bluish discoloration of her toenails and fingernails, as well as skin around her eyes, over her temples, and over and around her lips. She documented her facial pigmentation with photos in 2007 (Fig. 1). Since bluish discoloration was not reported with EZG/RTG use at that time, she was asked to consult a dermatologist. She had a punch biopsy from her right temple in May 2007, which revealed minimal perivascular lymphoplasmacytic infiltration and subtle particulate melanin deposits around the same vessels. It was then suspected that EZG/RTG was responsible for the pigmentation and the patient was offered to be taken off the medication, but chose to continue treatment due to improvement in seizure frequency. The dose of EZG/RTG was reduced by 200 mg in July 2007 and then by another 200 mg in February 2008. Because seizures continued, she was switched from carbamazepine to lamotrigine, and levetiracetam was added in October 2008, after which she became seizure-free. A taper of EZG/RTG was initiated in August 2009 and she stopped the medication in October 2009.
Fig. 1.
Pigmentation around eyes and over and around lips on photographs obtained 6 years after initiating ezogabine.
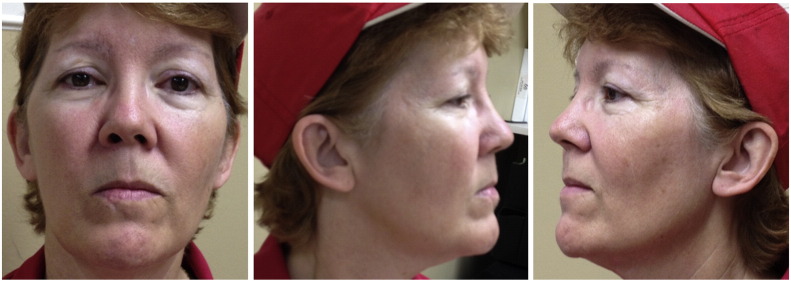
She reported gradual improvement in the skin bluish discoloration in April 2010. There was considerable improvement in the pigmentation by March 2011, and near complete resolution of the pigmentation in 2013. This resolution was complete by 2015 (Fig. 2).
Fig. 2.
Photographs obtained around 6 years after stopping ezogabine, showing resolution of pigmentation.
3. Discussion
Discoloration of the skin, nails, mucosa and retinal pigmentary abnormalities as an adverse effect of EZG/RTG have been described in the literature since 2014. The first report was of two patients with blue-gray skin dyspigmentation that was most pronounced on the face and lips [7]. There was also pigmentation on the nails, blue pigmentation on the hard palate, and black pigment deposits on the conjunctiva. Histopathologically, the main finding was that dermal cells were heavily laden with coarse melanin granules, mainly in peri-vascular and peri-eccrine distribution. Biopsy of the hard palate showed coarse pigment in the submucosa, inside macrophages as well as in the extracellular matrix, positive for melanin [7]. Another case report that followed described bluish-purple discoloration of the fingernails and hard palate after 2 years of treatment with EZG/RTG [8]. Our patient first reported bluish pigmentation after 5 years of treatment. Pigmentation has been reported in the retina and other mucosal membranes, including urinary bladder [6], [9]. Experimental studies on albino and pigmented rats suggest that the cause of skin discoloration is accumulation of free and/or melanin-bound pigment dimers of EZG/RTG and its N-acetyl metabolite [9]. The pigmentation changes seem to be time and dose-dependent. The most updated prescribing information reported approximately 10% of patients in long-term clinical trials developed skin discoloration, generally after 2 or more years of treatment and at higher doses of 900 mg or more per day [10]. However, a study available only in abstract form indicated that 108 out of 365 (30%) subjects examined had discoloration of lips, nails, skin, or mucosa, with a median time to onset of 4.4 years [11].
The FDA safety report suggested that skin discoloration may become permanent [12]. However, there were no data to support the notion that the discoloration is permanent. The first report on skin discoloration described improvement in skin, oral mucosa and nail discoloration four months after stopping EZG/RTG in one patient. There have not been further published reports on reversibility of the skin discoloration. Our subject demonstrates that skin discoloration caused by EZG/RTG is reversible following discontinuation of this medication. Improvement was noted in our patient within 6 months, with eventual total resolution within 6 years. While these findings are encouraging, they will have to be confirmed with additional observations in patients who have stopped EZG/RTG after developing skin pigmentation.
Conflicts of interest
none.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
We thank the patient for permission to use her photographs in publication for educational purposes.
Contributor Information
Sally V. Mathias, Email: sallyvmathias@yahoo.com.
Bassel W. Abou-Khalil, Email: bassel.abou-khalil@Vanderbilt.Edu.
References
- 1.Gunthorpe M.J., Large C.H., Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K + channel opener for the treatment of epilepsy. Epilepsia. 2012;53:412–424. doi: 10.1111/j.1528-1167.2011.03365.x. [DOI] [PubMed] [Google Scholar]
- 2.Brodie M.J., Lerche H., Gil-Nagel A., Elger C., Hall S., Shin P. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. 2010;75:1817–1824. doi: 10.1212/WNL.0b013e3181fd6170. [DOI] [PubMed] [Google Scholar]
- 3.French J.A., Abou-Khalil B.W., Leroy R.F., Yacubian E.M., Shin P., Hall S. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology. 2011;76:1555–1563. doi: 10.1212/WNL.0b013e3182194bd3. [DOI] [PubMed] [Google Scholar]
- 4.Porter R.J., Partiot A., Sachdeo R., Nohria V., Alves W.M. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology. 2007;68:1197–1204. doi: 10.1212/01.wnl.0000259034.45049.00. [DOI] [PubMed] [Google Scholar]
- 5.Brickel N., Gandhi P., VanLandingham K., Hammond J., DeRossett S. The urinary safety profile and secondary renal effects of retigabine (ezogabine): a first-in-class antiepileptic drug that targets KCNQ (K(v)7) potassium channels. Epilepsia. 2012;53:606–612. doi: 10.1111/j.1528-1167.2012.03441.x. [DOI] [PubMed] [Google Scholar]
- 6.2013. FDA anti-seizure drug Potiga (ezogabine) linked to retinal abnormalities and blue skin discoloration. [Google Scholar]
- 7.Garin Shkolnik T., Feuerman H., Didkovsky E., Kaplan I., Bergman R., Pavlovsky L. Blue-gray mucocutaneous discoloration: a new adverse effect of ezogabine. JAMA Dermatol. 2014;150:984–989. doi: 10.1001/jamadermatol.2013.8895. [DOI] [PubMed] [Google Scholar]
- 8.Beacher N.G., Brodie M.J., Goodall C. A case report: retigabine induced oral mucosal dyspigmentation of the hard palate. BMC Oral Health. 2015;15:122. doi: 10.1186/s12903-015-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescott J.S., Evans G.A., Saenz A.A. Pigmentary abnormalites (discoloration) associated with ezogabine/retigabine treatment: non clinical aspects. Epilepsy Curr. 2015;15(S1):339–340. (AES 2014 Annual Meeting Online Abstract Supplement) [Google Scholar]
- 10.2016. GlaxoSmithKline Potiga (ezogabine) tablets prescribing information. [Google Scholar]
- 11.Evans S., Saenz A.A., Harrington C., Kelly D., Walsh N., McDonald S. Pigmentary abnormalities (discoloration) associated with ezogabine/retigabine treatment: clinical aspects. Epilepsy Curr. 2015;15(S1):336–337. (AES 2014 Annual Meeting Online Abstract Supplement) [Google Scholar]
- 12.FDA FDA Drug Safety Communication . 2013. FDA approves label changes for anti-seizure drug Potiga (ezogabine) describing risk of retinal abnormalities, potential vision loss, and skin discoloration. [Google Scholar]