SUMMARY
Scarce data exist on the relationship between diabetes and extrapulmonary tuberculosis (EPTB). We evaluated whether diabetes impacts site of TB and risk of death in patients with EPTB. We evaluated a cohort of TB cases from the state of Georgia between 2009 and 2012. Patients aged ⩾16 years were classified by diabetes status according to medical records. Site of EPTB was determined by culture and/or state TB classification. Death was defined by all-cause mortality. Of 1325 eligible reported TB cases, 369 (27·8%) had any EPTB including 258 (19·5%) with only EPTB and 111 (8·4%) with pulmonary TB and EPTB. Of all TB cases, 158 had diabetes (11·9%). In multivariable analysis, the odds of any EPTB was similar in patients with and without diabetes [adjusted odds ratio 1·04, 95% confidence interval (CI) 0·70–1·56]. The risk of death was 23·8% in patients with EPTB and diabetes vs. 9·8% in those with no diabetes (P < 0·01); after adjusting for covariates the difference was not significant (aRR 1·19, 95% CI 0·54–2·63). Diabetes was common in patients with EPTB and risk of death was high. Improved understanding of the relationship between diabetes and EPTB is critical to determine the extent that diabetes affects TB diagnosis and clinical management.
Key words: Diabetes mellitus, extrapulmonary tuberculosis, tuberculosis
INTRODUCTION
Globally, there are an estimated 9 million annual incident cases of active tuberculosis (TB) [1, 2]. The 2014 estimated worldwide prevalence of type 2 diabetes was 8·3% (387 million persons) in adults, and is expected to surpass 590 million persons by 2035 [3]. The risk of active TB disease in patients with diabetes mellitus is estimated to be three times the risk of the general population and 15% of global active TB cases are attributable to diabetes [4–6]. Patients with diabetes may also have a greater risk of poor TB outcomes, including death and relapse [7]. Poor outcomes in patients with TB and diabetes may be due to immune impairment caused by diabetes [8, 9].
Nearly 15% of global TB cases are extrapulmonary tuberculosis (EPTB) cases. EPTB presents challenges to TB control because it is harder to diagnose [10] and certain forms are associated with worse outcomes [11]. Incidence of EPTB occurs more commonly in persons with impaired innate immunity [12, 13], renal disease [14], and HIV [15, 16]. Certain EPTB sites, including more severe sites such as central nervous system (CNS), are associated with HIV and lower CD4 cell count [17]. Although immune deficiency also occurs with diabetes, little is known about the epidemiological or clinical relationship between diabetes and EPTB.
Patients with diabetes are at increased risk of infections, including soft-tissue infections (S. aureus, Candidia albicans) [18, 19], group B streptococcus [20–22], and genitourinary infections (Enterobacteriaceae) [23, 24]. The immune mechanisms that place patients with diabetes at increased risk of infections, including TB, may also increase the risk of EPTB.
If diabetes creates differential risk of EPTB, then this would have important implications for the clinical diagnosis and treatment of TB in patients with diabetes. Few studies have examined the relationship between diabetes and likelihood of EPTB. Moreover, even less is known regarding the risk of mortality in patients with EPTB and diabetes. Therefore, we aimed to determine the association between diabetes and EPTB and to determine the association between diabetes and risk of all-cause mortality during TB treatment in patients with EPTB.
METHODS
We conducted a retrospective observational study of all TB cases reported to the state of Georgia between January 2009 and September 2012 as described previously [25]. Eligible participants included all patients with pulmonary TB (PTB) or EPTB, aged ⩾16 years, and whose case was reported to the Georgia state TB register during the study period.
Definitions
The study outcomes of interest included site of TB disease and all-cause mortality. Site of TB disease was determined by culture and radiography and categorized as PTB, EPTB, or both combined. Further location of EPTB was determined using a combination of clinical diagnosis, radiography results, and specimen culture type. All-cause mortality was determined from patient medical records. Death from any cause occurring before or during TB treatment was defined as a death for this analysis.
The primary exposure of interest for all analyses was diabetes mellitus status. All patients were asked if they had ever been diagnosed with diabetes; patients who self-reported having diabetes were categorized as having diabetes. Additionally, medical records were reviewed and used to determine if diabetes status was previously diagnosed. As per standard of care, clinicians performed baseline blood chemistry tests, including random blood glucose, on all patients with TB and the results were also used to indicate diabetes status in the Georgia state TB register.
Patients' demographic and clinical characteristics were abstracted from the Georgia state TB registry and from medical records as described previously [25]. Key covariates of interest in this study included HIV status, foreign place of birth, and end-stage renal disease (ESRD).
Data analyses
We examined the association between diabetes status and site of TB, and the association between diabetes and risk of all-cause mortality. We used bivariate analyses (χ2 test for categorical variables and Kruskal–Wallis test for continuous variables) to determine the association between diabetes, patients' characteristics, and the outcome site of TB: pulmonary, extrapulmonary, or both combined. Logistic regression was used to report odds ratios (OR), adjusted odds ratios (aOR), and 95% confidence intervals (CI) to compare the likelihood of having (1) EPTB-only, (2) both EPTB and PTB, or (3) any EPTB (patients with EPTB-only or both EPTB and PTB) by diabetes status and patients' clinical/demographic characteristics. For all three logistic models, the referent outcome comparison was to patients with PTB. To compare risk of all-cause mortality during EPTB treatment in patients with and without diabetes, we used log binomial regression to report risk ratios (RR), adjusted risk ratios (aRR), and 95% CI. Covariates included in regression models were chosen based on factors considered to be confounders (associated with both diabetes status and the models' outcome of interest) and using directed acyclic graph theory [26]. In multivariable analysis for the outcome site of TB, statistical interaction between diabetes and the covariates HIV, ESRD, and sex was assessed by including multiplicative terms in regression models [27]. For statistical tests, patients with HIV-positive results were compared to a composite group of all other patients (HIV negative and unknown HIV status). A two-sided P value ⩽0·05 was considered statistically significant throughout all analyses.
Ethical review
The study was approved by the Institutional Review Boards of Emory University and the Georgia Department of Public Health.
RESULTS
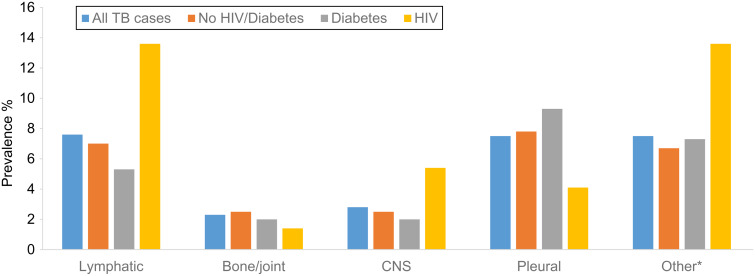
There were 1428 patients with active TB reported to the state of Georgia during the study period. A total of 1325 (93%) patients with active TB were eligible for inclusion in the present study. Overall, patients were mostly male (67%), US-born (55%), and African American (48%), with a median age of 45 years (interquartile range 31–57). HIV status was known for 1231 patients (93%), 54 patients were not offered an HIV test, 22 refused, and 18 had missing information. Diabetes was prevalent in 11·4% of patients, 11·1% had HIV, and 0·5% had both diabetes and HIV. EPTB was present in 369 (27.8%) patients: 258 (19.5%) had only EPTB and 111 (8·4%) had both PTB and EPTB (Table 1). Of patients with any EPTB, common sites of infection included lymphatic (n = 102, 27%), bone or joints (n = 31, 8·4%), CNS (n = 37, 10%), and pleural (n = 100, 27%) (Fig. 1). Of 42 patients with diabetes and any EPTB, lymphatic (n = 9, 21%) and pleural (n = 14, 33%) were the most common sites of infection (Supplementary Table 1). In addition, six (14%) of those with diabetes and any EPTB had ESRD.
Table 1.
Characteristics of patients with tuberculosis by pulmonary and extrapulmonary locations
| Pulmonary | Extrapulmonary | Pulmonary and extrapulmonary | ||
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Characteristic | 956 (72·1) | 258 (19·5) | 111 (8·4) | P value* |
| Diabetes mellitus | 116 (12·1) | 27 (10·5) | 15 (13·5) | 0·66 |
| HIV† | 95 (9·9) | 28 (10·9) | 31 (27·9) | <0·01 |
| Sex (male) | 645 (67·5) | 157 (60·8) | 81 (73·0) | 0·05 |
| Median age, years (IQR) | 46·0 (31·0–57·0) | 41·5 (30·0–57·0) | 44·0 (33–55·0) | 0·22 |
| Race/ethnicity | ||||
| NH White | 160 (16·8) | 31 (12·0) | 13 (11·7) | 0·08 |
| NH Black | 444 (46·5) | 132 (51·2) | 63 (56·8) | |
| NH Asian | 163 (17·1) | 55 (21·3) | 17 (15·3) | |
| Hispanic | 187 (19·6) | 40 (15·5) | 18 (16·2) | |
| Foreign born | 428 (44·9) | 122 (47·7) | 50 (45·0) | 0·73 |
| Recent homelessness | 104 (10·9) | 11 (4·4) | 12 (10·9) | <0·01 |
| Correctional facility‡ | 83 (8·7) | 15 (5·9) | 12 (10·9) | 0·21 |
| Heavy alcohol use | 172 (18·2) | 14 (5·6) | 18 (16·4) | <0·01 |
| Illicit drug use | 106 (11·2) | 12 (4·8) | 9 (8·2) | <0·01 |
| ESRD | 18 (1·9) | 13 (5·0) | 4 (3·6) | 0·02 |
| Previous TB treatment | 60 (6·3) | 12 (4·7) | 5 (4·5) | 0·53 |
| DST profile | ||||
| None to RIF/INH | 671 (88·6) | 129 (91·5) | 81 (89·0) | 0·75 |
| RIF or INH | 81 (10·7) | 12 (8·5) | 9 (9·9) | |
| Multidrug resistant | 5 (0·7) | 0 | 1 (1·1) | |
| Unavailable | 199 | 117 | 20 | |
| Death§ | 77 (8·0) | 28 (10·9) | 12 (10·8) | 0·28 |
AFB, Acid fast bacilli; DST, drug susceptibility test; ESRD, end-stage renal disease; INH, isoniazid; IQR, interquartile range; NH, non-Hispanic; RIF, rifampin.
Two-sided χ2 test.
HIV unknown categorized as negative.
Diagnosed with TB in a correctional facility.
All-cause mortality before or during or TB treatment.
Bold indicates statistically significant P value <0·05.
Fig. 1.
Distribution of extrapulmonary tuberculosis sites of infection (n = 1325). * Other includes genitourinary, breast, gastrointestinal, peritoneal, cranial, pericardium, appendages.
The prevalence of any EPTB-only in patients with diabetes was 26·6% compared to 28·0% in those without diabetes (P = 0·66). The prevalence of EPTB-only in patients with diabetes was 17·1% compared to 19·8% in those without diabetes and the prevalence of PTB and EPTB-only was 9·5% (diabetes) and 8·2% (no diabetes). Only ESRD, HIV, and male sex were significantly associated with EPTB. In multivariable analysis, patients with diabetes, compared to patients without diabetes, were non-significantly more likely to have both PTB and EPTB (aOR 1·39, 95% CI 0·75–2·58) after adjusting for age, sex, race/ethnicity, HIV status, ESRD and recent homelessness (Table 2).
Table 2.
Patients' characteristics associated with having extrapulmonary site of tuberculosis
| EPTB-only vs. PTB (N = 1214) | EPTB and PTB-only vs. PTB (N = 1067) | Any EPTB vs. PTB (N = 1325) | ||||
|---|---|---|---|---|---|---|
| Characteristic | OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) |
| Diabetes mellitus* | 0·85 (0·54–1·32) | 0·92 (0·57–1·47) | 1·13 (0·64–2·02) | 1·39 (0·75–2·58) | 0·93 (0·64–1·35) | 1·04 (0·70–1·56) |
| HIV† | 1·10 (0·71–1·72) | 1·15 (0·71–1·87) | 3·51 (2·21–5·60) | 3·46 (2·04–5·85) | 1·73 (1·22–2·45) | 1·82 (1·24–2·68) |
| Age, years | ||||||
| 16–34 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 |
| 35–44 | 0·89 (0·60–1·33) | 0·88 (0·58–1·33) | 1·76 (1·01–3·08) | 1·45 (0·81–2·59) | 1·09 (0·77–1·55) | 1·03 (0·72–1·48) |
| 45–54 | 0·57 (0·38–0·84) | 0·59 (0·38–0·90) | 1·13 (0·65–1·96) | 0·94 (0·52–1·70) | 0·70 (0·50–0·98) | 0·68 (0·47–0·97) |
| ⩾55 | 0·74 (0·52–1·04) | 0·72 (0·49–1·05) | 0·96 (0·56–1·65) | 0·93 (0·51–1·68) | 0·79 (0·58–1·07) | 0·78 (0·56–1·10) |
| Sex (female) | 1·33 (1·00–1·77) | 1·22 (0·91–1·64) | 0·77 (0·50–1·19) | 0·75 (0·47–1·18) | 1·14 (0·89–1·47) | 1·08 (0·83–1·40) |
| Race | ||||||
| NH White | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 |
| NH Black | 1·53 (1·00–2·36) | 1·40 (0·89–2·19) | 1·75 (0·94–3·26) | 1·32 (0·69–2·52) | 1·60 (1·10–2·32) | 1·39 (0·95–2·05) |
| NH Asian | 1·74 (1·07–2·85) | 1·41 (0·85–2·35) | 1·28 (0·60–2·73) | 1·21 (0·56–2·63) | 1·61 (1·04–2·48) | 1·40 (0·90–2·19) |
| Hispanic | 1·10 (0·66–1·85) | 0·94 (0·54–1·62) | 1·19 (0·56–2·49) | 0·96 (0·44–2·12) | 1·13 (0·72–1·76) | 0·97 (0·60–1·55) |
| Recent homelessness | 0·37 (0·19–0·69) | 0·41 (0·21–0·80) | 0·99 (0·53–1·87) | 0·60 (0·30–1·21) | 0·55 (0·35–0·87) | 0·49 (0·30–0·81) |
| ESRD | 2·77 (1·34–5·72) | 2·67 (1·25–5·71) | 1·95 (0·65–5·86) | 1·59 (0·51–4·99) | 2·52 (1·28–4·94) | 2·27 (1·13–4·57) |
aOR, Adjusted odds ratio; CI, confidence interval; EPTB, extrapulmonary tuberculosis; ESRD, end-stage renal disease; NH, non-Hispanic; OR, odds ratio; PTB, pulmonary tuberculosis.
Multivariable model adjusted for age, sex, race/ethnicity, HIV, ESRD, recent homelessness.
HIV unknown categorized as negative. Multivariable model adjusted for age, sex, race/ethnicity, ESRD, recent homelessness.
Of 369 patients with any EPTB, 42 (11%) died including 31% (13/42) before TB treatment initiation (Table 3). The unadjusted risk of death in EPTB patients with diabetes was 2·43 (95% CI 1·29–4·58). After adjusting for sex, age, foreign-born status, HIV, EPTB site, and ESRD, the adjusted risk of mortality in patients with diabetes was 1·19 (95% CI 0·54–2·63) times the risk in patients without diabetes. Factors significantly associated with mortality included HIV (aRR 2·18, 95% CI 1·06–4·46), CNS site of infection (aRR 3·79, 95% CI 1·32–8·47), and ESRD (aRR 3·48, 95% CI 1·43–8·47).
Table 3.
Risk factors for mortality during tuberculosis treatment in patients with EPTB
| Characteristic | Mortality risk | ||
|---|---|---|---|
| 42/369 (11·4%) | Crude RR | Adjusted RR* | |
| Died/N (%) | (95% CI) | (95% CI) | |
| Diabetes mellitus | |||
| No | 32/327 (9·8) | 1·00 | 1·00 |
| Yes | 10/42 (23·8) | 2·43 (1·29–4·58) | 1·19 (0·54–2·63) |
| HIV† | |||
| No | 30/310 (9·7) | 1·00 | 1·00 |
| Yes | 12/59 (20·3) | 2·10 (1·14–3·86) | 2·18 (1·06–4·46) |
| EPTB site | |||
| Lymphatic | 7/102 (6·9) | 1·00 | 1·00 |
| Bone/joint | 3/31 (9·7) | 1·41 (0·39–5·13) | |
| CNS | 10/37 (27·0) | 3·94 (1·62–9·59) | 3·79 (1·42–8·47)‡ |
| Pleural | 11/100 (11·0) | 1·60 (0·65–3·97) | |
| Other§ | 11/99 (11·1) | 1·61 (0·65–4·01) | |
| Sex | |||
| Female | 10/131 (7·6) | 1·00 | 1·00 |
| Male | 32/238 (13·5) | 1·76 (0·89–3·47) | 1·53 (0·75–3·15) |
| Age, years | |||
| 16–34 | 6/129 (4·7) | 1·00 | 1·00 |
| 35–44 | 4/72 (5·6) | 1·19 (0·35–4·09) | |
| 45–54 | 10/69 (14·5) | 3·12 (1·18–8·21) | 4·77 (2·16–10·51)|| |
| ⩾55 | 22/99 (22·2) | 4·78 (2·01–11·33) | |
| Race/ethnicity | |||
| NH White | 9/44 (20·5) | 1·00 | |
| NH Black | 27/195 (13·9) | 0·68 (0·34–1·34) | |
| NH Asian | 2/72 (2·8) | 0·14 (0·03–0·60) | |
| Hispanic | 4/58 (6·9) | 0·34 (0·11–1·02) | |
| Foreign born | |||
| No | 32/197 (16·2) | 1·00 | 1·00 |
| Yes | 10/172 (5·8) | 0·36 (0·18–0·71) | 0·72 (0·34–1·53) |
| Recent homelessness | |||
| No | 38/346 (11·0) | 1·00 | |
| Yes | 4/23 (17·4) | 1·58 (0·62–4·05) | |
| Correctional facility# | |||
| No | 42/342 (12·3) | 1·00 | |
| Yes | 0/27 (0) | n.a. | |
| Heavy alcohol use | |||
| No | 35/302 (10·4) | 1·00 | |
| Yes | 7/32 (21·9) | 2·11 (1·02–4·35) | |
| Illicit drug use | |||
| No | 40/348 (11·5) | 1·00 | |
| Yes | 2/21 (9·5) | 0·83 (0·21–3·20) | |
| ESRD | |||
| No | 35/352 (9·9) | 1·00 | 1·00 |
| Yes | 7/17 (41·2) | 4·14 (2·16–7·92) | 3·48 (1·43–8·47) |
| Previous TB treatment | |||
| No | 39/352 (11·1) | 1·00 | |
| Yes | 3/17 (17·7) | 1·59 (0·55–4·64) |
CNS, Central nervous system; CI, confidence interval; EPTB, extrapulmonary tuberculosis; ESRD, end-stage renal disease, NH, non-Hispanic; RR, risk ratio.
Multivariable model included diabetes mellitus, HIV, EPTB site, sex, age, foreign born, and ESRD.
HIV unknown categorized as negative.
In multivariable model, EPTB site was dichotomous as CNS vs. all other.
Other includes genitourinary, breast, gastrointestinal, peritoneal, cranial, pericardium, appendages.
In multivariable model, age was dichotomous as <45 and ⩾45 years.
TB diagnosed in a correctional facility.
Bold indicates statistically significant, P value <0·05.
In an assessment of statistical interaction, the effect of diabetes on prevalence of any EPTB was not significantly different by HIV, ESRD, or sex. However, there was a non-significant trend (P = 0·42) for increased prevalence of EPTB in those with diabetes and ESRD (aOR 4·22, 95% CI 1·11–16·13) compared to those without diabetes and ESRD (aOR 2·17, 95% CI 0·86–5·46). Similarly the effect of diabetes on risk of death in patients with EPTB was not significantly different by HIV, ESRD, or site of extrapulmonary infection.
DISCUSSION
In this retrospective cohort of adult patients with any EPTB from the state of Georgia, diabetes was common (11·4%) and lymphatic and pleural sites of infection were the most frequent sites of extrapulmonary disease in patients with diabetes and EPTB. Of patients with EPTB-only, the prevalence of diabetes (10·5%) was also high, and similar compared to the prevalence of HIV (10·9%). We did not find that patients with diabetes were more likely to have EPTB compared to PTB. We did observe a high proportion (23·8%) of all-cause mortality in patients with EPTB and diabetes, although the observed crude association between diabetes and risk of death was largely due to other patient factors associated with death in those with diabetes, most notably age and ESRD. Our study also confirmed previous findings that HIV is associated with EPTB [1] and an increased risk of death in patients with EPTB [11], and we reported ESRD to be associated with both EPTB and increased risk of death.
Few previous studies to date have examined the association between diabetes and EPTB. One population-based study of adult TB patients (n = 1194) in Galicia, Spain, reported a non-significant increased odds of having EPTB vs. PTB in patients with diabetes (OR 1·48, 95% CI 0·89–2·47) [28]. Another study analysing TB cases during 2007–2011 from Brazil's Notifiable Disease Surveillance System reported that 8·4% of patients with diabetes had EPTB compared to 14·7% in those without diabetes (P < 0·01) [29]. The multivariable model from the Brazilian analysis only adjusted for clinical characteristics and did not aim to estimate the association between diabetes and EPTB. Third, a Taiwanese hospital-based study of 766 patients with TB reported that diabetes was associated with decreased likelihood of EPTB (aOR 0·41, 95% CI 0·22–0·76) [14]. However, the Taiwanese study used stepwise regression methods and likely adjusted for factors on the causal pathway between diabetes and risk of TB leading to a biased effect of diabetes on risk of EPTB. Considering our results in the context of previous studies, the majority of existing data suggest that diabetes likely does not increase the risk of EPTB. Nonetheless, the global prevalence of diabetes will continue to increase substantially in the next 20 years, and given the paucity of studies that were specifically designed to examine the relationship between diabetes and EPTB, more investigations will be needed. Future diabetes EPTB studies will likely be improved by Xpert assay technology which has recently been demonstrated to be a sensitive and specific tool to detect and diagnose EPTB [10].
The high all-cause mortality rate (11·4%) in patients with EPTB observed in this study is also consistent with previous reports of mortality in patients with EPTB in the state of Georgia. For example, in a study conducted during 1995–2001, Kourbatova et al. reported the 12-month mortality rate in patients with EPTB at an inner-city Atlanta hospital was 14·6% [11]. In our study the unadjusted risk of death in patients with EPTB and diabetes was more than twice the risk in those without diabetes; however, this apparent increased risk of death was likely confounded by age and ESRD. In this study, age and ESRD were associated with both diabetes and with increased risk of death, after adjusting for age, ESRD, and other factors, the estimated effect of diabetes on risk of death was substantially attenuated (RR 1·19, 95% CI 0·54–2·63).
We did not detect significant statistical interaction between diabetes and HIV, ESRD, or sex. However, the finding that patients with both ESRD and diabetes had an increased trend towards any EPTB (aOR 4·22 95% CI 1·11–16·13) suggests there may be synergy between ESRD and diabetes with the risk of EPTB. Chronic renal disease is a risk factor for active TB [30] and previous studies reported a high prevalence of EPTB in patients on dialysis [31] and ESRD [14]. Consistent with our findings that ESRD is associated with more than twice the odds of EPTB, a study in Taiwan reported that the odds of EPTB was significantly higher in patients with ESRD (aOR 3·74, 95% CI 1·45–9·67) [14]. Kidney damage may lead to an increased risk of developing EPTB in part due to latent TB infection existing in kidneys. A recent study of 49 necropsy specimens from Mexican patients who died from causes other than TB found that 43 had mycobacterical DNA in EPTB sites, including 79% (34/43) with mycobacterial DNA in the kidneys [32]. Globally, the number of patients receiving renal replacement therapy doubled during 1990–2010 and is expected to double again by 2030, reaching 5·4 million people [33]. Given the aetiological connection between diabetes and ESRD, and the marked expected increases in both diseases, an improved understanding of the diabetes–ESRD–TB epidemiological relationships are needed. Specifically, whether the risk of EPTB in patients with ESRD is modified by diabetes should be evaluated in future studies.
Although not the primary focus of our study, we also found that ESRD and HIV were significantly associated with all-cause mortality, even after adjusting for multiple patient clinical characteristics. To our knowledge, our study is the first to report that ESRD is associated with all-cause mortality in patients with EPTB even after adjusting for key confounders such as age, HIV, and diabetes. Uraemia from ESRD is associated with persistent immune inflammation and reduced T-cell activation which may also contribute to increased mortality [34]. We previously reported HIV to be associated with CNS site of infection in patients with EPTB [17], and CNS site of infection is associated with increased risk of mortality [11]. Unlike this study that reports HIV to be significantly associated with all-cause mortality in adjusted analyses, Kourbatova et al. reported that HIV was associated with mortality only in unadjusted analysis [11].
This study is subject to some limitations. First, we did not systematically screen all patients in this study for diabetes using haemoglobin A1c or another widely accepted diagnostic technique to measure diabetes. Instead we relied on medical record abstraction and self-reported diabetes status, consequently our measure of diabetes was subject to misclassification. Nonetheless, all patients did receive general blood chemistry tests and therefore patients with high glucose levels would likely be identified as having diabetes. In addition, self-reported diabetes status has been found to have high specificity, in our study patients were probably not falsely misclassified as having diabetes. Second, our study was unable to assess the relationship between glucose control, duration of diabetes, or immune function with EPTB. While we did not observe an association between diabetes and EPTB or site of EPTB, patients with poorly controlled diabetes may have greater immune impairment and more likely to have certain sites of EPTB; we were unable to examine this association. Third, specimen cultures were not available for all patients with EPTB, therefore classification of EPTB and site of EPTB may be misclassified. Nonetheless, we performed a subgroup analysis in only patients who had a positive EPTB specimen culture (data not shown). The magnitude, direction, and statistical significance of the subgroup analyses was the same as reported in our primary analyses.
CONCLUSION
The increasing global prevalence of diabetes has important implications for public health management of TB. Improved understanding of the relationship between diabetes and risk of EPTB is also critical to characterize the extent to which diabetes will affect TB diagnosis and clinical management. Our study thoroughly examined the relationship between diabetes and EPTB and found that diabetes does not significantly increase the likelihood of EPTB. Nonetheless, as incident active TB cases continue to decrease in the United States, a larger proportion of new TB cases in the United States will arise due to reactivation [35, 36] and consequently a greater proportion due to diabetes. If a larger proportion of TB cases in the United States are attributable to diabetes, the relationship between diabetes and EPTB will require re-examination.
ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases [K23AI103044 (R.R.K.) and K24AI114444 (N.R.G.)], Bethesda, MD, USA, the Emory Centers for AIDS Research (P30 AI050409) and the Emory University Laney Graduate School, Atlanta, GA, USA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DECLARATION OF INTEREST
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816000364.
click here to view supplementary material
REFERENCES
- 1.Peto HM, et al. Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clinical Infectious Diseases 2009; 49: 1350–1357. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global tuberculosis report 2014. Geneva: World Health Organization, 2014. [Google Scholar]
- 3.IDF. Diabetes Atlas, 6th edn. Brussels: International Diabetes Federation, 2013. [Google Scholar]
- 4.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Medicine 2008; 5: e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker MA, et al. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clinical Infectious Diseases 2012; 54: 818–825. [DOI] [PubMed] [Google Scholar]
- 6.Lonnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes & Endocrinology 2014; 2: 730–739. [DOI] [PubMed] [Google Scholar]
- 7.Baker MA, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Medicine 2011; 9: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martens GW, et al. Tuberculosis susceptibility of diabetic mice. American Journal of Respiratory Cell and Molecular Biology 2007; 37: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar NP, et al. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. Journal of Infectious Diseases 2013; 208: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denkinger CM, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. European Respiratory Journal 2014; 44: 435–446. [DOI] [PubMed] [Google Scholar]
- 11.Kourbatova EV, et al. Risk factors for mortality among patients with extrapulmonary tuberculosis at an academic inner-city hospital in the US. European Journal of Epidemiology 2006; 21: 715–721. [DOI] [PubMed] [Google Scholar]
- 12.Sterling TR, et al. Human immunodeficiency virus-seronegative adults with extrapulmonary tuberculosis have abnormal innate immune responses. Clinical Infectious Diseases 2001; 33: 976–982. [DOI] [PubMed] [Google Scholar]
- 13.Antas PR, et al. Decreased CD4+ lymphocytes and innate immune responses in adults with previous extrapulmonary tuberculosis. Journal of Allergy and Clinical Immunology 2006; 117: 916–923. [DOI] [PubMed] [Google Scholar]
- 14.Lin JN, et al. Risk factors for extra-pulmonary tuberculosis compared to pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 2009; 13: 620–625. [PubMed] [Google Scholar]
- 15.Yang Z, et al. Identification of risk factors for extrapulmonary tuberculosis. Clinical Infectious Diseases 2004; 38: 199–205. [DOI] [PubMed] [Google Scholar]
- 16.Jones BE, et al. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. American Review of Respiratory Disease 1993; 148: 1292–1297. [DOI] [PubMed] [Google Scholar]
- 17.Leeds IL, et al. Site of extrapulmonary tuberculosis is associated with HIV infection. Clinical Infectious Diseases 2012; 55: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller LM, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clinical Infectious Diseases 2005; 41: 281–288. [DOI] [PubMed] [Google Scholar]
- 19.Peleg AY, et al. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes/Metabolism Research and Reviews 2007; 23: 3–13. [DOI] [PubMed] [Google Scholar]
- 20.Skoff TH, et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clinical Infectious Diseases 2009; 49: 85–92. [DOI] [PubMed] [Google Scholar]
- 21.Farley MM. Group B streptococcal disease in nonpregnant adults. Clinical Infectious Diseases 2001; 33: 556–561. [DOI] [PubMed] [Google Scholar]
- 22.Thomsen RW, et al. Impact of diabetes and poor glycaemic control on risk of bacteraemia with haemolytic streptococci groups A, B, and G. Journal of Infection 2011; 63: 8–16. [DOI] [PubMed] [Google Scholar]
- 23.Ishay A, Lavi I, Luboshitzky R. Prevalence and risk factors for asymptomatic bacteriuria in women with Type 2 diabetes mellitus. Diabetic Medicine 2006; 23: 185–188. [DOI] [PubMed] [Google Scholar]
- 24.Stapleton A. Urinary tract infections in patients with diabetes. American Journal of Medicine 2002; 113 (Suppl. 1A): 80S–84S. [DOI] [PubMed] [Google Scholar]
- 25.Magee MJ, et al. Diabetes mellitus and risk of all-cause mortality among patients with tuberculosis in the state of Georgia, 2009–2012. Annals of Epidemiology 2014; 24: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999; 10: 37–48. [PubMed] [Google Scholar]
- 27.Kleinbaum D, Klein M. Logistic Regression: A Self-Learning Text, Third Edition. New York, NY: Springer Science, 2010. [Google Scholar]
- 28.Garcia-Rodriguez JF, et al. Extrapulmonary tuberculosis: epidemiology and risk factors. Enfermedades Infecciosas y Microbiologia Clinica 2011; 29: 502–509. [DOI] [PubMed] [Google Scholar]
- 29.Gomes T, et al. Epidemiology of extrapulmonary tuberculosis in Brazil: a hierarchical model. BMC Infectious Diseases 2014; 14: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussein MM, Mooij JM, Roujouleh H. Tuberculosis and chronic renal disease. Seminars in Dialysis 2003; 16: 38–44. [DOI] [PubMed] [Google Scholar]
- 31.Lui SL, et al. Tuberculosis infection in Chinese patients undergoing continuous ambulatory peritoneal dialysis. American Journal of Kidney Diseases 2001; 38: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 32.Barrios-Payan J, et al. Extrapulmonary locations of mycobacterium tuberculosis DNA during latent infection. Journal of Infectious Diseases 2012; 206: 1194–1205. [DOI] [PubMed] [Google Scholar]
- 33.Liyanage T, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015; 385: 1975–1982. [DOI] [PubMed] [Google Scholar]
- 34.Meijers RW, et al. T-cell ageing in end-stage renal disease patients: Assessment and clinical relevance. World Journal of Nephrology 2014; 3: 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bock NN, et al. A tuberculin screening and isoniazid preventive therapy program in an inner-city population. American Journal of Respiratory and Critical Care Medicine 1999; 159: 295–300. [DOI] [PubMed] [Google Scholar]
- 36.Styblo K. Recent advances in epidemiological research in tuberculosis. Advances in Tuberculosis Research 1980; 20: 1–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816000364.
click here to view supplementary material