Abstract
Treatment options for gram-positive resistant bacteria are limited; therefore, efforts to evaluate therapy options in the critical care population are warranted. Cefepime has broad-spectrum activity against gram-negative and gram-positive organisms. We have previously demonstrated that the combination of cefepime with vancomycin, linezolid, or quinupristin-dalfopristin had an improved or enhanced effect against methicillin-resistant Staphylococcus aureus (MRSA). We investigated various regimens of cefepime alone and in combination against two clinical MRSA isolates (R2481 and R2484) in an established in vitro pharmacodynamic model. Human pharmacokinetic regimen simulations were as follows: cefepime, 2 g every 8 h (q8h) (C8) and 12 h (C12), continuous-infusion 2-g loading dose followed by 4 g alone or in combination with gentamicin and tobramycin (1.0 or 2.0 [G1 and G2 or TB1 and TB2] mg/kg of body weight q12h and 5.0 [G5 or TB5] mg/kg q24h), arbekacin (ARB) (100 mg q12h), linezolid (LIN) (600 mg q12h), tigecycline (TIG) (100 mg q24h), or daptomycin (DAP) (6 mg/kg q24h) for 48 h. The MICs for cefepime, gentamicin, tobramycin, ARB, LIN, TIG, and DAP for the two clinical MRSA isolates (R2481 and R2484) were 4 and 4, 0.25 and 0.5, 128 and 0.5, 0.5 and 0.125, 2 and 4, 0.25 and 0.25, and 0.0625 and 0.125 μg/ml, respectively. At 48 h, combinations of C12 and C8 plus ARB, G1, or G5 (range, −2.05- to −4.32-log10 decrease) demonstrated enhanced lethality against R2481 (resistant to tobramycin) (P < 0.05). A similar relationship was demonstrated against R2484 with cefepime plus ARB, gentamicin, or tobramycin (range, −2.05- to −3.63-log10 decrease) (P < 0.05). A 99.9% kill was achieved with cefepime plus aminoglycoside combinations as early as 2 h and maintained throughout the 48-h period. TIG was antagonistic when combined with C12 against both isolates. DAP alone achieved 99.9% kill for up to 48 h for both isolates and was the most active agent against R2481 and R2484 (−2.89- and −3.61-log10 decrease at 48 h); therefore, combination therapy did not enhance lethality. Overall, the most potent combinations noted were cefepime in combination with low- and high-dose aminoglycosides. Further investigations with combination therapies are warranted.
Gram-positive organisms, such as Staphylococcus aureus, are among the most common bacteria infecting patients in the intensive care units (13). Methicillin-resistant S. aureus (MRSA) is a major nosocomial pathogen that has caused severe morbidity and mortality worldwide (38). This organism is endemic in many hospitals and account for as much as 52% of all clinical isolates (43). Until recently, glycopeptide antibiotics, such as vancomycin, were often the only available treatment. The increase in vancomycin usage over the years and the development of resistance to this drug have led to the search for alternative therapy. (16).
Empirical therapy in the intensive care unit (ICU) should be broad enough to cover pathogens such as Pseudomonas aeruginosa and S. aureus, especially empirically until results of cultures are available (13, 17, 21, 22). In view of the rising prevalence of multidrug-resistant S. aureus, combination therapy may be useful in providing a greater antimicrobial empirical coverage and improving efficacy and in helping to prevent the further emergence of resistant organisms (39, 48).
Cefepime is a broad-spectrum cephalosporin active against many gram-negative and gram-positive organisms, including S. aureus. Data suggest that time above the MIC is the pharmacodynamically linked parameter that predicts cefepime's efficacy in human and animal models (14). This parameter also has been used as the basis to support consideration of administration of cephalosporin antibiotics by continuous infusion. Cefepime has high affinity for the penicillin-binding proteins of gram-negative and gram-positive bacteria and thus possesses excellent antibacterial activity against methicillin-susceptible S. aureus. Maximal efficacy for cephalosporins against S. aureus has been demonstrated at 40 to 50% of the dosing interval when serum levels are above MIC with animal infection models (14).
Previous investigations have evaluated the usefulness of various combinations of antibiotics against MRSA, including combinations of quinupristin-dalfopristin and cefepime, linezolid, doxycycline, and vancomycin(3). Several studies have demonstrated additive or synergistic activity when cefepime was combined with various antibiotics, such as vancomycin plus β-lactams, ampicillin-sulbactam ± arbekacin, and trimethoprim-sulfamethoxazole (3, 9, 26, 32, 47). Cefepime is commonly used in combination with an aminoglycosides for empirical therapy in the ICU for gram-negative organisms or for definitive treatment of P. aeruginosa infections in critical care settings (22). However, to date, there are limited data regarding the utility of combination therapy with cefepime for empirical treatment of gram-positive infections. We, therefore, evaluated cefepime alone and in combination with gentamicin, arbekacin, tobramycin, linezolid, tigecycline, or daptomycin against MRSA.
(A portion of this work was presented at the 14th European Congress of Clinical Microbiology and Infectious Diseases, Prague, Czech Republic, May 2004).
MATERIALS AND METHODS
Bacterial strains.
Two clinical strains of MRSA (R2481 and R2484) obtained from William Beaumont Hospital, Royal Oak, Mich., were utilized in all experiments.
Antimicrobial agents.
Cefepime (Elan Pharmaceuticals, Americas, San Diego, Calif.), gentamicin (Sigma Chemical Company, St. Louis, Mo.), and linezolid (Pharmacia-Upjohn Laboratories, Kalamazoo, Mich.) were commercially purchased. Arbekacin (Meiji Seika Kaisha, Ltd. Pharmaceutical Division, Tokyo, Japan) (49), daptomycin (Cubist Pharmaceuticals Inc., Lexington, Mass.), and tigecycline (Wyeth Research, Pearl River, N.Y.) were supplied by their respective manufacturers. Stock solutions of each antibiotic were freshly prepared on the day of the experiments and stored in the freezer at −4°C.
Medium.
All susceptibility testing and in vitro pharmacodynamic models used Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with 25 mg of calcium/liter and 12.5 mg of magnesium/liter (SMHB). Mueller-Hinton broth adjusted to physiologic ionized calcium concentrations (1.1 to 1.7 mmol/liter) according to NCCLS guidelines (daptomycin dependency on calcium for its mechanism of action) and 12.5 mg of magnesium/liter were used for all daptomycin experiments. Colony counts for all samples were determined by using tryptic soy agar (TSA) (Difco Laboratories) plates.
In vitro susceptibility.
MICs and minimum bactericidal concentrations (MBCs) were determined by using a broth microdilution technique with an inoculum of 5 × 106 CFU/ml according to NCCLS guidelines (31).
In vitro pharmacodynamic model.
The in vitro pharmacodynamic model consists of a 250-ml one-compartment glass chamber with multiple ports for the removal of SMHB, delivery of antibiotics, and collection of bacterial and antimicrobial samples was utilized (3). All experiments were conducted over 48 h and were performed in duplicate to ensure reproducibility. Prior to each experiment, bacterial colonies from an overnight growth on TSA were added to SMHB to obtain a suspension corresponding to a 0.5 McFarland standard. Then, 2.5 ml of this suspension was added to each of the pharmacodynamic models to produce a starting inoculum of 106 CFU/ml. The model was placed in a 37°C water bath for the duration of the experiment, with magnetic stir bars in the medium to allow for continuous mixing. A peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, Ill.) was used to continually replace antibiotic-containing medium with fresh SMHB (at a rate simulating the half-lives of the antibiotics). All antimicrobials were infused over approximately 1 min. Regimen simulations were as follows: cefepime, 2 g every 8 h (q8h) and q12h (estimated peak concentration and half-life of cefepime, 130 μg/ml; 2 h); cefepime continuous infusion (CI), 2-g loading dose followed 4 g q24h (20 μg/ml; 2 h) (6, 10); gentamicin and tobramycin, 1.0 and 2.0 mg/kg q12h (3 and 6 μg/ml; 3 h) and 5 mg/kg q24h (15 μg/ml; 3 h); arbekacin, 100 mg q12h (8 μg/ml; 3 h); linezolid, 600 mg q12h (18 μg/ml; 6 h); tigecycline, 100 mg q24h (1.17 μg/ml; 31 h) (34, 35, 36, 51); daptomycin, 6 mg/kg q24h (6.65 μg/ml simulated free concentration based upon 93% protein binding; 8 h) (2, 24, 50). The elimination rate for all combination models was set for the antibiotic with the shorter half-life, and the antibiotic with a longer half-life was supplemented (8).
Antibiotic assays.
Gentamicin, tobramycin, and arbekacin concentrations were determined by using a fluorescence polarization immunoassay (TDx Assay; Abbott Laboratories, Irving, Tex., and Dainabot Co., LTD, Tokyo, Japan). Coefficients of variation for gentamicin, tobramycin, and arbekacin are ≤3.6, ≤4, and ≤5.1%, respectively. The limits of detection for gentamicin, tobramycin, and arbekacin were 1.0, 1.0, and 2.0 μg/ml, respectively. Since the standards for these commercial kits are prepared in serum, we determined the accuracy of detection of aminoglycoside concentrations in SMHB by preparing sets of five spiked samples in both serum and broth for each aminoglycoside in triplicate. The results indicated that there were no appreciable differences demonstrated between serum and broth (1:1.01 to 1:1.06) for arbekacin, gentamicin, and tobramycin, respectively. Linezolid concentrations were determined at the Division of Infectious Diseases at the National Jewish Medical and Research Center (Denver, Colo.), which was previously described by Allen et al. (3). The standard curve for linezolid in SMHB ranged from 0.5 to 30 μg/ml. The within-sample precision (percent coefficient of variation) of validation for a single standard concentration was 0.69%, and the overall validation precision across all standards was 1.04 to 4.39. The limit of detection for linezolid was 0.5 μg/ml (3).
Concentrations of all other agents were determined by using standard agar diffusion bioassay procedures. Cefepime and daptomycin concentrations were determined by using antibiotic assay medium 5 with Micrococcus luteus ATCC 9341 and antibiotic assay medium 1 supplemented with 50 mg of calcium/liter and M. luteus ATCC 9341, respectively. Tigecycline concentrations were determined by using agarose medium and Bacillus cereus ATCC 11778 spore suspension. Standards and samples were tested in triplicate using blank 1/4-in. disks saturated with 20 μl of the appropriate solution. Concentrations of 150, 130, 20, 8, and 2 μg/ml, 10, 7.5, 5, 2.5, and 1.25 μg/ml, and 4, 2, 1, 0.5, 0.25, 0.12, and 0.06 μg/ml were used as standard curves for cefepime, daptomycin, and tigecycline, respectively. Coefficients of variation for cefepime, daptomycin, and tigecycline assays were <10, ≤8, and 8.4%, respectively. The limits of detection for each of the above assays were 2.0 μg/ml (cefepime), 1.25 μg/ml (daptomycin), and 0.06 μg/ml (tigecycline).
Pharmacokinetic analysis.
Antibiotic concentrations were determined from samples drawn in duplicate from each model at 0, 0.5, 1, 2, 4, 6, 8, 24, 28, 32, and 48 h. Samples were stored at −70°C until analysis. Antibiotic peak, trough concentrations, and half-life were calculated using concentration-time plots of the model samples. The area under the concentration-time curve from 0 to 24 h was calculated by using the linear trapezoid method and the PKANALYST program (version 1.10; MicroMath Scientific Software, Salt Lake City, Utah).
Pharmacodynamic analysis.
Samples (approximately 1.5 ml each) from each model were collected at 0, 1, 2, 4, 6, 8, 24, 28, 32, and 48 h. Samples were then serially diluted in 0.9% sodium chloride. Bacterial counts were determined by plating 100-μl samples of each diluted sample on TSA, using an automated spiral dispenser (Automatic Spiral Plater; Don Whitley Scientific Limited, West Yorkshire, England). All samples were diluted 10- to 100-fold before plating in order to minimize antibiotic carryover or by plating or vacuum filtration (sample washed through a 0.22-μm-pore-size filter with normal saline) when sample with predicted concentrations were close to the MIC for the experiment organisms. Plated samples were then incubated at 37°C for 24 h, and colony counts (log10 CFU/ml) were determined by using a laser bacteria colony counter (ProtoCOL, version 2.05.02; Synbiosis, Cambridge, England). The limit of detection for this method of colony count determination was 2-log10 CFU/ml. In vitro model time-kill curves were determined by plotting mean colony counts (log10 CFU/ml) from each model versus time. Bactericidal activity (99.9% kill) was defined as a ≥3-log10 CFU/ml reduction in colony count from the starting inoculum. Enhancement of activity was defined as an increase in kill of ≥2-log10 CFU/ml by combination of antimicrobials versus the most active single agent of that combination (3). Improvement was defined as >1- and <2-log10-CFU/ml increase in kill in comparison to the most active single agent, while combinations that resulted in ≥1-log10 bacterial growth in comparison to that with the least-active single agent were considered to represent antagonism (3). The terms “improvement” and “enhancement” were used because our simulations used therapeutically obtained serum concentrations, and this did not permit the mathematical modeling necessary to consider the standard terms “additivity” and “synergy” (3). “Indifferent” was defined as no differences. Reductions in colony counts and the ability to achieve a 99.9% kill were determined over a 48-h period and were compared between regimens.
Detection of resistance.
Samples (100 μl each) from each time point were plated onto TSA containing an antibiotic concentration of four and eight times the MIC for each organism and were incubated for 48 h at 37°C in order to monitor the development of resistance. Plates were visually inspected for growth of resistant subpopulations after 24, 32, and 48 h of incubation. The MIC for each organism was determined by using broth microdilution methods.
Statistical analysis.
Differences between regimens (log10 CFU/ml) at 24 and 48 h were determined using one-way analysis of variance with Tukey's post-hoc test. For all experiments, a P value of ≤0.05 was considered indicative of statistical significance. All statistical analyses were performed using SPSS (version 11.5; SPSS, Inc., Chicago, Ill.) (3).
RESULTS
Susceptibility testing.
The microdilution MICs and MBCs for all antimicrobials tested are shown in Table 1. Both strains, MRSA R2481 and R2484, were susceptible to all the antimicrobials tested, except tobramycin. MRSA R2484 was tobramycin susceptible, and MRSA R2481 was tobramycin resistant (MIC = 128 μg/ml).
TABLE 1.
Susceptibilities of the two MRSA clinical strains
| Antimicrobial agent | Activity (μg/ml) against strain:
|
|||
|---|---|---|---|---|
| MRSA R2481
|
MRSA R2484
|
|||
| MIC | MBC | MIC | MBC | |
| Cefepime | 4 | 8 | 4 | 8 |
| Gentamicin | 0.25 | 0.5 | 0.5 | 1 |
| Tobramycin | 128 | 256 | 0.5 | 1 |
| Arbekacin | 0.5 | 0.5 | 0.13 | 1 |
| Daptomycin | 0.06 | 0.13 | 0.13 | 0.5 |
| Linezolid | 2 | 32 | 4 | 16 |
| Tigecycline | 0.25 | 0.5 | 0.25 | 0.5 |
Pharmacokinetics.
Observed pharmacokinetic parameters achieved in the in vitro model experiments are listed in Table 2.
TABLE 2.
Pharmacokinetic parameters
| Antimicrobial agent (regimen) | Peak (μg/ml) | Trough (μg/ml) | Half-life (h) |
|---|---|---|---|
| Cefepime (q12H) | 140 ± 21.5 | 2.5 ± 0.69 | 2.0 ± 0.22 |
| Cefepime (q8H) | 137.4 ± 2.97 | 9.0 ± 0.19 | 2.0 ± 0.04 |
| Cefepime (LDa + continuous infusion) | 136.8 ± 6.60 | 19.4 ± 1.40 | N/Ab |
| Gentamicin (5.0 mg/kg q24H) | 14.7 ± 0.50 | 0.21 ± 0.01 | 2.6 ± 0.04 |
| Gentamicin (2.0 mg/kg q12H) | 5.4 ± 0.09 | 0.46 ± 0.06 | 3.2 ± 0.07 |
| Gentamicin (1.0 mg/kg q12H) | 2.5 ± 0.09 | 0.11 ± 0.01 | 3.0 ± 0.06 |
| Tobramycin (5.0 mg/kg q24H) | 15.4 ± 0.08 | 0.41 ± 0.01 | 3.1 ± 0.04 |
| Tobramycin (2.0 mg/kg q12H) | 6.2 ± 0.05 | 0.29 ± 0.01 | 2.6 ± 0.04 |
| Tobramycin (1.0 mg/kg q12H) | 3.15 ± 0.03 | 0.26 ± 0.01 | 3.2 ± 0.10 |
| Arbekacin | 7.9 ± 0.40 | 0.63 ± 0.08 | 3.3 ± 0.17 |
| Daptomycin | 6.8 ± 0.12 | 0.69 ± 0.10 | 7.3 ± 0.35 |
| Linezolid | 21.6 ± 1.00 | 4.0 ± 0.01 | 5.7 ± 0.35 |
| Tigecycline | 1.2 ± 0.02 | 0.69 ± 0.01 | 32 ± 2.0 |
LD, loading dose.
N/A, not applicable.
Pharmacodynamics.
The reduction in bacterial inoculum for each antimicrobial alone is shown in Table 3, and combination results are shown in Tables 4 and 5. Although there appeared to be some improvement in kill as a function of dosing interval (greatest effect with CI, followed by q8h dosing, followed by q12h dosing) for cefepime, this was not statistically significant. Daptomycin alone demonstrated the greatest activity against both MRSA isolates, and therefore, no enhanced or improved efficacy in combination was observed. Gentamicin and tobramycin alone did not demonstrate kill against both MRSA isolates at 48 h; however, a 99.9% kill was achieved at earlier time points. Linezolid and tigecycline were each bacteriostatic when administered alone, and no time point examined has achieved a 99.9% kill. We observed no effective activity for tobramycin alone and in combination with cefepime against R2481 due to the resistant susceptibility profile (data not shown).
TABLE 3.
Inoculum changes over 48 h obtained for each antimicrobial alone
| Antimicrobial agent | Change over 48 h (log10 CFU/ml) for strain:
|
|
|---|---|---|
| MRSA R2481 | MRSA R2484 | |
| Cefepime (2g q12h) | +1.51 | +1.39 |
| Cefepime (2g q8h) | −0.63 | −1.83 |
| Cefepime (continuous infusion) | −1.08 | −2.40 |
| Gentamicin (5.0 mg/kg q24h) | −0.73 | −1.70 |
| Gentamicin (2.0 mg/kg q12h) | −2.09 | −2.84 |
| Gentamicin (1.0 mg/kg q12h) | +0.28 | +0.44 |
| Tobramycin (5.0 mg/kg q24h) | —a | −1.06 |
| Tobramycin (2.0 mg/kg q12h) | +2.18 | −0.75 |
| Tobramycin (1.0 mg/kg q12h) | — | −0.35 |
| Arbekacin | −1.56 | −1.06 |
| Daptomycin | −2.89 | −3.61 |
| Linezolid | −2.65 | −1.78 |
| Tigecycline | −0.37 | −0.72 |
—, model was not performed.
TABLE 4.
R2481 versus cefepime combined with various antimicrobials at various dosing intervals
| Antimicrobial agent (regimen) | Activities of drug against MRSA R2481 in combination witha:
|
|||||
|---|---|---|---|---|---|---|
| Cefepime (2 g q12h)
|
Cefepime (2 g q8h)
|
Cefepime (continuous infusion)
|
||||
| Δ48-h log10 CFU/ml | Activities | Δ48-h log10 CFU/ml | Activities | Δ48-h log10 CFU/ml | Activities | |
| Gentamicin (5.0 mg/kg q24h) | −2.68** | ENC | −2.58* | ENC | −1.69* | IMP |
| Gentamicin (2.0 mg/kg q12h) | −1.69 | IMP | −1.34 | IMP | −1.54 | IMP |
| Gentamicin (1.0 mg/kg q12h) | −3.82** | ENC | −3.14** | ENC | −2.62*** | ENC |
| Arbekacin | −2.23* | ENC | −2.34* | ENC | −2.34*** | ENC |
| Daptomycin | 0.04 | IND | −0.85 | IND | −0.11 | IND |
| Linezolid | −0.39 | IND | −0.56 | IND | −0.15 | IND |
| Tigecycline | 1.75 | ANG | −0.03 | IND | −1.86 | IMP |
Change in 48-h log10 CFU of R2481/ml with cefepime (2g q12h, 2 g q8h, and continuous infusion) plus gentamicin (1.0 or 2.0 mg/kg q12h or 5.0 mg/kg q24h), arbekacin, linezolid, daptomycin, or tigecycline compared to results with the most potent agent alone. ENC, enhancement; IMP, improvement; ANG, antagonistic; IND, indifferent. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
TABLE 5.
R2484 versus cefepime combined with various antimicrobials at various dosing intervals
| Antimicrobial agent (regimen) | Activities of drug against MRSA R 2484 in combination witha:
|
|||||
|---|---|---|---|---|---|---|
| Cefepime (2 g q12h)
|
Cefepime (2 g q8h)
|
Cefepime (continuous infusion)
|
||||
| Δ48-h log10 CFU/ml | Activities | Δ48-h log10 CFU/ml | Activities | Δ48-h log10 CFU/ml | Activities | |
| Gentamicin (5.0 mg/kg q24h) | −3.85* | ENC | −2.05 | ENC | −1.46** | IMP |
| Gentamicin (2.0 mg/kg q12h) | 0.23 | IND | −0.93 | IND | −0.93 | IND |
| Gentamicin (1.0 mg/kg q12h) | −4.32*** | ENC | −2.05 | ENC | −1.46** | IMP |
| Tobramycin (5.0 mg/kg q24h) | −2.81** | ENC | −2.05 | ENC | −0.44 | IND |
| Tobramycin (2.0 mg/kg q12h) | −3.03*** | ENC | −2.05 | ENC | −1.46** | IMP |
| Tobramycin (1.0 mg/kg q12h) | −3.63*** | ENC | −2.05 | ENC | −1.46** | IMP |
| Arbekacin | −2.9** | ENC | −1.24 | IMP | −1.46** | IMP |
| Daptomycin | 0.9 | IND | 0.69 | IND | 0 | IND |
| Linezolid | −1.32 | IMP | −0.43 | IND | −0.24 | IND |
| Tigecycline | 2.06 | ANG | −0.28 | IND | −0.66 | IND |
Change in 48-h log10 CFU of R2484/ml with cefepime (2 g q12h, 2 g q8h, and continuous infusion) plus gentamicin (1.0 or 2.0 mg/kg q12h or 5.0 mg/kg q24h), tobramycin (1.0 or 2.0 mg/kg q12h or 5.0 mg/kg q24h), arbekacin, daptomycin, linezolid, or tigecycline compared to results with the most potent agent alone. ENC, enhancement; IMP, improvement; ANG, antagonistic; IND, indifferent. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
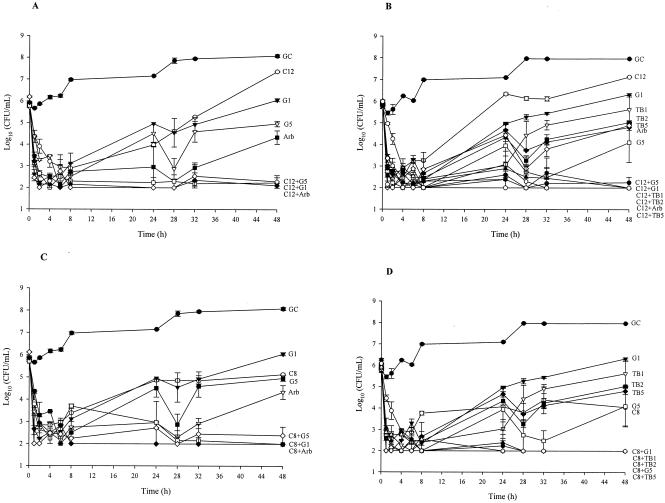
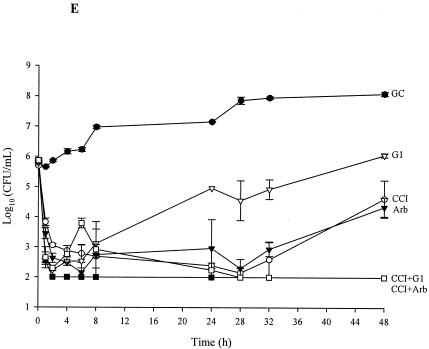
The results of combination regimens of inoculum changes for 48-h pharmacodynamic models for the tested strains are depicted in Fig. 1. Positive values indicate regrowth. Against R2481, significant enhancement of efficacy was observed with cefepime (CI), cefepime (q8h and q12h) with gentamicin (1 mg/kg q12h and 5 mg/kg q24h) or arbekacin (P ≤ 0.04). Significant enhancement of efficacy was also demonstrated against R2484 with cefepime (q8h and q12h) and both gentamicin (1 mg/kg q12h and 5 mg/kg q24h) and tobramycin (1.0 and 2 mg/kg q12h and 5.0 mg/kg q24h) or arbekacin (q12h only) (P ≤ 0.02). A 99.9% kill was achieved at 8, 24, and 48 h for the following combinations against both strains: cefepime, q12h, q8h, and CI, with gentamicin and tobramycin (5.0 mg/kg q24h), gentamicin and tobramycin (2.0 mg/kg q12h), or arbekacin. Cefepime (CI) was very active against R2484; therefore, no enhancement of effect was demonstrated against R2484 versus that of cefepime (CI) with any of the tested antimicrobials. Improved activity was demonstrated against R2481 with cefepime (q8h) plus gentamicin (2.0 mg/kg q12h) or cefepime (CI) plus gentamicin (1.0 and 2.0 mg/kg q12h), gentamicin (5.0 mg/kg q24h), or tigecycline (P > 0.05). Against R2484, improved activity was demonstrated with cefepime (q12h) plus linezolid or cefepime (q8h) plus arbekacin as well as cefepime (CI) plus gentamicin and tobramycin (1.0 mg/kg q12h), tobramycin (2.0 mg/kg), or arbekacin (P ≤ 0.009). A 99.9% kill was achieved at 8, 24, and 48 h for the following combinations against both strains: cefepime (q8h) plus gentamicin (2.0 mg/kg q12h) or plus arbekacin and cefepime (CI) plus gentamicin (5.0 mg/kg q24h), plus gentamicin and tobramycin (2.0 mg/kg q12h), plus gentamicin and tobramycin (1.0 mg/kg q12h), or plus tigecycline. Antagonism was noted only with cefepime (2 g q12h) plus tigecycline.
FIG. 1.
Activities of tested antimicrobials (alone and in combinations which demonstrated enhancement of activity): R2481 versus cefepime (2 g q12h) (A); R2484 versus cefepime (2 g q12h) (B); R2481 versus cefepime (2 g q8h) (C); R2484 versus cefepime (2 g q8h) (D); and R2481 versus cefepime (continuous infusion) (E). The key for each regimen is as follows: GC, growth control; C12, cefepime, 2g q12h; C8, cefepime, 2 g q8h; CCI, cefepime, continuous infusion; G1 and TB1, gentamicin and tobramycin, 1.0 mg/kg q12h; G2 and TB2, gentamicin and tobramycin, 2.0 mg/kg q12h; G5 and TB5, gentamicin and tobramycin, 5.0 mg/kg q24h; Arb, arbekacin; Lin, linezolid; Tig, tigecycline; Dap, daptomycin.
Detection of resistance.
Resistance was detected for tobramycin (12-fold the MIC) for monotherapy regimens. No evidence of resistance was observed with any other regimen tested, including models where significant bacterial regrowth occurred.
DISCUSSION
The inappropriate use of antibiotics has contributed to the emergence of resistance globally with gram-negative bacilli and gram-positive bacteria (28). Infections with antibiotic-resistant organisms are associated with increased mortality, increased length of stay in the ICU and hospital, and increased costs compared to infections with antibiotic-susceptible organisms (28). Some of the most problematic organisms are P. aeruginosa and MRSA (17, 20, 21, 22). Appropriate empirical choices in ICU settings are critical, due to the rise in multidrug-resistant P. aeruginosa and MRSA (28). The prevalence of MRSA has been reported to be as high as 52% in this setting (43). Empirical ICU coverage often comprises combinations of broad-spectrum antipseudomonal β-lactams, aminoglycosides (such as gentamicin, tobramycin, and amikacin), arbekacin (available in Japan only), and/or a glycopeptide (vancomycin and teicoplanin) or monotherapy with a potent broad-spectrum agent (such as the carbapenems) (22, 49, 52). Combination therapy is often recommended for empirical treatment of ICU infections, since monotherapy, even with a highly active agent, may not cover all potential organisms, and the emergence of resistant organisms is always a potential (22). Specific empirical combination therapy for S. aureus has not been advocated.
Cefepime is a broad-spectrum cephalosporin that offers broad activity against both gram-negative organisms, including P. aeruginosa, and gram-positive organisms, such as S. aureus (19, 42). In vitro data have suggested that the bactericidal activity of β-lactams is generally concentration independent, with a significant postantibiotic effect against gram-positive bacteria (14). Attempts have been made to establish optimal dosing regimens, and intermittent dosing continues to be the standard of practice. Though shorter intermittent dosing intervals will optimize the pharmacodynamic properties of most β-lactams, this is highly dependent on the organism and the specific antimicrobial agent (4, 10, 43, 44, 45). Time above the MIC is the most important pharmacodynamic parameter for predicting the outcome (27). The maximal efficacy for cephalosporins in animal infection models is observed when serum concentrations are greater than the MIC for 40 to 50% of dosing interval for S. aureus (14). Therefore, we evaluated cefepime dosing at continuous infusion versus every 8 h and every 12 h (4, 6, 7, 9). In our models, the percent time above the MIC was 100, 100, and 84% for continuous infusion, every 8 h, and every 12 h, respectively. Although not statistically significant, we observed a greater reduction in bacterial density as a function of dosing interval. This relationship has been previously shown with cefepime against gram-negative organisms (6, 9, 11, 12, 41).
The observed activity of cefepime against MRSA in this study may be related to some minimal activity against penicillin-binding protein 2a and/or due to a heteroresistance profile for beta-lactams in which some initial activity may be expected against a portion of the bacterial inoculum (40). We retrospectively performed subpopulation analysis versus oxacillin for both strains utilized in this experiment and compared the results to a strain (COL) that demonstrates a homogenous susceptibility profile for oxacillin (data not shown). As expected, both R2481 and R2484 demonstrated a heterogeneous profile for oxacillin. Although the activity of cefepime against MRSA is minimal, it has been observed that improved activity against MRSA can be demonstrated when cefepime is combined with vancomycin, quinupristin-dalfopristin, and linezolid (3, 17). Our data demonstrated improved kill with cefepime in combination with linezolid against R2484 which is similar to that reported by Allen et al. In this study, enhanced kill with cefepime plus vancomycin and improved kill with quinupristin-dalfopristin was also demonstrated. Raymond et al. obtained similar results with cefpirome (“fourth-generation” broad-spectrum cephalosporin structurally similar to cefepime) plus vancomycin against MRSA (37).
In our study, the most potent activity against MRSA was observed when cefepime was combined with an aminoglycoside. β-Lactams plus aminoglycoside have traditionally demonstrated synergy and additivity for a variety of pathogens, including P. aeruginosa, Enterobacteriaceae, and Acinetobacter (12, 29, 30, 33, 43, 46). We found that combinations of cefepime plus an aminoglycoside administered every 12 h or every 24 h demonstrated the most potent activity.
Cefepime is a broad-spectrum antibiotic commonly employed in critical care settings. In a recent study by Konstantinou et al. examining the impact of cefepime in an ICU setting, less resistance to therapy, less vancomycin-resistant enterococci, reduced vancomycin use, and shorter posttherapy hospitalization were observed with patients treated with cefepime than with those treated with ceftazidime (23). Araake et al. examined cefepime in combination with arbekacin or vancomycin and teicoplanin against a mixed culture of MRSA and P. aeruginosa (5). They demonstrated enhancement of effect with cefepime plus arbekacin against MRSA and P. aeruginosa but did not demonstrate enhancement with cefepime plus vancomycin or teicoplanin against MRSA (5). They suggested that arbekacin alone demonstrated good bactericidal activity and that combination with cefepime enhanced the bactericidal activity against MRSA (5).
A potential limitation of our research is the use of therapeutic concentrations only of all tested agents (1). This is in contrast to traditional synergy testing, such as checkerboard and killing-curve techniques, which are commonly conducted using a range of subinhibitory concentrations of one or both antimicrobials. While this typically leads to identification of a wider range of synergistic combinations, we chose to study clinically achievable concentrations in order to allow broader applicability of our results to clinical practice (1).
Despite this potential limitation, our data suggest that cefepime in combination with aminoglycoside offers reasonable coverage of MRSA if the isolate is susceptible to the aminoglycosides. Preliminary in vitro models (data not shown) performed with tobramycin and cefepime in combination against the tobramycin-resistant isolate (R2481) did not demonstrate significant activity against this isolate; therefore, additional combination studies against this pathogen were not pursued. Although we did not confirm the presence of an aminoglycoside-modifying enzyme in this organism, the aminoglycoside-modifying enzymes in MRSA that are most responsible for tobramycin resistance are the aminoglycoside-6-O-nucleotidyltransferase I and aminoglycoside-6′-N-acetyltransferase/2"-O-phosphoryltransferase (18, 25). The prevalence of MRSA resistant to aminoglycosides has been noted to be as high as 35.5% in a recent surveillance study, and this factor should be noted when applying this data to clinical setting (15).
In the present study we were able to show improved or enhanced activity through the use of a variety of antimicrobial combinations encompassing cefepime, gentamicin, tobramycin, arbekacin, and linezolid against MRSA. The combination of cefepime and an aminoglycoside against MRSA is encouraging; however, caution should be applied until these results can be verified clinically.
Acknowledgments
This work was supported by grants from Elan Pharmaceuticals.
We thank Abbott Laboratories for the use of the fluorescence polarization immunoassay analyzer for determining aminoglycoside concentrations. We also thank Chrissy Cheung for her assistance with the in vitro model experiments.
REFERENCES
- 1.Akins, R. L., and M. J. Rybak. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, R. L., and M. J. Rybak. 2000. In vitro activities of daptomycin, arbekacin, vancomycin, and gentamicin alone and/or in combination against glycopeptide intermediate-resistant Staphylococcus aureus in an infection model. Antimicrob. Agents Chemother. 44:1925-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, G. P., R. Cha, and M. J. Rybak. 2002. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 46:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrose, P. G., R. C. Owens, Jr., M. J. Garvey, and R. N. Jones. 2002. Pharmacodynamic considerations in the treatment of moderate to severe pseudomonal infections with cefepime. J. Antimicrob. Chemother. 49:445-453. [DOI] [PubMed] [Google Scholar]
- 5.Araake, M., T. Hara, A. Miyata, M. Tani, and H. Ogawa. 2001. Combination effect of arbekacin and cefepime on mixed culture of MRSA and P. aeruginosa. Jpn. J. Antibiot. 54:69-78. [PubMed] [Google Scholar]
- 6.Baririan, N., H. Chanteux, E. Viaene, H. Servais, and P. M. Tulkens. 2003. Stability and compatibility study of cefepime in comparison with ceftazidime for potential administration by continuous infusion under conditions pertinent to ambulatory treatment of cystic fibrosis patients and to administration in intensive care units. J. Antimicrob. Chemother. 51:651-658. [DOI] [PubMed] [Google Scholar]
- 7.Benko, A. S., D. M. Cappelletty, J. A. Kruse, and M. J. Rybak. 1996. Continuous infusion versus intermittent administration of ceftazidime in critically ill patients with suspected gram-negative infections. Antimicrob. Agents Chemother. 40:691-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaser, J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl. A):125-130. [DOI] [PubMed] [Google Scholar]
- 9.Bosso, J. A., B. A. Saxon, and J. M. Matsen. 1991. Comparative activity of cefepime, alone and in combination, against clinical isolates of Pseudomonas aeruginosa and Pseudomonas cepacia from cystic fibrosis patients. Antimicrob. Agents Chemother. 35:783-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess, D. S., R. W. Hastings, and T. C. Hardin. 2000. Pharmacokinetics and pharmacodynamics of cefepime administered by intermittent and continuous infusion. Clin. Ther. 22:66-75. [DOI] [PubMed] [Google Scholar]
- 11.Cappelletty, D. M. 1999. Evaluation of several dosing regimens of cefepime, with various simulations of renal function, against clinical isolates of Pseudomonas aeruginosa in pharmacodynamic infection model. Antimicrob. Agents Chemother. 43:129-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappelletty, D. M., S. L. Kang, S. M. Palmer, and M. J. Rybak. 1995. Pharmacodynamics of ceftazidime administered as continuous infusion or intermittent bolus alone and in combination with single daily-dose amikacin against Pseudomonas aeruginosa in an in vitro infection model. Antimicrob. Agents Chemother. 33:1797-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark, N. M., E. Hershberger, M. J. Zervos, and J. P. Lynch. 2003. Antimicrobial resistance among gram-positive organisms in the intensive care unit. Curr. Opin. Crit. Care 9:403-412. [DOI] [PubMed] [Google Scholar]
- 14.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 15.Diekema, D. A., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, M. Beach, and the SENTRY Participants Group. 2001. Survey of infections due to Staphylococcus species: frequency of occurrences and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32 (Suppl. 2):S114-S132. [DOI] [PubMed] [Google Scholar]
- 16.Enright, M. C., D. A. Robinson, G. Randle, E. J. Feil, H. Grundmann, and B. G. Spratt. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 99:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddadin, A. S., S. A. Fappiano, and P. A. Lipsett. 2002. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad. Med. J. 78:385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ida, T., R. Okamoto, C. Shimauchi, T. Okubo, A. Kuga, and M. Inoue. 2001. Identification of aminoglycoside-modifying enzymes by susceptibility testing: epidemiology of methicillin-resistant Staphylococcus aureus in Japan. J. Clin. Microbiol. 39:3115-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessler, R. E. 2001. Cefepime microbiologic profile and update. Pediatr. Infect. Dis. J. 20:331-336. [DOI] [PubMed] [Google Scholar]
- 20.Kollef, M. H. 2003. Antibiotic heterogeneity: should we use it? Crit. Care Med. 31:2074-2076. [DOI] [PubMed] [Google Scholar]
- 21.Kollef, M. H. 2001. Optimizing antibiotic therapy in the intensive care unit setting. Crit. Care. 5:189-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollef, M. H. 2003. The importance of appropriate initial antibiotic therapy for hospital-acquired infections. Am. J. Med. 115:582-584. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinou, K., K. Baddam, A. Lanka, K. Reddy, and M. J. Zervos. 2004. Cefepime versus ceftazidime for treatment of pneumonia. J. Int. Med. Res. 32:84-93. [DOI] [PubMed] [Google Scholar]
- 24.Lee, B. L., M. Sachdeva, and H. F. Chambers. 1991. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob. Agents Chemother. 35:2505-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemaitre, N., W. Sougakoff, A. Masmoudi, M. H. Fievet, R. Bismuth, and V. Jarlier. 1998. Characterization of gentamicin-susceptible strains of methicillin-resistant Staphylococcus aureus involved in nosocomial spread. J. Clin. Microbiol. 36:81-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozniewski, A., C. Lion, F. M. Weber, and M. Weber. 2001. In vitro synergy between cefepime and vancomycin against methicillin-resistant susceptible and -resistant Staphylococcus aureus and Staphylococcus epidermidis. J. Antimicrob. Chemother. 47:83-86. [DOI] [PubMed] [Google Scholar]
- 27.Manduru, M., L. B. Mihm, R. L. White, L. V. Friedrich, P. A. Flume, and J. A. Bosso. 1997. In vitro pharmacodynamics of ceftazidime against Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 41:2053-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masterton, R., G. L. Drusano, D. L. Paterson, and G. Park. 2003. Appropriate antimicrobial treatment in nosocomial infections—the clinical challenges. J. Hosp. Infect. 55:1-12. [DOI] [PubMed] [Google Scholar]
- 29.McGrath, B. J., E. M. Bailey, K. C. Lamp, and M. J. Rybak. 1992. Pharmacodynamics of Once-daily amikacin in various combinations with cefepime, aztreonam, and ceftazidime against Pseudomonas aeruginosa in an in vitro infection model. Antimicrob. Agents Chemother. 36:2741-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mimoz, O., A. Jacolot, S. Leotard, N. Hidri, K. Samii, P. Nordmann, and O. Petitjean. 1998. Efficacies of cefepime, ceftazidime, and imipenem alone or in combination with amikacin in rats with experimental pneumonia due to ceftazidime-susceptible or -resistant Enterobacter cloacae strains. Antimicrob. Agents Chemother. 42:3304-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. 2002. Methods for determining bactericidal activity of antimicrobial agents. NCCLS document M-26. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 32.Otsuka, Y., T. Yoshibe, M. Namioka, and T. Ezaki. 2000. Combination effect of teicoplanin and beta-lactams on MRSA. Jpn. J. Antibiot. 53:643-651. [PubMed] [Google Scholar]
- 33.Owns, R. C., Jr., M. A. Banevicius, D. P. Nicolau, C. H. Nightingale, and R. Quintiliani. 1997. In vitro synergistic activities of tobramycin and selected beta-lactams against gram-negative clinical isolates. Antimicrob. Agents Chemother. 41:2586-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel, R., M. S. Rouse, K. E. Piper, and J. M. Steckelberg. 2000. In vitro activity of GAR-936 against vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 38:177-179. [DOI] [PubMed] [Google Scholar]
- 35.Petersen, P. J., P. A. Bradford, W. J. Weiss, T. M. Murphy, P. E. Sum, and S. J. Projan. 2002. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens. Antimicrob. Agents Chemother. 46:2595-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen, P. J., N. V. Jacobus, W. J. Weiss, P. E. Sum, and R. T. Testa. 1999. In vitro and in vivo antibacterial activities of a novel glycylcline, the 9-t-butylglyclamido derivative of minocycline (GAR-936). Antimicrob. Agents Chemother. 43:738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raymond, J., G. Vedel, and M. Bergeret. 1996. In-vitro bactericidal activity of cefpirome in combination with vancomycin against Staphylococcus aureus and coagulase-negative staphylococci. J. Antimicrob. Chemother. 38:1067-1071. [DOI] [PubMed] [Google Scholar]
- 38.Rybak, M. J., and R. L. Akins. 2001. Emergence of methicillin-resistant Staphylococcus aureus with intermediate glycopeptide resistance: clinical significance and treatment options. Drugs 61:1-7. [DOI] [PubMed] [Google Scholar]
- 39.Rybak, M. J., and B. J. McGrath. 1996. Combination antimicrobial therapy for bacterial infections: guidelines for the clinician. Drugs 52:390-405. [DOI] [PubMed] [Google Scholar]
- 40.Sieradzki, K., and A. Tomasz. 1997. Suppression of beta-lactam antibiotic resistance in a methicillin-resistant Staphylococcus aureus through synergic action of early cell wall inhibitors and some other antibiotics. J. Antimicrob. Chemother. 39(Suppl. A):47-51. [DOI] [PubMed] [Google Scholar]
- 41.Sprauten, P. F., P. M. Beringer, S. G. Louie, T. W. Synold, and M. A. Gill. 2003. Stability and antibacterial activity of cefepime during continuous infusion. Antimicrob. Agents Chemother. 47:1991-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sultan, N., M. Y. Cirak, and D. Erbas. 2000. Synergistic effect of cefepime on the phagocytic killing of Staphylococcus aureus by human polymorphonuclear leucocytes and the determination of this effect by means of nitrite production. Microbios 103:97-106. [PubMed] [Google Scholar]
- 43.Tallis, E., B. Rudensky, D. Attias, d. Raveh, Y. Schlesinger, and A. M. Yinnon. 1999. In-vitro activity of cefepime and other broad-spectrum antimicrobials against several groups of gram-negative bacilli and Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 35:121-126. [DOI] [PubMed] [Google Scholar]
- 44.Tam, V. H., P. S. McKinnon, R. L. Akins, G. L. Drusano, and M. J. Rybak. 2003. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob. Agents Chemother. 47:1853-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tam, V. H., P. S. McKinnon, R. L. Akins, M. J. Rybak, and G. L. Drusano. 2002. Pharmacodynamics of cefepime in patients with gram-negative Infections. J. Antimicrob. Chemother. 50:425-428. [DOI] [PubMed] [Google Scholar]
- 46.Tessier, P. R., D. P. Nicolau, C. O. Onyeji, and C. H. Nightingale. 1999. Pharmacodynamics of intermittent- and continuous-infusion cefepime alone and in combination with once-daily tobramycin against Pseudomonas aeruginosa in an in vitro infection model. Chemotherapy 45:284-295. [DOI] [PubMed] [Google Scholar]
- 47.Vouillamoz, J., J. M. Entenza, C. Feger, M. P. Glauser, and P. Moreillon. 2000. Quinupristin-dalfopristin combined with beta-lactams for treatment of experimental endocarditis due to Staphylococcus aureus constitutively resistant to macrolide-lincosamide-streptogramin B antibiotics. Antimicrob. Agents Chemother. 44:1789-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh, T. R., and R. A. Howe. 2002. The prevalence and mechanisms of vancomycin resistance in Staphylococcus aureus. Annu. Rev. Microbiol. 56:657-675. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe, T., K. Ohashi, K. Matsui, and T. Kubota. 1997. Comparative studies of the bactericidal, morphological and post-antibiotic effects of arbekacin and vancomycin against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 39:471-476. [DOI] [PubMed] [Google Scholar]
- 50.Wise, R., T. Gee, J. M. Andrews, B. Dvorchik, and G. Marshall. 2002. Pharmacokinetics and inflammatory fluid penetration of intravenous daptomycin in volunteers. Antimicrob. Agents Chemother. 46:31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wyeth Research. 2000. Investigator's brochure: effects in humans, vol. 3, p. 60-73. Wyeth Research, Pearl River, N.Y. [Google Scholar]
- 52.You, I., R. Kariyama, M. J. Zervos, H. Kumon, and J. W. Chow. 2000. In-vitro activity of arbekacin alone and in combination with vancomycin against gentamicin- and methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 36:37-41. [DOI] [PubMed] [Google Scholar]