Abstract
GM2-gangliosidosis, AB variant is an extremely rare autosomal recessive inherited disorder caused by mutations in the GM2A gene that encodes GM2 ganglioside activator protein (GM2AP). GM2AP is necessary for solubilisation of GM2 ganglioside in endolysosomes and its presentation to β-hexosaminidase A. Conversely GM2AP deficiency impairs lysosomal catabolism of GM2 ganglioside, leading to its storage in cells and tissues. We describe a 9-year-old child with an unusual juvenile clinical onset of GM2-gangliosidosis AB. At the age of 3 years he presented with global developmental delay, progressive epilepsy, intellectual disability, axial hypertonia, spasticity, seizures and ataxia, but without the macular cherry-red spots typical for GM2 gangliosidosis. Brain MRI detected a rapid onset of diffuse atrophy, whereas whole exome sequencing showed that the patient is a compound heterozygote for two mutations in GM2A: a novel nonsense mutation, c.259G > T (p.E87X) and a missense mutation c.164C > T (p.P55L) that was recently identified in homozygosity in patients of a Saudi family with a progressive chorea-dementia syndrome. Western blot analysis showed an absence of GM2AP in cultured fibroblasts from the patient, suggesting that both mutations interfere with the synthesis and/or folding of the protein. Finally, impaired catabolism of GM2 ganglioside in the patient's fibroblasts was demonstrated by metabolic labeling with fluorescently labeled GM1 ganglioside and by immunohistochemistry with anti-GM2 and anti-GM3 antibodies. Our observation expands the molecular and clinical spectrum of molecular defects linked to GM2-gangliosidosis and suggests novel diagnostic approach by whole exome sequencing and perhaps ganglioside analysis in cultured patient's cells.
Keywords: Gangliosidosis, GM2 ganglioside, GM2 ganglioside activator protein, Lysosomal storage disease
1. Introduction
GM2-gangliosidosis, AB variant (MIM#272750) is an autosomal recessive disorder of lysosomal ganglioside catabolism caused by mutations in the GM2A gene (reviewed in [1]). GM2A encodes the GM2 ganglioside activator protein (GM2AP, MIM #613109). A small (~25 kDa) amphiphilic protein GM2AP facilitates hydrolysis of GM2 ganglioside at the surfaces of the intra-endosomal membranes (IMs) generated during endocytosis [2], [3]. At the acidic pH of the endosome, GM2AP is protonated and binds to IMs rich in the anionic lipid, bis(monoacylglycero)phosphate [4], [5]. Once bound to the IM surface, GM2AP forms a specific 1:1 complex with GM2 ganglioside, extracts a ganglioside molecule from the membrane and presents it to β-hexosaminidase A (HexA) for the hydrolytic cleavage of β-N-acetylglucosamine residue from the glycan chain, producing GM3 ganglioside for further conversion into lactosylceramide by the ganglioside neuraminidases Neu3 and Neu4 [6].
All previously described patients with GM2-gangliosidosis AB presented with clinical phenotypes similar to those of acute infantile Tay-Sachs or Sandhoff diseases, though perhaps slightly milder [7], [8]. Cherry red macular spots have been reported in all cases. Recently however, three members of a Saudi family homozygous for the c.164C > T (p.P55L) mutation in GM2A have been reported. The patients presented at 7–13 years with a progressive chorea-dementia syndrome characterised by spastic quadriparesis, limb dystonia, generalized chorea and generalized cerebral atrophy [9]. The patients lacked the macular cherry red spots characteristic of GM2 gangliosidosis AB, and there was no report on whether GM2 ganglioside has been stored in their tissues.
Here we describe a 9–year-old boy with an atypical delayed clinical phenotype of GM2-gangliosidosis AB. He presented with global developmental delay, progressive epilepsy, intellectual disability, axial hypertonia, spasticity, seizures and ataxia, but similarly to recently described Saudi patients without retinal cherry spots. Molecular and biochemical analysis showed that the patient is a compound-heterozygote for 2 mutations in GM2A, one similar to that described by Salih et al. [9] and a novel nonsense mutation c.259G > T (p.E87X). These mutations result in drastically reduced levels of GM2AP and impaired GM2 catabolism in the patient's cells.
2. Subject/materials and methods
2.1. Patient, biochemical and molecular analyses
The patient is followed at Sainte-Justine university hospital center (CHU Ste-Justine) in Montreal. Activities of lysosomal enzymes in blood leucocytes and cultured skin fibroblasts, the levels of urinary glycosaminoglycans and other biochemical tests were performed in the Biochemical Medical Genetics Laboratory of CHU Ste-Justine. Whole exome sequencing was performed on the leucocyte DNA samples using an Illumina HiSeq 2000 sequencer at the Whole Genome Laboratory in Baylor College of Medicine, in Houston, TX.
2.2. Immunocytochemistry
Cultured skin fibroblasts of the patient obtained from skin biopsies of the patient and of three normal health controls from the cell repository of the Medical Genetics Division of CHU Ste-Justine were cultured in DMEM containing 10% FBS (Wisent BioProducts) and 1% antibiotic-antimycotic (Life Technologies) on glass slides in 6-well plates until 70% confluency, fixed with 4% PFA, permeabilized with 0.25% (v/v) Triton X-100, and stained with the antibodies against GM2 (KM966, 1:500), GM3 (M2590, Cosmo Bio Co., Ltd., 1:100) or LAMP-2 (mouse anti-human H4B4, DSHB, 1:100) followed by appropriate secondary antibodies (Alexa fluor 488 goat anti-human IgG, 1:500; Alexa fluor 555 goat anti-mouse IgG, 1:400) and Draq5™ solution (all ThermoFisher Scientific). For quantification, images of 40 randomly selected cells were acquired with the LSM510 Zeiss or Leica TCS SPE confocal microscopes (× 63 glycerol immersion objectives, N.A. 1.4) and analysed using the ImageJ software. The percentage of GM2-positive cells was measured by manually counting the cells in randomly selected field images acquired with a Nikon Eclipse E800 fluorescence microscope (× 40 objective).
2.3. Analysis of the GM2AP expression by Western blot
Human fibroblasts or Cos7 cells transfected with the pcDNA3.1-TOPO plasmid expressing human GM2AP [12] were cultured in 10 cm dishes until 80–90% confluency, harvested, and homogenized in H2O by sonication (3 × 10 s). The protein was quantified using the Bradford reagent (Bio-Rad). After separation by SDS PAGE on a 15% gel, the proteins were transferred to a nitrocellulose membrane and hybridized with antibodies against GM2AP [10] (1:1000) or α-tubulin (12G10, DSHB, 1:15,000) followed by the appropriate secondary antibodies (sc-2020, Santa Cruz Biotechnology, 1:4000 or 7076S, Cell Signaling, 1:10,000). The membrane was developed with Pierce® ECL Western Blotting substrate (Thermo Scientific) and the signal detected using G:Box Chemi XQR system (Syngene).
2.4. Analysis of ganglioside catabolism in cultured fibroblasts
Fibroblasts were plated at a density of 2 × 104 cells/cm2 in 10 cm dishes. After 18 h the medium was supplemented with BODIPY® FL C5-Ganglioside GM1 (Life Technologies) at a final concentration of 1.45 μM and the cells were further cultured for 72 h. Gangliosides were purified from the cell pellets and analysed by thin layer chromatography (TLC) on silica plates (Merck) as previously described [11]. The plates were analysed under UV light and images acquired using G:Box Chemi XQR system. The intensity of GM1 and GM2 ganglioside band was quantified using ImageJ software.
3. Results
3.1. Clinical course
The patient is a nine-year-old boy. He came to medical attention because of seizures at 3 years of age. He is the second child of unrelated French Canadian parents with a negative family history for neurodegenerative problems. Following a normal term pregnancy and spontaneous vaginal delivery he was born weighing 3.5 kg with a head circumference of 33.0 cm. Apgar scores were 9 at one, five and 10 min and he was discharged after 48 h.
His initial development was felt to be normal although in retrospect, signs of motor dysfunction were present before 12 months of age. He sat at 6 months, walked without support at 1 year, but tended to walk in a tiptoe fashion. He climbed stairs in alternating fashion at 2 years. He was toilet trained by 2 years of age. He was never able to run. He had poor fine motor skills, although he succeeded in feeding himself. He pronounced single words with difficulty by 12 months of age and spoke in sentences by 3 years. A speech therapist diagnosed marked verbal dyspraxia at the age of three years. He had limited interests and disliked changes in his routine. He was suspected clinically to have absence seizures from about 3 years of age but electroencephalographic studies were normal on four occasions. His first proven seizure, at 5 years of age, was a prolonged focal convulsion with secondary generalization lasting about 3 min with a postictal state lasting several minutes. Focal seizures then recurred about once a week, accompanied by loss of contact with his environment and facial twitching. They were treated initially with valproic acid. Soon thereafter the patient was noted to have ataxia and treatment was empirically switched to levetiracetam. Between 5 and 6 years of age he progressively deteriorated, with progressive ataxia, mild spasticity and loss of previous abilities like dressing himself and fecal continence. There was progressive loss of expressive speech, decreased understanding of simple sentences and interest in activities. He developed marked sialorrhea. Swallowing difficulties required thickening of oral fluids. Convulsions increased in frequency to 3–4 per day.
Cerebral magnetic resonance imaging at 5 years of age was normal, but at the age of 5 years 11 months re-examination of the brain and spinal cord revealed diffuse cortical and subcortical atrophy of supratentorial and infratentorial structures and ventricular enlargement. No abnormalities of signal were identified. The spinal cord was normal. Electroencephalography at 7.5 years of age showed abnormal diffuse slow wave activity and independent bifrontal, parieto-occipital and temporal epileptic foci. Ophthalmology evaluations at the ages of 5 years 9 months and 8 years 9 months were normal, with normal fundoscopic findings and absence of cherry red spots.
Diagnostic investigations initially focused on lysosomal storage diseases and the levels of glycosaminoglycans in the urine and activities of lysosomal hydrolases in the blood white cells were tested. All tests were normal including the levels of total β-hexosaminidase (3180 nmol/h/mg of protein, normal 1687–4509), HexA measured by thermoinactivation (1246 nmol/h/mg of protein (61%), normal 719–1942 (56–80%)), and HexA measured with specific sulfated substrate (366 nmol/h/mg of protein, normal 154–545).
These negative results lead to extensive further investigations. Positive findings are noted here, although their relationship if any with the primary condition is not obvious. They include mild persistent elevation of plasma aspartate aminotransferase (79–113 units/L, normal 11–43), and recently of alanine aminotransferase (65–160 units/L, normal 5–25) and mild hypercholesterolemia (5.81 mmol/L, normal 3.20–4.40) with normal glucose and triglyceride levels. Normal results were obtained for plasma amino acids, total homocystine, acylcarnitines, lactate, pyruvate, uric acid, creatine kinase, folate, vitamin B12, very long chain fatty acids, phytanic acid, pipecolic acid, copper and ceruloplasmin. Transferrin isoelectric focusing was normal. Urine glycosaminoglycans, organic acids, creatine, guanidinoacetoacetate, purine and pyrimidine metabolites, α-aminoadipic semialdehyde and copper were normal. Cerebrospinal fluid revealed normal levels of proteins (0.32 g/L, normal 0.15 to 0.40), glucose, lactate, 5-hydroxyindoleacetic acid, homovanillic acid, 3-O-methyldopa, neopterin, tetrahydrobiopterin, 5-methyltetrahydrofolate, amino acids and pyridoxal-5-phosphate.
Treatment with thiamine, biotin and riboflavin was started empirically, without noticeable effect. A trial of ketogenic diet for refractory seizures was stopped after six weeks due to lack of improvement, and worsening feeding difficulties with severe dysphagia. After exome sequencing revealed the mutations in GM2A as described below, vitamin therapy was stopped and a trial of corticotherapy (prednisone 2 mg/kg/day) was given over 3 months, because of its potential anticonvulsant and antiinflammatory effects. Corticotherapy reduced seizure frequency and severity, increased awake periods and increased appetite, but had no effect on the other aspects of the disease and progression. Due to the development of complications, prednisone was tapered and stopped over 3 months. Because of severe dysphagia and feeding difficulties, frequent aspiration and suboptimal hydration, a feeding gastrostomy was accepted by the family shortly before nine years of age. The patient had severe multifactorial sleep disturbances related to seizures, neuropathic pain and possibly to feeding difficulties and insufficient caloric intake. After gastrostomy and treatment with melatonin (6 mg po hs), his sleep pattern almost normalized. He is currently also followed by palliative care. He stopped walking at 7.5 years. He has severe spasticity and contractures in all limbs despite a daily program of stretching exercises. He has no visual contact. His ophthalmological exam remains normal without cherry red spots. Addition of phenytoin, substantially decreased the seizure frequency. He has focal right motor seizures, once or twice daily, usually in the morning. The major problem remains spasticity and neuropathic pain, for which different treatments have been tried without any major improvement.
3.2. Discovery and investigation of GM2AP deficiency
Whole exome sequencing was performed on leucocyte DNA from the patient. A novel heterozygous nonsense mutation c.259G > T (p.E87X) in exon 3 of GM2A gene was detected and confirmed by Sanger sequencing. The father was heterozygous for the c.259G > T change. This variant was not present in the Exome Aggregation Consortium (ExAC) or 1000 Genomes databases. Additionally, the missense mutation c.164C > T (p.P55L), recently reported in homozygosity in patients with progressive chorea-dementia [9], was identified in exon 2 of GM2A. By Sanger sequencing, the patient and his mother were both heterozygous. The sequences of other genes potentially implicated in neurological diseases including that of GLB1 were normal.
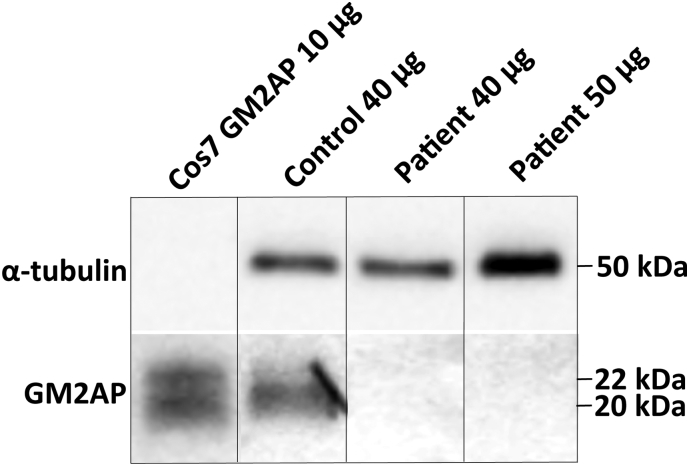
In order to study GM2AP production in the patient's cells we have established fibroblast cultures from patient's skin biopsy and analysed total protein extract by Western blot using goat polyclonal anti-GM2AP antibodies [10]. Our experiments (Fig. 1) showed that the patient's fibroblasts lacked the ~ 20 kDa protein band corresponding to the size of mature GM2AP [12], [13]. This band was present in fibroblast lines from 3 healthy controls, while the same antibodies detected 2 bands of ~ 22 and ~ 20 kDa (most likely GM2AP precursor and mature form, respectively [13]) in the total protein extract of Cos7 cells transfected with a plasmid encoding human GM2AP [12]. These results demonstrate that the GM2AP is reduced in the patient's cells to below detection level and suggest that both p.E87X and p.P55L mutations interfere with the synthesis and/or stability of GM2AP. We further tried to increase the level of GM2AP by treating cells in culture with ambroxol, which previously has been shown to increase GM2A expression at least 3 fold, presumably by activating TFEB transcription factor [14]. We also tried to rescue the folding of the mutant GM2AP by culturing fibroblasts at 30 °C [15]. In both cases, however the amount of GM2AP remained below the detection level of Western blot (data not shown).
Fig. 1.
GM2AP is not detected in the patient's cultured fibroblasts by Western blot.
Either 40 or 50 μg of total protein extracted from cultured fibroblasts of the patient or normal healthy controls (N = 2) were resolved on SDS PAGE gels, transferred to nitrocellulose membrane and hybridized with anti-GM2AP antibody. A mature ~ 20 kDa GM2AP band was detected in control fibroblasts but not in the patient's fibroblasts. The two bands detected in the protein extract of Cos7 cells transfected with a plasmid encoding GM2AP correspond to the mature ~ 20 kDa protein and its ~ 22 kDa precursor. α-Tubulin was used as a loading control.
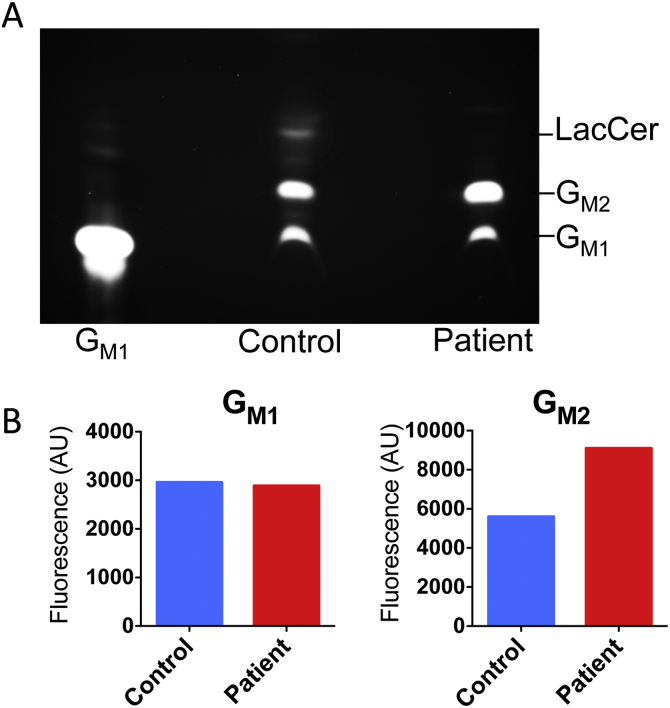
The catabolism of GM2 ganglioside in the patient's and normal healthy control fibroblasts was analysed by metabolic labeling with fluorescently (BODIPY)-labeled GM1 ganglioside for 48 h. Analysis by TLC of the fluorescently labeled gangliosides extracted from the cell pellets (Fig. 2A) showed that control cells converted the fluorophore-labeled GM1 to GM2 and further, to lactosylceramide (LacCer). Patient's cells also generated GM2 but failed to produce LacCer. In contrast, the amount of GM2 was significantly increased indicating that this ganglioside is stored in the patient's cells. The results of TLC analysis of the neutral lipid fraction (Supplemental Fig. 1) are also consistent with the above hypothesis indicating that the cells from normal controls contained multiple fluorescently labeled lipid bands (presumably BODIPY-labeled LacCer, glucosylceramide, and ceramide) undetectable in the patient's cells.
Fig. 2.
Thin-layer chromatography of fluorescently labeled gangliosides reveals accumulation of GM2 ganglioside in the patient's cultured fibroblasts.
Fibroblasts of the patient or a normal healthy control subject were cultured in DMEM containing 1.45 μM of fluorescently labeled BODIPY® FL C5 GM1 ganglioside for 72 h. Total lipids were extracted from cell pellets with 1:1 chloroform/methanol mixture and the gangliosides were separated from neutral lipids and analysed by TLC on silica plates.
(A) The fluorescence image of the TLC plate acquired on G:Box Chemi XQR system. The position of fluorescent GM1 ganglioside used as a standard is shown.
(B) GM1 and GM2 ganglioside bands were quantified in control and patient's fibroblasts by ImageJ software.
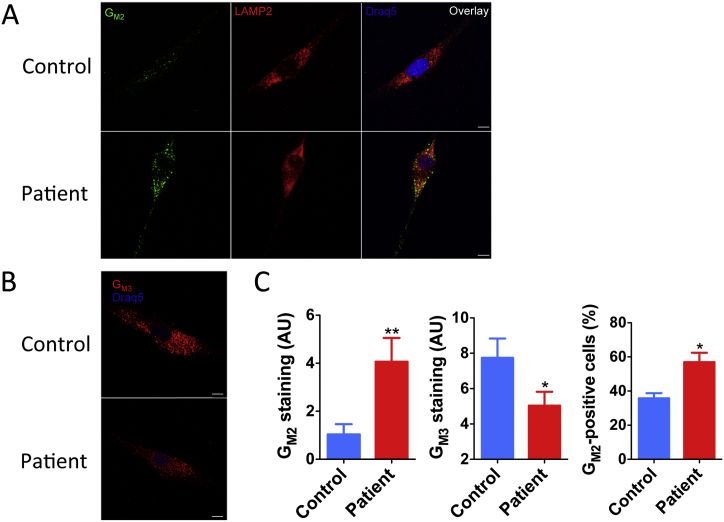
To determine if GM2 was stored in lysosomes of cultured fibroblasts from the patient, we analysed them by immunohistochemistry using monoclonal antibodies specific to GM2 ganglioside or its catabolic product GM3 ganglioside (Fig. 3). The cells were stained either with anti-GM2 or anti-GM3 monoclonal antibodies and the antibodies against the lysosomal marker protein, LAMP-2. The intensity of GM2 and GM3 staining was quantified using ImageJ software in randomly selected isolated fibroblasts and the percent of cells with strong staining against GM2 was counted in randomly selected fields by a person who was unaware of the cell genotype. Our results (Fig. 3A and B) indicate that the patient's cells show significantly increased staining for GM2 and decreased staining for GM3 ganglioside consistent with compromised conversion of GM2 into GM3. In the patient's (but not in the control cells) GM2 staining co-localized with the lysosomal marker LAMP-2 (Fig. 3A).
Fig. 3.
Increased lysosomal GM2 and reduced GM3 ganglioside in the patient's cultured fibroblasts.
(A) Fibroblasts from the patient and two normal healthy controls cultured on glass coverslips were fixed and stained with monoclonal humanized anti-GM2 and mouse anti-LAMP-2 antibodies followed by anti-human IgG Alexa 488-labeled (green) and anti-mouse IgG Alexa 555-labeled (red) secondary antibodies. Nuclei were stained with Draq5 (blue). The patient's fibroblasts show a higher intensity of GM2 staining (green) than control fibroblasts and increased co-localization of GM2 and LAMP-2 staining.
(B) Fibroblasts from the patient and two normal healthy controls were stained with monoclonal mouse anti-GM3 antibody followed by anti-mouse IgG Alexa-555-labeled secondary antibody (red). Nuclei were stained with Draq5 (blue).
The images were acquired in a Leica SP8 confocal microscope with a 63 × objective. Bar represents 10 μM. The panels show representative images of at least 40 studied for each cell type.
(C) Mean GM2 and GM3 staining intensity and a fraction of GM2-positive cells in control and patient's fibroblasts. Mean staining intensities per μm2 were measured with ImageJ software. Data show mean (± SEM) of individual values measured for 35 randomly selected cells. **P < 0.01, *P < 0.05 in unpaired two-tailed t-test. GM2-positive cells were manually counted in three randomly selected microscope fields (~ 150 cells each). Data show mean values (± SEM) of 3 independent experiments. *P < 0.05 in unpaired two-tailed t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We report the clinical course of a unique patient with GM2 gangliosidosis AB with a juvenile onset and without ocular features such as progressive blindness and macular cherry-red spots. The patient illustrates several points of clinical importance. First, the diagnosis of GM2 gangliosidosis AB must be suspected in the presence of neurodegenerative signs if β-hexosaminidase values in leucocytes or cultured fibroblasts are normal. Second, gene sequencing is therefore likely to be the first line diagnostic technique for atypical cases of GM2 gangliosidosis AB. This patient received an extensive metabolic workup that was otherwise negative. GM2 gangliosidosis AB was suspected only after GM2A mutations were identified by exome sequencing. Biochemical diagnosis of GM2 gangliosidosis AB is essential for confirming the biological activity of DNA sequence variants but it requires a high level of expertise and will likely remain confined to a small number of specialized laboratories. This suggests that GM2A sequencing should be included in broad panels for neurodegenerative conditions.
The phenotypic spectrum of GM2 gangliosidosis AB may be broader than previously thought. Macular cherry red spots were absent in this patient. Cherry red spots are characteristic of infantile Tay-Sachs and Sandhoff diseases, but are rarely present in juvenile or subacute cases [16], [17], [18], [19], [20], [21], [22], [23]. The lack of cherry red spots may also apply to later-onset cases of GM2 gangliosidosis AB. However, patients with neurodegenerative conditions with normal β-hexosaminidase activity and without cherry red spots would probably not have been submitted for detailed biochemical testing for the possibility of GM2 gangliosidosis. Therefore, it is possible that higher clinical suspicion or unbiased genomic testing will identify new patients and reveal the complete phenotypic spectrum of GM2 gangliosidosis AB. Technically, our results also show that the accumulation of GM2 ganglioside in the cultured fibroblasts from the patients affected with GM2 gangliosidosis AB can be demonstrated by immunohistochemistry even without supplementing the cell medium with an excess of GM1 or GM2 ganglioside. This method may therefore be useful for confirmation of diagnosis in some particular cases.
The reported patient is a compound heterozygote for two mutations in the GM2A gene: a novel nonsense mutation c.259G > T (p.E87X) and a missense mutation c.164C > T (p.P55L) recently reported by Salih et al. [9] and according to our analysis classified as potentially damaging by both SIFT and Polyphen-2 software. The novel point mutation c.259G > T results in a transcript containing a premature termination codon. The GM2A mRNA containing c.259G > T will likely undergo mRNA nonsense-mediated decay [24], [25]. In turn, Pro55 conserved among at least 17 mammalian species is important for supporting the conformation of the turn between two β-strands of GM2AP [26], suggesting that its replacement with the bulky hydrophobic Leu interferes with the tertiary structure of the protein. A misfolded mutant GM2AP may be then targeted for degradation by the Endoplasmatic Reticulum-Associated Degradation pathway (ERAD). This interpretation is consistent with the results of the Western blot analysis, which did not detect GM2AP cross-reactive band in cultured patient's fibroblasts.
Until recently seven GM2A mutations have been described in the AB-variant of GM2 gangliosidosis [8], [27], [28]. Only two of them were missense: p.Cys138Arg was identified in a female patient who died at the age of 14 months and was diagnosed after death [29], [30] and p.Arg169Pro, also identified in infantile-onset patient [10]. Both patients were homozygous for the corresponding mutations. Further biochemical studies in patient fibroblasts identified that both of these mutations caused retention and degradation of the mutant proteins in the endoplasmic reticulum [10], [31].
In contrast, the later onset and milder symptoms of the patient reported here might be due to the presence of residual levels of active or partially active GM2AP carrying P55L substitution. As it is the case in many lysosomal diseases, the severity and onset of GM2 gangliosidosis inversely correlates with the residual rate of GM2 catabolism. Specifically, in infantile Tay-Sachs and Sandhoff patients the residual GM2 conversion rate does not exceed 0.5%, in juvenile and adult forms it reaches 2–4% of normal, while the residual rates above about 10% of normal seem to be compatible with the healthy state [32], [33]. Interestingly, the three recently reported patients homozygous for p.P55L in GM2AP presented with a neurological movement disease without ocular features and an onset at the age of 7–8 years [9]. Although this study did not describe if the patients had GM2 storage it is tempting to speculate that our patient had milder phenotype due to the presence of the Leu55 allele. The amount of the GM2AP protein in the cultured fibroblasts from the patient remained however below detection level even when we tried to increase its synthesis by treating cells with ambroxol to activate its expression [14] or by culturing them at 30 °C, to rescue the fold of the GM2AP mutant [15]. A precise quantification of the GM2 conversion rate is necessary therefore to prove this hypothesis. Alternatively, relatively milder phenotype of our patient can be related to the effects of other genes, which remain to be investigated.
The following is the supplementary data related to this article.
Fluorescently labeled neutral glycosphingolipids are decreased in patient's fibroblasts. Fibroblasts of the patient or normal healthy control subject were cultured in the presence of 1.45 μM fluorescently labeled BODIPY® FL C5 GM1 ganglioside for 72 h. Total lipids were extracted from cell pellets with 1:1 chloroform/methanol mixture and the neutral lipids were separated from gangliosides and analysed by TLC on silica plates. The fluorescence image of the TLC plate was acquired on G:Box Chemi XQR system.
Funding
Supported in part by the operating grant (111068) from Canadian Institutes of Health Research to A.V.P. and the Ph.D. scholarship (SFRH/BD/84929/2012) from the Fundação para a Ciência e a Tecnologia (FCT, Portugal) financed by POPH/FSE to C.M.
Acknowledgements
We thank the patient and his parents for their support and participation in this study, Dr. Nobuo Hanai, Dr. Akiko Furuya and Kyowa Hakko Kirin Co., Ltd. for a generous gift of monoclonal antibodies against GM2 ganglioside, Dr. Xuefang Pan for the help with studying gangliosides by TLC and Dr. Gaziella Di Christo for providing access to confocal microscopy facilities. We are also grateful to Dr. Thierry Levade (Institut de Médecine Moléculaire de Rangueil, Université Toulouse III Paul-Sabatier, Equipe 14, IFR31, Toulouse) and Dr. Mila Ashmarina (CHU Ste-Justine) for helpful advice.
Footnotes
The authors do not have conflict of interests concerning this work.
References
- 1.Sandhoff K., Harzer K. Gangliosides and gangliosidoses: principles of molecular and metabolic pathogenesis. J. Neurosci. 2013;33:10195–10208. doi: 10.1523/JNEUROSCI.0822-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkhardt J.K., Huttler S., Klein A., Mobius W., Habermann A., Griffiths G., Sandhoff K. Accumulation of sphingolipids in SAP-precursor (prosaposin)-deficient fibroblasts occurs as intralysosomal membrane structures and can be completely reversed by treatment with human SAP-precursor. Eur. J. Cell Biol. 1997;73:10–18. [PubMed] [Google Scholar]
- 3.Mobius W., Herzog V., Sandhoff K., Schwarzmann G. Intracellular distribution of a biotin-labeled ganglioside, GM1, by immunoelectron microscopy after endocytosis in fibroblasts. J. Histochem. Cytochem. 1999;47:1005–1014. doi: 10.1177/002215549904700804. [DOI] [PubMed] [Google Scholar]
- 4.Gallala H.D., Sandhoff K. Biological function of the cellular lipid BMP-BMP as a key activator for cholesterol sorting and membrane digestion. Neurochem. Res. 2011;36:1594–1600. doi: 10.1007/s11064-010-0337-6. [DOI] [PubMed] [Google Scholar]
- 5.Gallala H.D., Breiden B., Sandhoff K. Regulation of the NPC2 protein-mediated cholesterol trafficking by membrane lipids. J. Neurochem. 2011;116:702–707. doi: 10.1111/j.1471-4159.2010.07014.x. [DOI] [PubMed] [Google Scholar]
- 6.Smutova V., Albohy A., Pan X., Korchagina E., Bovin N., Cairo C.W., Pshezhetsky A.V. Structural basis for substrate specificity of mammalian neuraminidases. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravel R., Kaback M.M., Proia R.L., Sandhoff K., Suzuki K. The GM2 gangliosidoses. In: Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. The Metabolic and Molecular Bases of Inherited Diseases. McGraw-Hill New York; NY (USA): 2001. pp. 3827–3876. [Google Scholar]
- 8.Sheth J., Datar C., Mistri M., Bhavsar R., Sheth F. Shah K GM2 gangliosidosis AB variant: novel mutation from India - a case report with a review. BMC Pediatr. 2016;16:88. doi: 10.1186/s12887-016-0626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salih M.A., Seidahmed M.Z., El Khashab H.Y. Mutation in GM2A leads to a progressive chorea-dementia syndrome. Tremor Other Hyperkinet. Mov. (N Y) 2015;5:306. doi: 10.7916/D8D21WQ0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroder M., Schnabel D., Hurwitz R., Young E., Suzuki K., Sandhoff K. Molecular genetics of GM2-gangliosidosis AB variant: a novel mutation and expression in BHK cells. Hum. Genet. 1993;92:437–440. doi: 10.1007/BF00216446. [DOI] [PubMed] [Google Scholar]
- 11.Seyrantepe V., Canuel M., Carpentier S. Mice deficient in Neu4 sialidase exhibit abnormal ganglioside catabolism and lysosomal storage. Hum. Mol. Genet. 2008;17:1556–1568. doi: 10.1093/hmg/ddn043. [DOI] [PubMed] [Google Scholar]
- 12.Schepers U., Glombitza G., Lemm T., Hoffmann A., Chabas A., Ozand P., Sandhoff K. Molecular analysis of a GM2-activator deficiency in two patients with GM2-gangliosidosis AB variant. Am. J. Hum. Genet. 1996;59:1048–1056. [PMC free article] [PubMed] [Google Scholar]
- 13.Glombitza G.J., Becker E., Kaiser H.W., Sandhoff K. Biosynthesis, processing, and intracellular transport of GM2 activator protein in human epidermal keratinocytes. The lysosomal targeting of the GM2 activator is independent of a mannose-6-phosphate signal. J. Biol. Chem. 1997;272:5199–5207. doi: 10.1074/jbc.272.8.5199. [DOI] [PubMed] [Google Scholar]
- 14.McNeill A., Magalhaes J., Shen C. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain. 2014;137:1481–1495. doi: 10.1093/brain/awu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meijer O.L., Welling L., Valstar M.J. Residual N-acetyl-alpha-glucosaminidase activity in fibroblasts correlates with disease severity in patients with mucopolysaccharidosis type IIIB. J. Inherit. Metab. Dis. 2016;39:437–445. doi: 10.1007/s10545-016-9916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nardocci N., Bertagnolio B., Rumi V., Angelini L. Progressive dystonia symptomatic of juvenile GM2 gangliosidosis. Mov. Disord. 1992;7:64–67. doi: 10.1002/mds.870070113. [DOI] [PubMed] [Google Scholar]
- 17.Nalini A., Christopher R. Cerebral glycolipidoses: clinical characteristics of 41 pediatric patients. J. Child Neurol. 2004;19:447–452. doi: 10.1177/088307380401900610. [DOI] [PubMed] [Google Scholar]
- 18.Maegawa G.H., Stockley T., Tropak M. The natural history of juvenile or subacute GM2 gangliosidosis: 21 new cases and literature review of 134 previously reported. Pediatrics. 2006;118:e1550–e1562. doi: 10.1542/peds.2006-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozenberg R., Kok F., Burin M.G., Sa Miranda M.C. Diagnosis and molecular characterization of non-classic forms of Tay-Sachs disease in Brazil. J. Child Neurol. 2006;21:540–544. doi: 10.1177/08830738060210061101. [DOI] [PubMed] [Google Scholar]
- 20.Wortmann S.B., Lefeber D.J., Dekomien G. Substrate deprivation therapy in juvenile Sandhoff disease. J. Inherit. Metab. Dis. 2009;32(Suppl. 1):S307–S311. doi: 10.1007/s10545-009-1261-2. [DOI] [PubMed] [Google Scholar]
- 21.Levit A., Nutman D., Osher E., Kamhi E., Navon R. Two novel exonic point mutations in HEXA identified in a juvenile Tay-Sachs patient: role of alternative splicing and nonsense-mediated mRNA decay. Mol. Genet. Metab. 2010;100:176–183. doi: 10.1016/j.ymgme.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Smith N.J., Winstone A.M., Stellitano L., Cox T.M., Verity C.M. GM2 gangliosidosis in a UK study of children with progressive neurodegeneration: 73 cases reviewed. Dev. Med. Child Neurol. 2012;54:176–182. doi: 10.1111/j.1469-8749.2011.04160.x. [DOI] [PubMed] [Google Scholar]
- 23.Georgiou T., Christopoulos G., Anastasiadou V. The first family with Tay-Sachs disease in Cyprus: genetic analysis reveals a nonsense (c.78G > A) and a silent (c.1305C > T) mutation and allows preimplantation genetic diagnosis. Meta Gene. 2014;2:200–205. doi: 10.1016/j.mgene.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maquat L.E. When cells stop making sense: effects of nonsense codons on RNA metabolism in vertebrate cells. RNA. 1995;1:453–465. [PMC free article] [PubMed] [Google Scholar]
- 25.Holbrook J.A., Neu-Yilik G., Hentze M.W., Kulozik A.E. Nonsense-mediated decay approaches the clinic. Nat. Genet. 2004;36:801–808. doi: 10.1038/ng1403. [DOI] [PubMed] [Google Scholar]
- 26.Wright C.S., Li S.C., Rastinejad F. Crystal structure of human GM2-activator protein with a novel beta-cup topology. J. Mol. Biol. 2000;304:411–422. doi: 10.1006/jmbi.2000.4225. [DOI] [PubMed] [Google Scholar]
- 27.Mahuran D.J. Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim. Biophys. Acta. 1999;1455:105–138. doi: 10.1016/s0925-4439(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 28.Renaud D., Brodsky M. GM2-gangliosidosis, AB variant: clinical, ophthalmological, MRI, and molecular findings. JIMD Rep. 2015 doi: 10.1007/8904_2015_469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroder M., Schnabel D., Suzuki K., Sandhoff K. A mutation in the gene of a glycolipid-binding protein (GM2 activator) that causes GM2-gangliosidosis variant AB. FEBS Lett. 1991;290:1–3. doi: 10.1016/0014-5793(91)81211-p. [DOI] [PubMed] [Google Scholar]
- 30.de Baecque C.M., Suzuki K., Rapin I., Johnson A.B., Whethers D.L. GM2-gangliosidosis, AB variant: clinico-pathological study of a case. Acta Neuropathol. 1975;33:207–226. doi: 10.1007/BF00688395. [DOI] [PubMed] [Google Scholar]
- 31.Xie B., Rigat B., Smiljanic-Georgijev N., Deng H., Mahuran D. Biochemical characterization of the Cys138Arg substitution associated with the AB variant form of GM2 gangliosidosis: evidence that Cys138 is required for the recognition of the GM2 activator/GM2 ganglioside complex by beta-hexosaminidase A. Biochemistry. 1998;37:814–821. doi: 10.1021/bi971211s. [DOI] [PubMed] [Google Scholar]
- 32.Sandhoff K. My journey into the world of sphingolipids and sphingolipidoses. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012;88:554–582. doi: 10.2183/pjab.88.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke J.T., Mahuran D.J., Sathe S., Kolodny E.H., Rigat B.A., Raiman J.A., Tropak M.B. An open-label phase I/II clinical trial of pyrimethamine for the treatment of patients affected with chronic GM2 gangliosidosis (Tay-Sachs or Sandhoff variants) Mol. Genet. Metab. 2011;102:6–12. doi: 10.1016/j.ymgme.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescently labeled neutral glycosphingolipids are decreased in patient's fibroblasts. Fibroblasts of the patient or normal healthy control subject were cultured in the presence of 1.45 μM fluorescently labeled BODIPY® FL C5 GM1 ganglioside for 72 h. Total lipids were extracted from cell pellets with 1:1 chloroform/methanol mixture and the neutral lipids were separated from gangliosides and analysed by TLC on silica plates. The fluorescence image of the TLC plate was acquired on G:Box Chemi XQR system.