Abstract
Single doses of MAALOX TC and ranitidine were administered separately with 1,400 mg of fosamprenavir (FPV). MAALOX TC decreased the area under the concentration-time curve from 0 to 24 h (AUC0-24) for plasma amprenavir (APV) by 18% and the maximum concentration of drug in serum (Cmax) by 35%; the plasma APV concentration at 12 h (C12) increased by 14%. Ranitidine at 300 mg decreased the AUC0-24 for plasma APV by 30% and Cmax by 51%; C12 was unchanged. FPV may be coadministered with antacids without concern and without separation in dosing; however, caution is recommended when FPV is coadministered with histamine2- receptor antagonists or proton pump inhibitors.
Fosamprenavir (FPV, GW433908) has been approved for treatment of human immunodeficiency virus (HIV)-infected adult patients. FPV, a phosphoester prodrug, is rapidly and extensively hydrolyzed to the HIV protease inhibitor amprenavir (APV) during absorption, with minimal systemic FPV exposure (12).
Due to the chemical properties of FPV, an interaction with both antacids and histamine2-receptor antagonists is possible. FPV exhibits pH-dependent solubility, with maximal solubility at pH 3.3 and reduced solubility at higher pHs (5). The phosphate group on FPV could bind to the metal cations contained in antacids, which could either alter solubility or prevent presystemic conversion of FPV to APV. This study assessed the effects of antacids and ranitidine on single-dose plasma APV pharmacokinetics following administration of FPV.
This single-dose, open, randomized, three-way balanced crossover study included administration of 1,400 mg of FPV alone, 1,400 mg of FPV immediately following 30 ml of oral antacid (MAALOX TC; Novartis Consumer Health), 1,800 mg of magnesium hydroxide and 3,600 mg of aluminum hydroxide dried gel (2,754 mg of aluminum hydroxide), and 1,400 mg of FPV 1 h after 300 mg of ranitidine. There was a 4- to 7-day washout between each treatment. Subjects fasted overnight, continuing until 4 h after dosing. Water was permitted ad libitum during the overnight fast. The study drug was administered with 180 ml of water, and additional water was permitted ad libitum from 2 h after dosing. The container in which MAALOX TC was administered was rinsed, and the water was consumed. Blood samples were collected in sodium citrate-containing tubes (Vacutainers; Becton-Dickinson) at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 16, and 24 h after dosing. Prior to analysis, plasma was stored at or below 30°C, at which temperature stability has been confirmed for 32 months. Plasma APV and FPV concentrations were measured within 4 months of initial dosing using a validated high-performance liquid chromatography assay with tandem mass spectrometric detection following solid-phase extraction (the linear range for APV was 10 to 10,000 ng/ml, and that for FPV was 5 to 1,000 ng/ml). For APV, intra-assay precision, interassay precision (percent coefficient of variation), and accuracy (percent bias) were ≤13.83, ≤2.57, and ≤10.47, respectively. Pharmacokinetic analysis of plasma APV concentration-time data was conducted utilizing noncompartmental model 200 (for extravascular administration), with the log linear trapezoidal calculation method, of the WinNonLin Professional version 3.0 software package (Pharsight Corporation, Mountain View, Calif.). Assuming an intrasubject log area under the concentration-time curve (AUC) and a standard deviation for log maximum concentration of drug in serum (Cmax) of 0.26 (based upon previous FPV pharmacokinetic studies), it was estimated that 24 evaluatable subjects were needed to provide 80% power for the 90% confidence intervals (CIs) of the treatment ratios to fall within 0.75 to 1.33 for the AUC from 0 h to infinity (AUC0-∞) of plasma APV and 0.70 to 1.43 for Cmax. The CI of 0.75 to 1.33 was considered clinically meaningful for the AUC0-∞ of plasma APV; a wider CI was used for Cmax since this parameter is considered less clinically relevant for performance indexes. Analysis of variance, considering sequence, period, and treatment as fixed effects and subject within sequence as a random effect, was performed using the SAS (version 6.12) mixed linear models procedure. Combination treatments were compared to FPV alone.
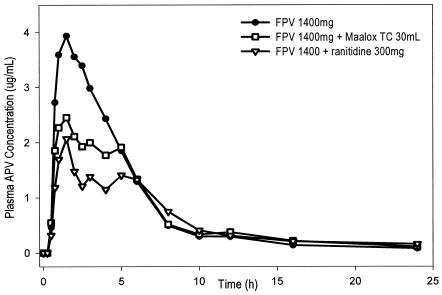
Thirty healthy subjects gave written informed consent, and 26 (24 male and 2 female) subjects completed the study. There were no serious adverse events during the study, and no subject withdrew due to a drug-related adverse event. Figure 1 depicts the median plasma APV concentration-versus-time profile for each of the three treatments. Plasma APV pharmacokinetic parameters and geometric least-squares mean treatment ratios (90% CI) are summarized in Table 1. When FPV was administered immediately following 30 ml of MAALOX TC, plasma APV AUC0-∞, AUC0-24, and Cmax were reduced by 15, 18, and 35%, respectively. When FPV was administered 1 h after 300 mg of ranitidine, the AUC0-∞, AUC0-24, and Cmax of plasma APV were reduced by 26, 30, and 51%, respectively. Neither coadministration of MAALOX TC or of ranitidine with FPV resulted in a statistically significant difference in APV plasma concentration at 12 h (C12). The plasma APV maximum half-life (tmax) was increased with ranitidine pretreatment. Plasma FPV concentrations were very low (range, below the limit of quantification [0.005 μg/ml] to 0.057 μg/ml) and quantifiable at few time points (within 4 h postdose). However, more subjects receiving FPV alone (22 of 26; 85%) had quantifiable plasma FPV concentrations than subjects receiving FPV with MAALOX TC (1 of 26; 4%) or subjects receiving FPV after ranitidine (10 of 26; 38%).
FIG. 1.
Median plasma APV concentration-versus-time profiles following single-dose administration of 1,400 mg of FPV, 1,400 mg of FPV plus 30 ml of MAALOX TC, and 1,400 mg of FPV 1 h after 300 mg of ranitidine (number of subjects, 26 per group).
TABLE 1.
Plasma APV pharmacokinetic parameters and treatment comparisonsa
| Treatment or comparison | AUC0-24 (μg · h/ml) | AUC0-∞ (μg · h/ml) | Cmax (μg/ml) | C12 (μg/ml) | tmax (h)d |
|---|---|---|---|---|---|
| Treatmentsb | |||||
| FPV | 20.67 (17.31-24.69) | 22.05 (18.50-26.30) | 4.73 (4.11-5.45) | 0.32 (0.25-0.40) | 1.50 (0.75-5.00) |
| FPV + MAALOX TC | 16.98 (14.32-20.14) | 18.71 (15.72-22.26) | 3.09 (2.69-3.54) | 0.36 (0.28-0.48) | 1.50 (0.75-5.00) |
| FPV + ranitidine | 14.45 (11.46-18.22) | 16.03 (12.55-20.48) | 2.31 (1.86-2.87) | 0.32 (0.24-0.42) | 1.75 (0.75-5.00) |
| Comparisonsc | |||||
| FPV + MAALOX TC vs FPV | 0.82 (0.74-0.91) | 0.85 (0.77-0.94) | 0.65 (0.57-0.76) | 1.14 (0.93-1.39) | 1.18 (0.84-1.39) |
| FPV + ranitidine vs FPV | 0.70 (0.63-0.78) | 0.74 (0.66-0.82) | 0.49 (0.42-0.57) | 0.99 (0.81-1.21) | 1.37 (1.03-1.71) |
All data are based on results for a group of 26 subjects, except for the AUC0-∞ data, which are based on a group of 24 because λz (apparent terminal phase rate constant) was not estimated from plasma APV concentration-time profiles for two subjects. All dosages were as follows: FPV, 1,400 mg; MAALOX TC, 30 ml; ranitidine, 300 mg.
For all treatment data except tmax values, geometric means are given with 95% CIs in parentheses.
For all comparison data except tmax values, geometric least-squares mean ratios are given with 90% CIs in parentheses.
The tmax treatment and comparison data are expressed as medians with ranges in parentheses and least-squares mean ratios with 90% CIs in parentheses, respectively.
In this study, both MAALOX TC and ranitidine decreased plasma APV exposure. Although no statistical comparison between the two test treatments was made, ranitidine appeared to result in a larger decrease in plasma APV exposure than MAALOX TC. Given that ranitidine does not have a chelation effect but results in a more durable increase in gastric pH than MAALOX TC (11), the mechanism of these interactions was likely related to increased gastric pH, although a component of phosphate binding by the antacid cannot be ruled out. Increased gastric pH has also decreased the absorption of the HIV protease inhibitors atazanavir (2) and indinavir (3) but to a much greater magnitude (>80% decrease in plasma atazanavir and indinavir exposures) than that observed for FPV.
Both steady-state plasma protease inhibitor AUCs (AUCτsss [area under the curve over the dosing interval at steady state]) and trough concentrations (Cτsss [plasma concentration at the end of the dosing interval at steady state]) have been correlated with antiviral activity and development of resistance (1, 4, 6-8, 10). Plasma APV Cτss was determined to be a better predictor of viral load reduction at 4 weeks than AUCτss (9; G. L. Drusano, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-16, 1997). If it is assumed that single-dose plasma APV C12 is a surrogate for plasma APV Cτss, the lack of effect of either MAALOX or ranitidine on plasma APV C12 suggests that plasma APV Cτss would be unaffected by these absorption interactions, and thus, antiviral activity may not be compromised.
Given the minor reduction in plasma APV AUC and lack of change in C12, FPV may be coadministered with antacids, such as MAALOX TC, without concern and without separation in dosing. The clinical significance of the moderate reduction in plasma APV AUC without a corresponding decrease in C12 following coadministration of FPV and ranitidine is unclear. Therefore, FPV and histamine2-receptor antagonists should be coadministered with caution, because reduced plasma APV concentrations may result in a lowered virologic response. The impact of proton pump inhibitors on plasma APV pharmacokinetics following coadministration with FPV will be evaluated in a future study. Until data are available, the combination of FPV and proton pump inhibitors should also be used with caution.
REFERENCES
- 1.Acosta, E. P., K. Henry, L. Baken, L. M. Page, and C. V. Fletcher. 1999. Indinavir concentrations and antiviral effect. Pharmacotherapy 19:708-712. [DOI] [PubMed] [Google Scholar]
- 2.Bristol-Myers Squibb. July 2004. REYETAZ (atazanavir sulfate) product information. Bristol-Myers Squibb, Princeton, N.J.
- 3.Bristol-Myers Squibb. January 2004. VIDEX (didanosine) product information. Bristol-Myers Squibb, Princeton, N.J.
- 4.Fletcher, C. V., R. C. Brundage, R. P. Remmel, L. M. Page, D. Weller, N. R. Calles, C. Simon, and M. W. Kline. 2000. Pharmacologic characteristics of indinavir, didanosine, and stavudine in human immunodeficiency virus-infected children receiving combination therapy. Antimicrob. Agents Chemother. 44:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furfine, E. S., C. T. Baker, M. R. Hale, D. J. Reynolds, J. A. Salisbury, A. D. Searle, S. D. Studenberg, D. Todd, R. D. Tung, and A. Spaltenstein. 2004. Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir. Antimicrob. Agents Chemother. 48:791-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas, D. W., E. Arathoon, M. A. Thompson, R. D. J. Pedro, J. E. Gallant, D. E. Uip, J. Currier, L. M. Noriega, D. S. Lewi, P. Uribe, L. Benetucci, P. Cahn, D. Paar, A. C. White, Jr., A. C. Collier, C. H. Ramirez-Ronda, C. Harvey, M. O. Chung, D. Mehrotra, J. Chodakewitz, and B. Y. Nguyen. 2000. Comparative studies of two-times-daily versus three-times-daily indinavir in combination with zidovudine and lamivudine. AIDS 14:1973-1978. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzi, P., S. Yerly, K. Abderrakim, M. Fathi, O. T. Rutschmann, J. von Overbeck, D. Leduc, L. Perrin, B. Hirschel, et al. 1997. Toxicity, efficacy, plasma drug concentrations and protease mutations in patients with advanced HIV infection treated with ritonavir plus saquinavir. AIDS 11:F95-F99. [DOI] [PubMed] [Google Scholar]
- 8.Molla, A., M. Korneyeva, Q. Gao, S. Vasavanonda, P. J. Schipper, H. M. Mo, M. Markowitz, T. Chernyavskiy, P. Niu, N. Lyons, A. Hsu, G. R. Granneman, D. D. Ho, C. A. Boucher, J. M. Leonard, D. W. Norbeck, and D. J. Kempf. 1996. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat Med. 2:760-766. [DOI] [PubMed] [Google Scholar]
- 9.Sadler, B. M., C. Gillotin, Y. Lou, and D. S. Stein. 2001. Pharmacokinetic and pharmacodynamic study of the human immunodeficiency virus protease inhibitor amprenavir after multiple oral dosing. Antimicrob. Agents Chemother. 45:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein, D. S., D. G. Fish, J. A. Bilello, S. L. Preston, G. L. Martineau, and G. L. Drusano. 1996. A 24-week open-label phase I/II evaluation of the HIV protease inhibitor MK-639 (indinavir). AIDS 10:485-492. [DOI] [PubMed] [Google Scholar]
- 11.Watson, R. G., B. T. Johnston, T. C. Tham, and K. Kersey. 1996. Effervescent and standard formulations of ranitidine—a comparison of their pharmacokinetics and pharmacology. Aliment. Pharmacol. Ther. 10:913-918. [DOI] [PubMed] [Google Scholar]
- 12.Wood, R., K. Arasteh, H. J. Stellbrink, E. Teofilo, F. Raffi, R. B. Pollard, J. Eron, J. Yeo, J. Millard, M. B. Wire, and O. J. Naderer. 2004. Six-week randomized controlled trial to compare the tolerabilities, pharmacokinetics, and antiviral activities of GW433908 and amprenavir in human immunodeficiency virus type 1-infected patients. Antimicrob. Agents Chemother. 48:116-123. [DOI] [PMC free article] [PubMed] [Google Scholar]