Abstract
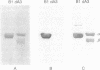
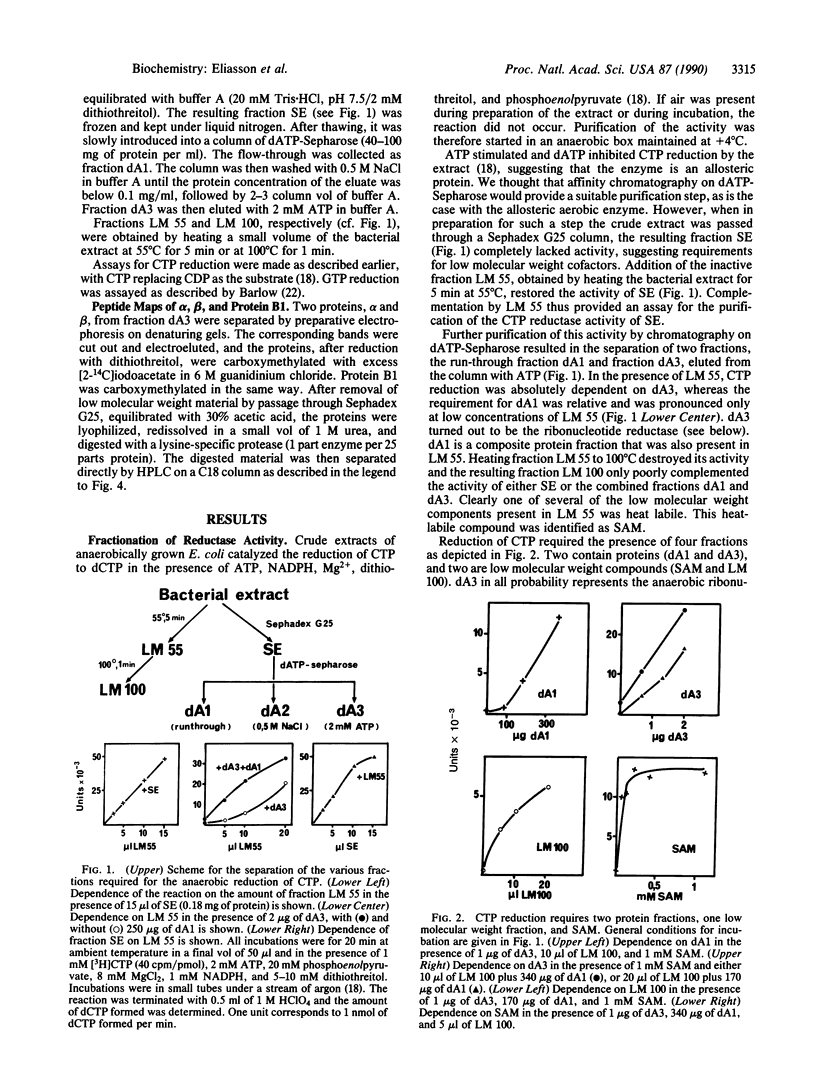
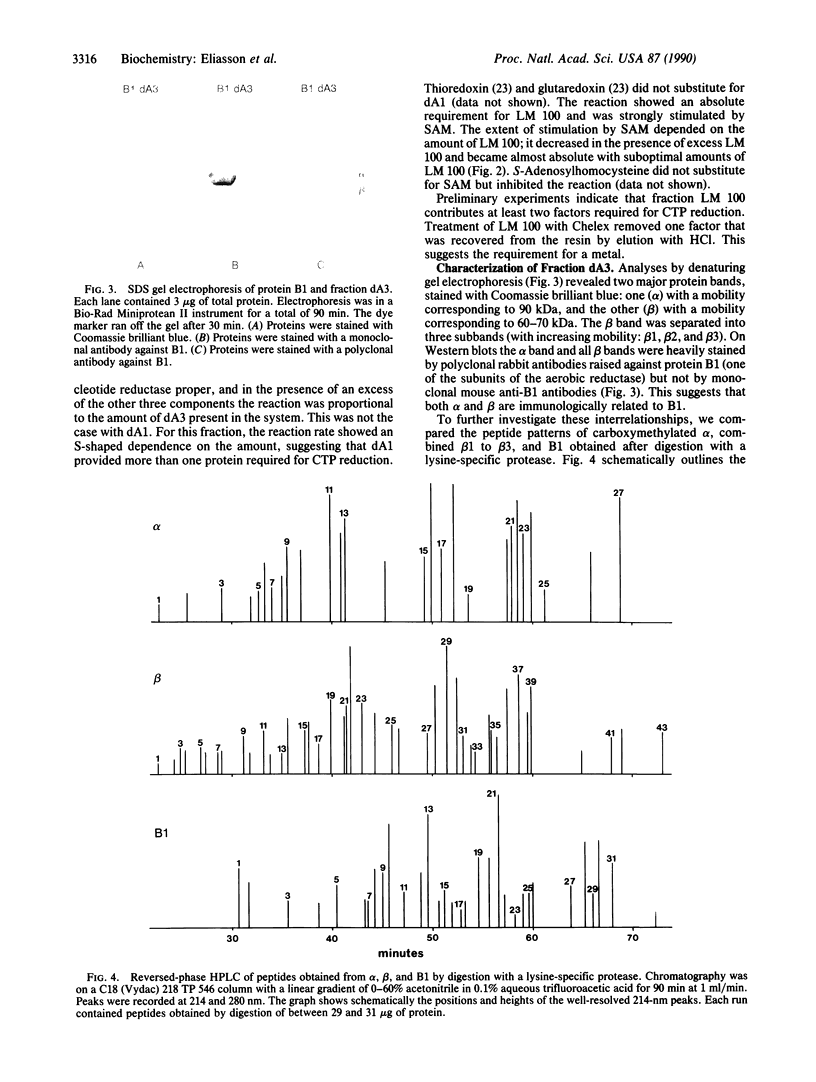
Extracts from anaerobically grown Escherichia coli contain an oxygen-sensitive activity that reduces CTP to dCTP in the presence of NADPH, dithiothreitol, Mg2+ ions, and ATP, different from the aerobic ribonucleoside diphosphate reductase (2'-deoxyribonucleoside-diphosphate: oxidized-thioredoxin 2'-oxidoreductase, EC 1.17.4.1) present in aerobically grown E. coli. After fractionation, the activity required at least five components, two heat-labile protein fractions and several low molecular weight fractions. One protein fraction, suggested to represent the actual ribonucleoside triphosphate reductase was purified extensively and on denaturing gel electrophoresis gave rise to several defined protein bands, all of which were stained by a polyclonal antibody against one of the two subunits (protein B1) of the aerobic reductase but not by monoclonal anti-B1 antibodies. Peptide mapping and sequence analyses revealed partly common structures between two types of protein bands but also suggested the presence of an additional component. Obviously, the preparations are heterogeneous and the structure of the reductase is not yet established. The second, crude protein fraction is believed to contain several ancillary enzymes required for the reaction. One of the low molecular weight components is S-adenosylmethionine; a second component is a loosely bound metal. We propose that S-adenosylmethionine together with a metal participates in the generation of the radical required for the reduction of carbon 2' of the ribosyl moiety of CTP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A., Barlow T., Pontis E., Reichard P. Production and characterization of monoclonal antibodies against the two subunits proteins B1 and B2 of Escherichia coli ribonucleotide reductase. Biochemistry. 1986 Feb 25;25(4):860–867. doi: 10.1021/bi00352a018. [DOI] [PubMed] [Google Scholar]
- Baraniak J., Moss M. L., Frey P. A. Lysine 2,3-aminomutase. Support for a mechanism of hydrogen transfer involving S-adenosylmethionine. J Biol Chem. 1989 Jan 25;264(3):1357–1360. [PubMed] [Google Scholar]
- Barlow T. Evidence for a new ribonucleotide reductase in anaerobic E. coli. Biochem Biophys Res Commun. 1988 Sep 15;155(2):747–753. doi: 10.1016/s0006-291x(88)80558-8. [DOI] [PubMed] [Google Scholar]
- Benner S. A., Ellington A. D., Tauer A. Modern metabolism as a palimpsest of the RNA world. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7054–7058. doi: 10.1073/pnas.86.18.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund O., Eckstein F. Synthesis of ATP- and dATP-substituted sepharoses and their application in the purification of phage-T4-induced ribonucleotide reductase. Eur J Biochem. 1972 Aug 4;28(4):492–496. doi: 10.1111/j.1432-1033.1972.tb01936.x. [DOI] [PubMed] [Google Scholar]
- Brown N. C., Reichard P. Ribonucleoside diphosphate reductase. Formation of active and inactive complexes of proteins B1 and B2. J Mol Biol. 1969 Nov 28;46(1):25–38. doi: 10.1016/0022-2836(69)90055-2. [DOI] [PubMed] [Google Scholar]
- Brown N. C., Reichard P. Role of effector binding in allosteric control of ribonucleoside diphosphate reductase. J Mol Biol. 1969 Nov 28;46(1):39–55. doi: 10.1016/0022-2836(69)90056-4. [DOI] [PubMed] [Google Scholar]
- Chirpich T. P., Zappia V., Costilow R. N., Barker H. A. Lysine 2,3-aminomutase. Purification and properties of a pyridoxal phosphate and S-adenosylmethionine-activated enzyme. J Biol Chem. 1970 Apr 10;245(7):1778–1789. [PubMed] [Google Scholar]
- Conradt H., Hohmann-Berger M., Hohmann H. P., Blaschkowski H. P., Knappe J. Pyruvate formate-lyase (inactive form) and pyruvate formate-lyase activating enzyme of Escherichia coli: isolation and structural properties. Arch Biochem Biophys. 1984 Jan;228(1):133–142. doi: 10.1016/0003-9861(84)90054-7. [DOI] [PubMed] [Google Scholar]
- Fontecave M., Eliasson R., Reichard P. Oxygen-sensitive ribonucleoside triphosphate reductase is present in anaerobic Escherichia coli. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2147–2151. doi: 10.1073/pnas.86.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Characterization of an iron sensitive Mud1 mutant in E. coli lacking the ribonucleotide reductase subunit B2. Arch Microbiol. 1988;149(4):344–349. doi: 10.1007/BF00411654. [DOI] [PubMed] [Google Scholar]
- Harder J., Follmann H., Hantke K. Deoxyribonucleotide synthesis in an Escherichia coli mutant (H 1491) which lacks ribonucleotide reductase subunit B2. Z Naturforsch C. 1989 Jul-Aug;44(7-8):715–718. doi: 10.1515/znc-1989-7-827. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Jamison C. S., Adler H. I. Mutations in Escherichia coli that effect sensitivity to oxygen. J Bacteriol. 1987 Nov;169(11):5087–5094. doi: 10.1128/jb.169.11.5087-5094.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe J., Neugebauer F. A., Blaschkowski H. P., Gänzler M. Post-translational activation introduces a free radical into pyruvate formate-lyase. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1332–1335. doi: 10.1073/pnas.81.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorre D. G., Kurbatov V. A., Samukov V. V. General method for the synthesis of ATP gamma-derivatives. FEBS Lett. 1976 Nov;70(1):105–108. doi: 10.1016/0014-5793(76)80736-3. [DOI] [PubMed] [Google Scholar]
- Panagou D., Orr M. D., Dunstone J. R., Blakley R. L. A monomeric, allosteric enzyme with a single polypeptide chain. Ribonucleotide reductase of Lactobacillus leichmannii. Biochemistry. 1972 Jun 6;11(12):2378–2388. doi: 10.1021/bi00762a025. [DOI] [PubMed] [Google Scholar]
- Reichard P., Ehrenberg A. Ribonucleotide reductase--a radical enzyme. Science. 1983 Aug 5;221(4610):514–519. doi: 10.1126/science.6306767. [DOI] [PubMed] [Google Scholar]
- Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- Stubbe J. A. Protein radical involvement in biological catalysis? Annu Rev Biochem. 1989;58:257–285. doi: 10.1146/annurev.bi.58.070189.001353. [DOI] [PubMed] [Google Scholar]
- Thelander L., Eriksson S., Akerman M. Ribonucleotide reductase from calf thymus. Separation of the enzyme into two nonidentical subunits, proteins M1 and M2. J Biol Chem. 1980 Aug 10;255(15):7426–7432. [PubMed] [Google Scholar]
- Thelander L. Reaction mechanism of ribonucleoside diphosphate reductase from Escherichia coli. Oxidation-reduction-active disulfides in the B1 subunit. J Biol Chem. 1974 Aug 10;249(15):4858–4862. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Willing A., Follmann H., Auling G. Ribonucleotide reductase of Brevibacterium ammoniagenes is a manganese enzyme. Eur J Biochem. 1988 Jan 4;170(3):603–611. doi: 10.1111/j.1432-1033.1988.tb13740.x. [DOI] [PubMed] [Google Scholar]