Abstract
Background
Since the beginning of the twentieth century, infection has emerged as a fundamental aspect of cancer causation with a growing number of pathogens recognized as oncogenic. Meanwhile, oncolytic viruses have also attracted considerable interest as possible agents of tumor destruction.
Discussion
Lost in the dichotomy between oncogenic and oncolytic agents, the indirect influence of infectious organisms on carcinogenesis has been largely unexplored. We describe the various ways – from functional aspects to evolutionary considerations such as modernity mismatches – by which infectious organisms could interfere with oncogenic processes through immunity. Finally, we discuss how acknowledging these interactions might impact public health approaches and suggest new guidelines for therapeutic and preventive strategies both at individual and population levels.
Summary
Infectious organisms, that are not oncogenic neither oncolytic, may play a significant role in carcinogenesis, suggesting the need to increase our knowledge about immune interactions between infections and cancer.
Electronic supplementary material
The online version of this article (doi:10.1186/s12885-017-3234-4) contains supplementary material, which is available to authorized users.
Keywords: Immunity, Infection, Cancer, Evolution, Personal history of infection
Background
Since the beginning of the twentieth century, accumulating evidence shows that some infections may be directly linked to cancer incidence. First, a growing number of pathogens are recognized to be oncogenic, i.e. infection is a prerequisite for maintaining or initiating the growth of cancer cells (Table 1) [1]. Identification of infectious agents that contribute to oncogenesis, i.e. transformation of normal cells into cancer cells, constitutes a priority for cancer prevention mainly because effective preventive measures exist for some of them [2]. Second, oncolytic pathogens, that selectively destroy tumor tissue, have also been studied for more than a century as experimental agents for eliminating cancer cells (Table 2) [3].
Table 1.
Principal oncogenic agents and their participation to associated cancers
| Oncogenic agents | Associated cancer | Contribution | Transmission | Prevention or elimination methods | Carcinogens classification | Ref |
|---|---|---|---|---|---|---|
| Macro-Parasites | [90, 91] | |||||
| Schistosoma haematobium | Bladder cancer | 30% | Water | Anti-helminthics | ||
| Indirect | ||||||
| Opisthorchis viverrini and Clonorchis sinensis | Cholangioma liver cancer | 15% | Food | Anti-helminthics | ||
| Bacteria | ||||||
| Helicobacter pylori | Stomach cancer | 80% | Water, sanitation, food, saliva | Antibiotics, sanitation | Indirect | [92, 93] |
| Viruses | [92, 94, 95] | |||||
| Epstein Barr Virus | Burkitt’s lymphoma, nasoparyngeal cancer | 10–30% | Saliva | Antivirals for some illnesses | ||
| Hepatitis B and C | Liver cancer | 80% | needles, sex | Vaccination (HBV), antivirals, blood screening | ||
| Human T lymphotropic virus | Adult T cell leukaemia | Almost 100% | Sex, needle, milk | No treatment | Direct and indirect | |
| Human Papillomavirus | Cervical cancer | 100% | Sex, saliva | Vaccination, pap smear | ||
| Human Herpesvirus 8 | Kaposi sarcoma | Almost 100% | Sex, saliva | No treatment | ||
| Merkel cell polyomavirus | Merkel cell cancer | Almost 100% | Saliva | No treatment | ||
Today, the World Health Organization acknowledges that at least 20% of cancers have an infectious origin [96]. For transmission section, “needles” includes blood transfusion, contaminated medical syringes and illicit intravenous drug use. A classification of oncogenic organisms has been proposed on the basis of their contribution to carcinogenesis [1]. When infection leads to introduction of viral oncogenes into the host genome, pathogens are considered to be direct carcinogens. These pathogens exploit the host in ways that interfere with mechanisms of cancer prevention (cell cycle arrest, apoptosis, restriction of telomerase and cell adhesion). Infectious organisms that induce immunosuppression, chronic inflammation and/or chromosomal instability, are referred to as indirect carcinogens as they may drive mutations and promote cancer cell proliferation
Table 2.
Oncolytic agents
| More than a century ago, observations revealed that certain natural viral infections (e.g., West Nile virus and mumps virus) were associated with spontaneous cancer remissions [3]. These viruses were subsequently shown to have a natural preference for cancer cells and infection with these oncolytic viruses (OVs) triggers lysis of infected cells as well as activation of anti-tumoral immunity [97]. Advances in molecular biology have also allowed the modification of other viruses to make them specific to neoplastic tissues and/or to combined them with immune reagents to break tumor-induced immune tolerance [98]. For instance, recombinant measles viruses have been used to treat human patients with bone-marrow cancer [36]. Interestingly, this treatment only led to a significant resolution of tumor in two patients who were measles-seronegative. Recently, a genetically engineered virus called T-VEC virus has been approved by the US Food and Drug Administration to treat advanced melanoma [99]. Several studies have also focused on biological anticancer agents based on oncolytic bacteria. In 2014, Roberts and colleagues tested the oncolytic potential of Clostridium novyi, a bacterium extremely sensitive to oxygen that permits the specific targeting of cancer cells, in the center of solid tumor, that are in a hypoxic environment [100]. A derivative of the wild-type strain (C. novyi-NT) has been engineered to become inoffensive for the host [101] and tested via intratumoral injection against natural canine tumors as well as on advanced leiomyosarcoma in human patients [102]. C. novyi-NT destroys cancer cells, but also induces a rapid and robust local antitumoral response. Such experiments pave the way for considering pathogens as new therapeutic opportunities to eradicate neoplastic tissues. |
One alternative and underexplored way to study the relationship between infections and oncogenic events is to investigate the indirect role of infectious organisms1 (i.e., viruses, bacteria, fungi, protozoans and metazoans that exploit other organisms, called hosts, to complete their life cycle) that are not considered to be oncogenic or oncolytic in carcinogenesis. These links may result from interactions between immune pathways involved in protection against infectious agents and cancer cells. As the immune system plays a critical role in the control and suppression of malignant cells through immunosurveillance [4], any disequilibrium in immune system homeostasis may enhance or constrain cancer cell proliferation. In addition, infectious organisms could interfere with transmission of oncogenic agents2 through partial cross-immunity or immune facilitation, a phenomenon increasingly documented between non-oncogenic pathogens [5, 6]. Therefore, we suggest that oncogenic and oncolytic agents represent the two extremes of a continuum of organisms that play an indirect role during oncogenesis. Since infectious organisms are ubiquitous [7] and co-infections through the course of life remains the norm rather than exception [8], it calls for an urgent need to understand how pathogen communities may prevent or exacerbate carcinogenesis.
Discussion
Responses of immune system against proliferation of cancer cells and infections
While the complex links between the immune system and cancer have been already fully described elsewhere [9–11], it is nevertheless worth pointing out the primary immune mechanisms involved both in infection process and cancerogenesis. For instance, it has been shown that cancer cells are able to evade immune system through numerous mechanisms in advanced stages of tumor [4, 12]. Therefore, we could assume that infections may have a significant role at the beginning of carcinogenesis, i.e. during immunosurveillance. The immune recognition of specific antigens expressed by cancer cells, called tumor-associated antigens (TAA), is a necessary first step to initiate an anti-tumoral response. Receptors expressed on antigen-presenting cells bind and present TAA to T helper (Th) lymphocytes in the lymph nodes. Such Th1-polarized lymphocytes activate cytotoxic T cells and macrophages which in turn destroy cancer cells. In agreement with this, a Th1-polarized response has been mainly recognized to be protective against several cancers [13, 14]. Finally, to avoid auto-immunity and chronic inflammation regulatory T cells (Treg) and other immunosuppressive cells are recruited at the tumor site. Without ignoring that the immune phenotype of an individual results from complex interactions between cellular and humoral effectors, we suggest that mounting an immune response against invading infectious agents could interfere with anti-tumoral protection (Fig. 1).
Fig. 1.
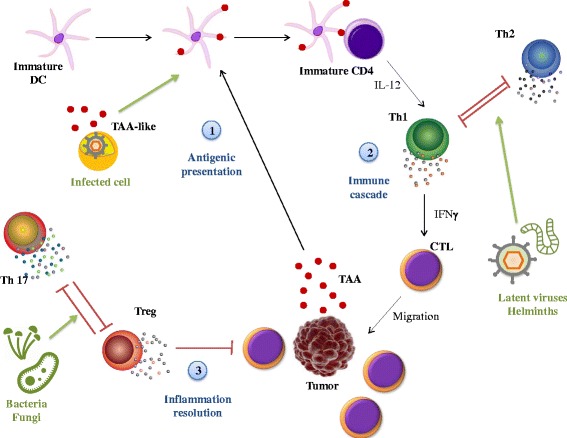
Shared immune responses to infections and cancer cells. The immune system’s action on cancer cells relies on three main steps: antigenic presentation, immune cascades and inflammation resolution. Infected cells can express TAA-like antigen which will activate DC subset. DC will prime Th cells to differentiate into Th1 cells. However, latent viruses and helminths could polarize Th cells toward a Th2 response. Finally, bacterial and fungal infections could disequilibrate inflammation resolution by activating Th17 cells that down-regulate Treg cells. (DC: dendritic cells; TAA: tumor associated antigens; Th: T helper cell; CTL: cytotoxic T cells, Treg: regulatory T cells; IFNγ: interferon γ; IL: interleukin)
Indeed, several lines of evidence back up this hypothesis. First, the presence of antibodies against TAA has been observed in cancer-free patients [15], and it has been suggested that some pathogens might have epitopes sharing common features with TAA. In this case, infection with pathogens expressing TAA might play the role of priming the immune response and improve, concomitantly or on a longer time scale, the effectiveness of immunosurveillance. Different infectious agents are known to selectively polarize the immune system towards a Th1 or a Th2 response. Given the reciprocal inhibition between Th1 and Th2 effectors [16], the nature of the infections can have a profound effect on the elimination of cancer cells by immune effectors. This idea is supported by the finding that patients with a Th2-polarized immune response have poor prognosis when suffering from lung, breast, colorectal, pancreatic cancers [17]. Finally, responses against extracellular bacteria and fungi could increase cancer risk through a Th17-mediated inflammation that may also inhibit resolution of inflammation by Fox P3 regulatory T cells (Treg) [18].
As humans are usually exposed to a variety of infectious agents during their lives, it could be expected that the chronology and typology of infections we face, from childhood to old age, might not only shape the functioning of our immune system but also our susceptibility to cancer. Here, we would like to stress that infections are likely to interfere with cancer dynamics at the individual scale depending on i) the personal history of infection (because of the shared immune responses to cancer cells and infections), and ii) the interactions between species within the pathogen communities; but also at the population scale depending on iii) the mismatches3 between the environment experienced by our ancestors and our current one (Additional file 1: Figure S1). Finally, we will discuss the possible public health consequences of underestimating such indirect interactions and call for a more integrative view of infectious disease control and cancer prevention strategies.
Personal history of infection can interfere with destruction of cancer cells
The community of organisms which have infected an individual during its life represents the personal history of infection. Accumulating evidence suggests that taking into account the past occurrence of infection is important for a better understanding of cancer epidemiology (Fig. 2).
Fig. 2.
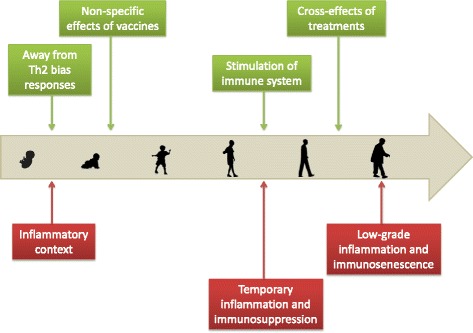
Indirect links between cancer and infections across human life. Green boxes and red boxes represent beneficial and detrimental links respectively. Childhood diseases and infection events occurring during the life of an individual could reduce cancer risk as they may enhance immune efficiency to eliminate cancer cells. In addition, some vaccines and treatments against infectious diseases have been reported to reduce cancer risk through the activation of anti-tumoral immunity. At the opposite end of the spectrum, infections may create inflammation or immunosuppression episodes that allow cancer cells to proliferate. Finally, chronic exposure to infections could account for age-related immune disorders and the inability to eliminate cancer cells
Infections occur as early as the first year of life and may impact the immune system and cancer risk. The increase in antigenic exposure, after birth through viral/bacterial infections, may be essential for newborns to switch from a Th2 biased [19] to a balanced Th1/Th2 immunity as well as to develop immunological memory [20]. Also, childhood diseases may activate specific anti-tumoral responses. For instance, mumps may lead to immune recognition of TAA present on ovarian cancer cells, resulting in an effective immunosurveillance [21]. However, childhood diseases could be associated with inflammation, and the persistence of this inflammatory process in adulthood may increase the risk of mutations in normal cells, giving an example of antagonistic pleiotropy4. In fact, individuals that have experienced major childhood illness are twice at risk to develop a cancer [22]. Leukemia is a specific example where childhood infections seem to play an ambiguous role [23]. A protective role of infections was first suggested by observational studies for Acute Lymphoid Leukemia (ALL) [24] and has recently been supported by an epidemiological study for Chronic Lymphoid Leukemia (CLL) [25]. However, another study has reported that the probability of developing ALL increases with the number of infectious diseases encountered in the first year of life [26].
Infection occurring later in life could also have a significant impact on the capacity of the immune system to keep in check cancer cells. Indeed, protection against lung cancer has been observed in humans frequently exposed to cattle in the dairy industry [27]. It has been suggested that protection is provided by endotoxins present in the dust which are known to be potent immune stimulating factors [28]. Furthermore, in a lung-cancer model, mice infected with influenza virus were better able to challenge the tumor [29]. It was suggested that influenza viruses might produce TAA which induces immune memory providing life-long immunosurveillance to cancer cells. The role of respiratory tract infection has also been highlighted by a significant positive association between personal history of pneumonia and CLL risk [30, 31]. Lastly, personal history of infection may also help to explain age-related immunodeficiency, i.e., immunosenescence [32], which is correlated with the reduced capacity to eliminate cancer cells [33]. By increasing exposure to antigens, a longer lifespan may induce chronic low-grade inflammation, contributing to immune disorders, which may, in turn, lead to accumulation of cancer cells in older individuals [34].
Acknowledging the role of personal history of infection in cancer initiation and progression might improve cancer prevention, for instance, through prophylactic cancer vaccination [35]. Consideration of personal infection history could also be useful in treatment strategies as it could alter patient response to therapy. For instance, Russell et al. [36] showed that injection of attenuated measles virus could treat bone-marrow cancer only if patients have never been infected by the virus in the past [36]. This result suggests that immune stimulation may not be high enough when the patient has already been infected by the virus and that the decision to use oncolytic viruses as therapeutic agents has to be made based on the personal history of infection.
Finally, personal history of infection may be related to the personal history of medications and vaccination. Medications that ameliorate symptoms of infection (fever, headache…) may influence carcinogenesis, as is the case for anti-inflammatory drugs. Daily consumption of aspirin, for example, has been recognized to decrease cancer mortality, in part by inhibiting metastasis [37]. Second, medications could be used against specific infectious agents. For instance, the anti-malarial artesunate shows an anti-tumoral activity comparable to other cancer drugs [38]. Also, a range of antibiotics disrupting mitochondrial functions have also been reported to eradicate stem cells of different tumor types [39]. Finally, vaccination against specific infectious agents could be used to prevent cancer. In fact, several studies report protection against melanoma, lymphoma or leukemia after BCG, vaccinia or yellow fever vaccination [40, 41]. These findings might be explained by non-specific effects of vaccines through the shifting of the immune response towards a Th1 profile or through cross reactivity [42]. Vaccines may also contain pathogen antigens with amino-acid sequences that are homologous with those of certain TAA [43]. By this cross reaction effect, vaccination allows eliminating malignant cells as soon as they appear. For instance, a prior immunization with BCG vaccine, which has antigenic similarity with human endogenous retroviruses (HERV-K-MEL), expressed in 95% of malignant melanocytes, has been associated with better survival in patients with melanoma [44].
Infectious organisms can modify transmission of oncogenic agents
While many pathogens can alter anti-tumoral immunity, some infections can also influence transmission of oncogenic pathogens. Indeed, as with any free organisms, species that form pathogen communities do interact in a synergistic or antagonistic ways [45], with effects on the epidemiology of each species within the community. On non-oncogenic pathogens, it has been shown, for instance, that HIV is responsible for a 37-fold increase in the risk to contract tuberculosis [46] whereas convalescence period induced by measles impacts the dynamics of the epidemic of Bordetella pertussis (the causative agent of a whooping cough) [47]. Here, we suggest that this type of interactions has the potential to influence cancer epidemiology by altering the transmission of oncogenic agents.
Endemic Burkitt Lymphoma has been associated with Epstein-Barr Virus infection in infancy and is geographically linked to holoendemic Plasmodium falciparum [48]. This association may result from reciprocal benefits for the two species (Fig. 3a). On the one hand, P. falciparum antigens can directly induce EBV reactivation and decrease EBV-specific T-cells during malaria infection [49, 50]. On the other hand, EBV in the lytic cycle is associated with suppressed B-cells [51] which play a role in the control of P. falciparum [52].
Fig. 3.
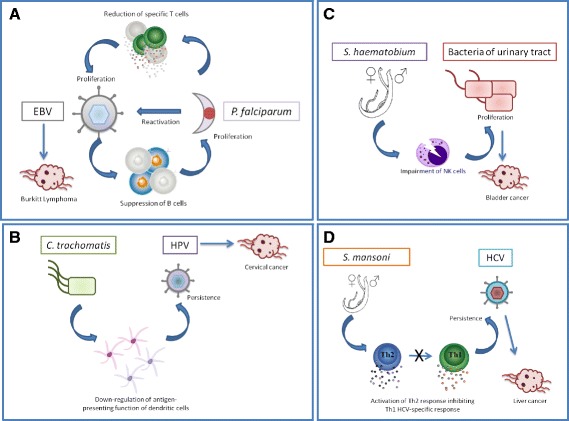
Interactions between infectious agents and oncogenic agents. a Reciprocal benefits between Epstein Barr Virus (EBV) and Plasmodium falciparum. While EBV suppresses B cells involved in the control of P. falciparum, the latter one induces EBV reactivation and decreases EBV-specific T-cells. b Helper function of Chlamydia trachomatis toward human papillomavirus. C. trachomatis products decrease antigenic presentation by dendritic cells allowing the oncogenic agent to persist. c Interactions between Schistosoma haematobium and bacteria. S. haematobium induces the impairment of NKT cells promoting bacterial infections of the urinary tract. d Co-infection with Hepatitis C virus (HCV) and Schistosoma mansoni. In the presence of HCV, S. mansoni has been shown to alter the CD4+ T cell proliferative response toward a Th2 profile, preventing the elimination of the virus by specific Th1 response
Second, human papillomavirus (HPV) persistence is the major cause of cervical cancer. Epidemiological studies have shown that Chlamydia trachomatis infection is also associated with this cancer [53] and increases the risk for persistence of HPV infection [54]. One potential mechanism of this interaction may rely on C. trachomatis products which may impact immunity allowing the oncogenic agent to persist. In fact, Chlamydia infection induces COX2 protein expression in epithelial cells and promotes PGE2 release [55]. PGE2 has been identified to down-regulate IL-12 production and the antigen-presenting function of dendritic cells [56]. Therefore, C. trachomatis infection may increase transmission of HPV by inhibiting cell-mediated immunity but also by creating a pro-inflammatory environment [57] favorable to HPV persistence (Fig. 3b).
Third, Schistosoma haematobium, an African trematode that has recently spread into Mediterranean Europe [58], is associated with urinary bladder cancer [59]. Interestingly, several studies have reported a high percentage of bacterial co-infection in the urinary tract [60, 61]. This pattern can be explained by the fact that helminths can induce an impairment of NKT cells promoting bacterial infections [62]. However, bacterial infections of the urinary tract have also been reported to increase the risk of bladder cancer through the production of nitrosamines, which are carcinogenic compounds [63]. Therefore S. haematobium could have two facilitating roles in carcinogenesis: a direct role through inflammation-induced DNA damages [64] and an indirect role in immune facilitation (Fig. 3c).
In addition to these well-described examples, evidence of interactions between infectious organisms and oncogenic agents are accumulating for other co-occurrences. For instance, co-infection with Hepatitis C virus (HCV), the causative agent of liver cancer, and Schistosoma mansoni has been linked to an increase in viral persistence [65]. In the presence of HCV, S. mansoni has been shown to alter the CD4+ T cell proliferative response toward a Th2 profile [66], preventing the HCV-specific Th1 response and thus its elimination (Fig. 3d). Specific interactions through the immune system may also occur in the following co-infections: HHV8/Mansonella perstans [67] and Merkel cell virus/Pseudomonas aeruginosa [68], however, the mechanisms have not been fully identified yet.
Finally, all interactions described above are associated with an increase in persistence/transmission of oncogenic agents while examples of co-infection conferring protection are scarce. We suggest that protection might come from co-infection involving closely related pathogenic species. For example, the immune response against Helicobacter pylori (stomach cancer) and H. bilis (biliary tract cancer [69]) may be very similar [70], and cross-immunity could result in reciprocal protection. The same mechanism could be applied to co-infections with varicella and HHV8 and/or EBV as they all belong to the Herpesvirus genus for which type I interferon plays a central role [71].
Infectious organisms and cancer susceptibility: an evolutionary perspective
Throughout evolutionary history, humans have been exposed to a great diversity of infectious agents, and the composition of the community has also fluctuated greatly over time [72]. In wealthy countries, mankind has experienced a significant decrease in infectious pressures due to public health strategies, including antibiotics, vaccines, and improved sanitation. The reduced prevalence of infectious diseases has however been paralleled by an increased incidence of many immune disorders, inflammatory diseases, and cancers. One evolutionary hypothesis relies on the mismatch that has rapidly (within a century) occurred between our current infectious environment and the one that our ancestors have been exposed to for thousands of years [73].
Infections could drive carcinogenesis by trade-offs5 at individual and population scales
The idea that cancer might result from antagonistic pleiotropy (improving early survival and/or reproduction at the expenses of late fitness6 (Additional file 1: Fig. S1)) is currently considered to be a viable hypothesis [74]. Nevertheless, very few studies have explored whether traits that help to limit the cost of infection might promote carcinogenesis later in life.
More specifically, resistance against infections could impact pro-oncogenic inflammation. The early immune response to infection relies on acute inflammation [75] which is also accepted as a hallmark of cancer [12]. Despite these oncogenic consequences, the inflammatory response still confers a fitness benefit in environments with high infectious burdens, because it improves the survival prospect at early life. Accordingly, fast-paced species rely more on pro-inflammatory responses whereas slow-paced species tend more toward anti-inflammatory responses [76]. In pathogen-rich environments, pro-inflammatory genes could have been favored, as fitness benefits that arise from early protection against infection would be greater than fitness costs arising later in life, like increased risk of cancer. Pro-inflammatory genes that have been positively selected during human evolutionary history may now be involved in the increased incidence of cancers in modern environments with reduced pathogen loads.
An example of such mismatch comes from the relative vulnerability of African Americans to malignant diseases compared with people of Caucasian origins in the USA [77]. Relocation of Africans from tropical countries, where pro-tumoral inflammation following Th2 activation was beneficial, into North America, and the consequent change in infection risk, may expose them to a higher risk of cancer [78]. Thus, the eradication of some infectious agents – notably those that co-evolved with us – may drive the vulnerability to immune-related disorders, with consequences for cancer susceptibility. While the use of helminths, or at least their immunomodulatory products, has been suggested in treatments of some inflammatory disorders [79], we hypothesize that they could reduce pro-tumoral inflammation, thus pro-tumoral mutation and accumulation of cancer cells. A caveat for such arguments derives from the fact that helminths, like other infectious organisms, evolve characteristics that enhance their own fitness; it is, therefore, naïve to expect that they could have uniformly positive immunological effects on human chronic diseases. If helminths, by their immunoregulatory role, suppress inflammation, they could reduce inflammation-induced oncogenesis. If, however, persistent infection by helminths generates a net increase in inflammation, they could contribute to oncogenesis, an effect that occurs in trematode-associated bladder cancer and cholangiocarcinoma [80].
Long-term co-evolution and persistence of oncogenic agents
From an evolutionary perspective, interactions between oncogenic agents and non-oncogenic infectious agents are of considerable importance for understanding the dynamics of co-evolution among geographically structured populations evolving under different ecological pressures. When an infectious agent is detrimental to host fitness, selection should favor resistance genes. However, when infections result in net fitness advantages, susceptibility genes should be maintained in the host population. For example, it has recently been suggested that H. pylori confers protection against tuberculosis (a lethal disease without appropriate medication) through enhancing IFNγ and Th1-like response to specific tuberculosis antigens [81]. In areas where tuberculosis is highly prevalent, susceptibility to H. pylori might have been favored by natural selection (Fig. 4). These conflicting selection pressures could potentially explain the wide distribution of H. pylori. Since 1950’s, antibiotics and vaccines have dramatically decreased tuberculosis prevalence in developed countries [82], suggesting that host resistance against H. pylori could be selected. Nevertheless, the appearance of resistant strains of M. tuberculosis in these populations combined with the increase of HIV transmission could together maintain susceptibility to H. pylori. Finally, in countries with low parasite pressure, the persistence of H. pylori could also be explained by its protective role against another cancer as it has been reported that elimination of the bacterium comes with an increase in esophageal adenocarcinoma incidence [83].
Fig. 4.
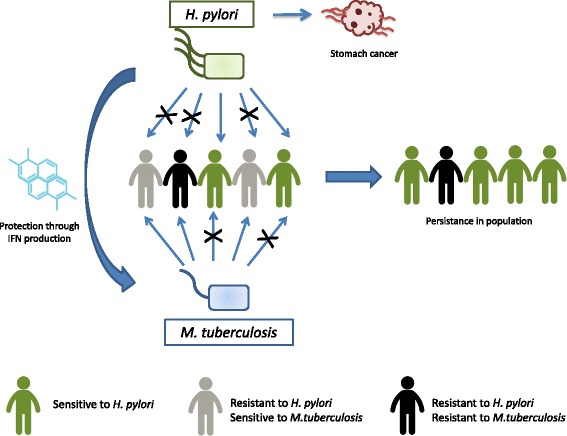
Long term interaction between Mycobacterium tuberculosis and Helicobacter pylori. H. pylori confers protection against M. tuberculosis through an increase in IFN production. In countries with a high prevalence of tuberculosis, infection with H. pylori might confer a selective benefit allowing the maintenance of H. pylori susceptibility genes
Conclusion
In this paper, we put forward several arguments suggesting that the links between infectious organisms and carcinogenesis through the immune system are varied and complex, and cannot be restricted to the study of oncogenic and oncolytic agents. These interactions can operate over the short-term through an altered immunosurveillance (Table 3 summarizes such examples when proximal mechanism has been identified) or via antagonistic/synergistic interactions between oncogenic and non-oncogenic agents, but also on a long-term leading to mismatches. Our arguments stress the need to broaden the view on the interactions between infections and oncogenesis. The interactions, described here to give a glimpse of the overall complexity, also include the microbiota and its possible role on carcinogenesis [84]. Therefore, rather than just studying a simple interaction between one individual and its cancer, we need to explore the intimate connections that could exist with its symbionts sensu largo in a given environment.
Table 3.
Examples of indirect interactions between infectious organisms and cancer through immunity for which the exact mechanism has been identified.TNF (Tumor Necrosis Factor)
| Impact on cancer | Infectious organisms | Mechanism implied | Immune compartment | Cancers | References |
|---|---|---|---|---|---|
| Exacerbating | Human Immunodeficiency virus | Destruction of CD4 + T cells | CD4+ T cells | Several cancers (including those with infectious origin) | [103–106] |
| Fusobacterium nucleatum (intra-tumoral bacteria) | Inhibition by contact between bacterial Fap2 protein and immune cell receptor TIGIT | Natural Killer cells | Various tumors | [107] | |
| Cytomegalovirus (infecting cancer cells) | Secretion of immunoregulatory protein (cmvIL-10) | Dentritic cells | Gliomas | [108, 109] | |
| Constraining | Streptococcus pyogenes/ Serratia marcescens | Secretion of high quantity of TNF | Global immune system | Sarcoma | [110, 111] |
| Attenuated Bacillus Calmette-Guérin (BCG) | Local stimulation of CD4+ T cells and Th1 immune response. Diminution of Treg cells. | T cell subsets | Bladder cancer | [112, 113] |
From an applied perspective, the stimulation of the immune system is a promising way to target cancer cells without damaging the healthy ones [85]. Most studies have focused on the relationship between immunity and cancer cells elimination based on the understanding of immunological mechanisms underlying the dialog between T-helper cells. Specific antibodies blocking CTLA-4 function enhance T-cell stimulation and promote anti-tumor immunity [86]. T-cell therapies, e.g., those using tumor-infiltrating lymphocytes (TIL) and chimeric antigen receptors (CAR), are promising [87]. Similarly, antibodies have been engineered to block the action of the Th17 cell subset, which secretes interleukin 17, with consistent results in mice where antibody injection was followed by a decrease in the number of tumors [88]. In this paper, we suggest that personal history of infection/medication, including childhood diseases, could modify how the immune system responds to immunotherapy possibly altering its efficiency.
The increase in cancer prevalence has been associated with lifestyle changes, such as an increased caloric intake, urbanization, and sedentary habits [89]. However, infection prophylaxis, improved medicine, and sanitation can also modify the strength of the interactions between infectious agents. In this context, the impact of infectious disease control on cancer epidemiology must be considered. Further work should focus on the potential effect of infectious organisms on cancer incidence and the consequences of infectious disease treatments on cancer risk at different scales. Such a global perspective is indispensable to anticipate the possible consequences of our current public health strategies.
Acknowledgments
This manuscript has been written thanks to the invaluable help of Pr Harald Zur Hausen. We also want to thank the reviewers for their highly pertinent comments that have greatly improved our manuscript. We are grateful to Tracey C. Russel for English editing.
Funding
This work was supported by the ANR (Blanc project EVOCAN), by the CNRS (INEE) and by André HOFFMANN (Fondation MAVA).
Availability of data and materials
Not applicable.
Authors’ contributions
CJ, BR and FT have designed the study and drafted the manuscript. AT, GS, BU, FM, DM, FR and PWE have contributed to different parts of the manuscript and to manuscript revisions. All authors have significantly contributed to the manuscript and approved the final version.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional.
affiliations.
Additional file
Antagonistic pleiotropy and mismatch concept. Antagonistic pleiotropy describes a situation where particular genes (e.g. inflammatory genes) have opposite effects on fitness at different ages, such that their effects are beneficial in early life, when natural selection is strong (following infections for instance), but harmful at later ages, when selection weakens. Whereas, mismatches between genotype and environment arise when a phenotype or genotype that were selected in a particular context (e.g. in a high parasitic burden) becomes detrimental in a new environment. (PPT 239 kb)
Footnotes
Infectious organisms: organisms that live obligatorily at the expense of another organism, called the host. The relation is beneficial for the infectious agent but detrimental for the host. This broad definition includes pathogens (virus, bacteria, fungi) and parasites (protozoans, helminthes, ticks among others).
Oncogenic agents: Infectious organisms recognized to have a direct and significant contribution to carcinogenesis. At the opposite, we refer to non-oncogenic agents when there is no direct evidence for a contribution in tumoral process.
Mismatches between genotype and environment arise when a phenotype or genotype that were selected in a particular context (e.g. in a high parasitic burden) becomes detrimental in a new environment.
Antagonistic pleiotropy describes a situation where particular genes (e.g. inflammatory genes) have opposite effects on fitness at different ages, such that their effects are beneficial in early life, when natural selection is strong (following infections for instance), but harmful at later ages, when selection weakens.
Trade-off: balance between the cost and the benefit of biological mechanisms regarding the fitness of the organism. It underlies that both aspects compete for a common resource.
Fitness: capacity of an individual to produce viable offspring, in other words contribution of an individual to the future generation. Also described as lifetime reproductive success.
Contributor Information
Camille Jacqueline, Email: camille.jacqueline@ird.fr.
Aurélie Tasiemski, Email: aurelie.tasiemski@univ-lille1.fr.
Gabriele Sorci, Email: gabriele.sorci@u-bourgogne.fr.
Beata Ujvari, Email: beata.ujvari@deakin.edu.au.
Fatima Maachi, Email: fatima.maachi@pasteur.ma.
Dorothée Missé, Email: dorothee.misse@ird.fr.
François Renaud, Email: francois.renaud@ird.fr.
Paul Ewald, Email: pw.ewald@louisville.edu.
Frédéric Thomas, Email: frederic.thomas2@ird.fr.
Benjamin Roche, Email: roche.ben@gmail.com.
References
- 1.Zur Hausen H, Villiers ED. Cancer ‘causation’ by infections—individual contributions and synergistic networks. Semin Oncol. 2015;41:860–875. doi: 10.1053/j.seminoncol.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Söderlund-Strand, A., Uhnoo, I. & Dillner, J. Change in Population Prevalences of Human Papillomavirus after Initiation of Vaccination: The High-Throughput HPV Monitoring Study. Cancer Epidemiol Biomarkers Prev. 2014; 2757–2765. doi:10.1158/1055-9965.EPI-14-0687 [DOI] [PubMed]
- 3.Kelly E, Russell SJ. History of Oncolytic Viruses : genesis to genetic engineering. Mol Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 5.Mideo N. Parasite adaptations to within-host competition. Trends Parasitol. 2009;25:261–268. doi: 10.1016/j.pt.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Ferguson N, Anderson R. Chaos, persistence, and evolution of strain structure in Antigenically diverse infectious agents. Science. 1998;80:795. doi: 10.1126/science.280.5365.912. [DOI] [PubMed] [Google Scholar]
- 7.Poulin R, Morand S. The Diversity of Parasites. The Quarterly Review of Biology. 2000;75:277–293. [DOI] [PubMed]
- 8.Poulin R. Evolutionary ecology of parasites. 2. Princeton: Princet. Univ. Press; 2007. [Google Scholar]
- 9.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 10.Bindea G, Mlecnik B, Fridman WH, Pagès F, Galon J. Natural immunity to cancer in humans. Curr Opin Immunol. 2010;22:215–222. doi: 10.1016/j.coi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Review hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Ingels A, et al. T-helper 1 immunoreaction influences survival in muscle-invasive bladder cancer: proof of concept. Ecancermedicalscience. 2014;8:486. doi: 10.3332/ecancer.2014.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haabeth OAW, et al. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun. 2011;2:240. doi: 10.1038/ncomms1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vella LA, et al. Healthy individuals have T-cell and antibody responses to the tumor antigen cyclin B1 that when elicited in mice protect from cancer. Proc Natl Acad Sci U S A. 2009;106:14010–14015. doi: 10.1073/pnas.0903225106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- 17.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 18.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 19.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janeway CA, Travers P, Walport M, Shlomchik MJ. The immune system in health and disease. 2001. [Google Scholar]
- 21.Cramer DW, Ave L. Mumps and ovarian cancer: moder interpretation of an historic association. Cancer Causes Control. 2011;21:1193–1201. doi: 10.1007/s10552-010-9546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackwell DL, Hayward MD, Crimmins EM. Does childhood health affect chronic morbidity in later life? Soc Sci Med. 2001;52:1269–1284. doi: 10.1016/S0277-9536(00)00230-6. [DOI] [PubMed] [Google Scholar]
- 23.Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer. 2006;6:193–203. doi: 10.1038/nrc1816. [DOI] [PubMed] [Google Scholar]
- 24.Ma, X. et al. Daycare attendance and risk of childhood acute lymphoblastic leukaemia. 2003 1419–1424. doi:10.1038/sj/bjc/6600274 [DOI] [PMC free article] [PubMed]
- 25.Parodi S, et al. Childhood infectious diseases and risk of leukaemia in an adult population. Int J Cancer. 2013;133:1892–1899. doi: 10.1002/ijc.28205. [DOI] [PubMed] [Google Scholar]
- 26.Crouch S, et al. Infectious illness in children subsequently diagnosed with acute lymphoblastic leukemia: modeling the trends from birth to diagnosis. Am J Epidemiol. 2012;176:402–408. doi: 10.1093/aje/kws180. [DOI] [PubMed] [Google Scholar]
- 27.Mastrangelo G, et al. Lung cancer risk: effect of dairy farming and the consequence of removing that occupational exposure. Am J Epidemiol. 2005;161:1037–1046. doi: 10.1093/aje/kwi138. [DOI] [PubMed] [Google Scholar]
- 28.Rylander R. Endotoxin in the environment--exposure and effects. J Endotoxin Res. 2002;8:241–252. doi: 10.1179/096805102125000452. [DOI] [PubMed] [Google Scholar]
- 29.Iheagwara UK, et al. Influenza virus infection elicits protective antibodies and T cells specific for host cell antigens also expressed as tumor associated antigens: a new view of cancer immunosurveillance. Cancer Immunol Res. 2015;2:263–273. doi: 10.1158/2326-6066.CIR-13-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landgren O, Rapkin J. Respiratory tract infections and subsequent risk of chronic lymphocytic leukemia. Blood. 2007;109:2198–2201. doi: 10.1182/blood-2006-08-044008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson L, Landgren O, Engels E. Commun community acquired infections and subsequent risk of chronic lymphocytic leukemia. Br J Haematol. 2010;147:444–449. doi: 10.1111/j.1365-2141.2009.07849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas-vaslin V, et al. Immunodepression and Immunosuppression during aging. 2009. [Google Scholar]
- 33.Fulop T, et al. Potential role of immunosenescence in cancer development. Ann N Y Acad Sci. 2010;1197:158–165. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- 34.Vasto S, et al. Inflammation, ageing and cancer. Mech Ageing Dev. 2009;130:40–45. doi: 10.1016/j.mad.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Hobohm U. Toward general prophylactic cancer vaccination. BioEssays. 2009;31:1071–1079. doi: 10.1002/bies.200900025. [DOI] [PubMed] [Google Scholar]
- 36.Russell SJ, et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc. 2014;89:926–933. doi: 10.1016/j.mayocp.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothwell PM, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 38.Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar C. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001 doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 39.Lamb R, et al. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget. 2015;6:4569–4584. doi: 10.18632/oncotarget.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villumsen M, et al. Risk of lymphoma and leukaemia after bacille Calmette-Guérin and smallpox vaccination: a Danish case-cohort study. Vaccine. 2009;27:6950–6958. doi: 10.1016/j.vaccine.2009.08.103. [DOI] [PubMed] [Google Scholar]
- 41.Mastrangelo G, et al. Does yellow fever 17D vaccine protect against melanoma? Vaccine. 2009;27:588–591. doi: 10.1016/j.vaccine.2008.10.076. [DOI] [PubMed] [Google Scholar]
- 42.Grange JM, Stanford JL, Stanford CA, Ko KF. Vaccination strategies to reduce the risk of leukemia and melanoma. J R Soc Med. 2003;96:389–392. doi: 10.1258/jrsm.96.8.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krone B, Kölmel KF, Henz BM, Grange JM. Protection against melanoma by vaccination with Bacille Calmette-Guerin (BCG) and/or vaccinia: an epidemiology-based hypothesis on the nature of a melanoma risk factor and its immunological control. Eur J Cancer. 2005;41:104–117. doi: 10.1016/j.ejca.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Kölmel KF, et al. Prior immunisation of patients with malignant melanoma with vaccinia or BCG is associated with better survival. An European Organization for Research and Treatment of cancer cohort study on 542 patients. Eur J Cancer. 2005;41:118–125. doi: 10.1016/j.ejca.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen AB, Fenton A. Emphasizing the ecology in parasite community ecology. Trends Ecol Evol. 2007;22:133–139. doi: 10.1016/j.tree.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 47.Rohani P, Green CJ, Mantilla-Beniers NB, Grenfell BT. Ecological interference between fatal diseases. Nature. 2003;1979:885–888. doi: 10.1038/nature01542. [DOI] [PubMed] [Google Scholar]
- 48.Morrow RH, Gutensohn N, Smith PG. Epstein-Barr virus-malaria interaction models for Burkitt ’ s lymphoma: implications for preventive trials Epstein-Barr virus-malaria interaction models for Burkitt ’ s lymphoma: implications for preventive trials 1. 1976. pp. 667–669. [PubMed] [Google Scholar]
- 49.Moormann AM, Snider CJ, Chelimo K. The company malaria keeps : how co-infection with Epstein-Barr virus leads to endemic Burkitt lymphoma. Curr Opin Infect Dis. 2011;24:435–441. doi: 10.1097/QCO.0b013e328349ac4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chêne A, et al. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog. 2007;3:e80. doi: 10.1371/journal.ppat.0030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat. Rev. Immunol. 2003;3:801–812. doi: 10.1038/nri1201. [DOI] [PubMed] [Google Scholar]
- 52.Dups JN, Pepper M, Cockburn IA. Antibody and B cell responses to Plasmodium sporozoites. Front Microbiol. 2014;5:625. doi: 10.3389/fmicb.2014.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Madeleine MM, et al. NIH public access. Int J Cancer. 2013;125:2621–2629. [Google Scholar]
- 54.Silins I, et al. Chlamydia trachomatis infection and persistence of human papillomavirus. Int J Cancer. 2005;116:110–115. doi: 10.1002/ijc.20970. [DOI] [PubMed] [Google Scholar]
- 55.Fukuda EY, et al. Activation of lipid metabolism contributes to interleukin-8 production during. Society. 2005;73:4017–4024. doi: 10.1128/IAI.73.7.4017-4024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harizi H, Juzan M, Pitard V, Moreau J-F, Gualde N. Cyclooxygenase-2-issued prostaglandin E2 enhances the production of endogenous IL-10 which down-regulates Dendritic cell functions. J Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 57.Paavonen J, Lehtinen M. Chlamydial pelvic inflammatory disease in adolescents. Hum Reprod Update. 1996;2:519–529. doi: 10.1093/humupd/2.6.519. [DOI] [PubMed] [Google Scholar]
- 58.Gautret P, et al. Local and international implications of Schistosomiasis acquired in Corsica France. Emerg Infect Dis. 2015;21:1865–1868. doi: 10.3201/eid2110.150881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.IARC. IARC monographs on the evaluation of carcinogenic risks to humans Schistosomes, Liver Flukes and Helicobacter pylori. Volume 61. 1994. [PMC free article] [PubMed]
- 60.Ossai OP, et al. Bacteriuria and urinary schistosomiasis in primary school children in rural communities in Enugu State, Nigeria, 2012. Pan Afr Med J. 2014;18:4–8. [DOI] [PMC free article] [PubMed]
- 61.Adeyeba OA, Ojeaga SGT. Urinary Schistosomiasis and concomitant urinary tract pathogens among school children in metropolitan Ibadan. Afr J Biomed Res. 2002;5:103–108. [Google Scholar]
- 62.Hsieh Y-J, Fu C-L, Hsieh MH. Helminth-induced interleukin-4 abrogates invariant natural killer T cell activation-associated clearance of bacterial infection. Infect Immun. 2014;82:2087–2097. doi: 10.1128/IAI.01578-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis CP, Cohen MS, Gruber MB, Anderson MD, Warren MM. Urothelial hyperplasia and neoplasia: a response to chronic urinary tract infection in rats. J Urol. 1984;132:1025–1031. doi: 10.1016/s0022-5347(17)49992-7. [DOI] [PubMed] [Google Scholar]
- 64.Ma N, et al. Nitrative DNA damage and Oct3/4 expression in urinary bladder cancer with Schistosoma haematobium infection. Biochem Biophys Res Commun. 2011;414:344–349. doi: 10.1016/j.bbrc.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 65.Kamal S, et al. Clinical, virological and histopathological features: long-term follow-up in patients with chronic hepatitis C co-infected with S. mansoni. Liver. 2000;20:281–289. doi: 10.1034/j.1600-0676.2000.020004281.x. [DOI] [PubMed] [Google Scholar]
- 66.Kamal SM, et al. Specific cellular immune response and cytokine patterns in patients Coinfected with hepatitis C virus and Schistosoma mansoni. J Infect Dis. 2001;184:972–982. doi: 10.1086/323352. [DOI] [PubMed] [Google Scholar]
- 67.Wakeham K, et al. Parasite infection is associated with Kaposi’s sarcoma associated herpesvirus (KSHV) in Ugandan women. Infect Agent Cancer. 2011;6:15. doi: 10.1186/1750-9378-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iaria M, et al. Detection of KI WU and Merkel cell polyomavirus in respiratory tract of cystic fibrosis patients. Clin Microbiol Infect. 2015;21(603):e9–603.e15. doi: 10.1016/j.cmi.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 69.Murata H, et al. Helicobacter bilis infection in biliary tract cancer. Aliment Pharmacol Ther. 2004;20:90–94. doi: 10.1111/j.1365-2036.2004.01972.x. [DOI] [PubMed] [Google Scholar]
- 70.Pisani P, et al. Cross-reactivity between immune responses to Helicobacter bilis and Helicobacter pylori in a population in Thailand at high risk of developing cholangiocarcinoma. Clin Vaccine Immunol. 2008;15:1363–1368. doi: 10.1128/CVI.00132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mossman KL, Ashkar ALIA. Review Herpesviruses and the innate immune response. Viral Immunol. 2005;18:267–281. doi: 10.1089/vim.2005.18.267. [DOI] [PubMed] [Google Scholar]
- 72.Barnes E. Diseases and human evolution. Albuquerque Univ: New Mex Press; 2005. [Google Scholar]
- 73.Oikonomopoulou K, Brinc D, Kyriacou K, Diamandis EP. Infection and cancer: revaluation of the hygiene hypothesis. Clin Cancer Res. 2013;19:2834–2841. doi: 10.1158/1078-0432.CCR-12-3661. [DOI] [PubMed] [Google Scholar]
- 74.Smith KR, Hanson HA, Mineau GP, Buys SS. Effects of BRCA1 and BRCA2 mutations on female fertility. Proc Biol Sci. 2012;279:1389–1395. doi: 10.1098/rspb.2011.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 76.Lee K. A. Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol. 2006;46:1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- 77.Walker B, Figgs LW, Zahm SH. Differences in cancer incidence, mortality, and survival between African Americans and whites. Environ Health Perspect. 1995;103:275–281. doi: 10.1289/ehp.95103s8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Byrne KJ, Dalgleish AG. Evolution, immune response, and cancer. Lancet. 2000;356:1033–1034. doi: 10.1016/S0140-6736(05)72656-8. [DOI] [PubMed] [Google Scholar]
- 79.Finlay CM, Walsh KP, Mills KHG. Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases. Immunol Rev. 2014;259:206–230. doi: 10.1111/imr.12164. [DOI] [PubMed] [Google Scholar]
- 80.Botelho MC, Oliveira PA, Lopes C, Correia da Costa JM, Machado JC. Urothelial dysplasia and inflammation induced by Schistosoma haematobium total antigen instillation in mice normal urothelium. Urol Oncol. 2015;29:809–814. doi: 10.1016/j.urolonc.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 81.Perry S, et al. Infection with Helicobacter pylori is associated with protection against tuberculosis. PLoS One. 2010;5:e8804. doi: 10.1371/journal.pone.0008804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gillespie SH. Tuberculosis: evolution in millennia and minutes. Biochemical Society Transactions. 2007;35:9–12. [DOI] [PubMed]
- 83.Blaser MJ. Disappearing microbiota: Helicobacter pylori protection against esophageal adenocarcinoma. Cancer Prev Res (Phila) 2008;1:308–311. doi: 10.1158/1940-6207.CAPR-08-0170. [DOI] [PubMed] [Google Scholar]
- 84.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ledford H. The killer within. 2014. [Google Scholar]
- 86.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14:559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 88.Qi H, et al. Therapeutic efficacy of IL-17A antibody injection in preventing the development of colitis associated carcinogenesis in mice. Immunobiology. 2015;220:54–59. doi: 10.1016/j.imbio.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 90.Mostafa MH, Sheweita SA. Relationship between Schistosomiasis and Bladder Cancer Evidence Supporting The Relationship Between Schistosomiasis And Bladder. 1999;12:97–111. doi: 10.1128/cmr.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Choi BI, Han JK, Hong ST, Lee KH. Clonorchiasis and cholangiocarcinoma: etiologic relationship and imaging diagnosis. Clin Microbiol Rev. 2004;17:540–52. [DOI] [PMC free article] [PubMed]
- 92.Zur Hausen H. The search for infectious causes of human cancers: where and why. Virology. 2009;392:1–10. doi: 10.1016/j.virol.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Aziz RK, Khalifa MM, Sharaf RR. Contaminated water as a source of Helicobacter pylori infection. J Adv Res. 2013;6:539–547. doi: 10.1016/j.jare.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ewald PW, Swain Ewald HA. Infection, mutation, and cancer evolution. J Mol Med (Berl). 2012;90:535–41. [DOI] [PubMed]
- 95.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 96.De Martel C, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 97.Russell SJ, Peng K-W, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bell J, McFadden G. Viruses for tumor therapy. Cell Host Microbe. 2014;15:260–265. doi: 10.1016/j.chom.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ledford H. Cancer-fighting viruses near market. Nature. 2015;526 [DOI] [PubMed]
- 100.Kim Y, Lin Q, Glazer PM, Yun Z. Hypoxic tumor microenvironment and cancer cell differentiation. Curr Mol Med. 2010;9:425–434. doi: 10.2174/156652409788167113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Agrawal N, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci U S A. 2004;101:15172–15177. doi: 10.1073/pnas.0406242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roberts NJ, et al. Intratumoral injection of clostridium novyi-NT spores induces antitumor responses. Sci. Transl Med. 2014;6:249ra111. doi: 10.1126/scitranslmed.3008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palefsky JM, Holly EA. Chapter 6: Immunosuppression and co-infection with HIV. J Natl Cancer Inst Monogr. 2003;31:5–10. [DOI] [PubMed]
- 104.Gopal S, et al. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32:876–880. doi: 10.1200/JCO.2013.53.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mesri EA, Cesarman E, Boshoff C. Kaposi‘s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chirenje ZM. HIV and cancer of the cervix. Best Pract Res Clin Obstet Gynaecol. 2005;19:269–276. doi: 10.1016/j.bpobgyn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 107.Gur, C. et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 344–355 (2015). doi:10.1016/j.immuni.2015.01.010 [DOI] [PMC free article] [PubMed]
- 108.Spencer JV, et al. Potent immunosuppressive activities of cytomegalovirus- encoded interleukin-10. J Virol. 2002;76:1285–1292. doi: 10.1128/JVI.76.3.1285-1292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cobbs CS. Evolving evidence implicates cytomegalovirus as a promoter of malignant glioma pathogenesis. Herpesviridae. 2011;2:10. doi: 10.1186/2042-4280-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the streptococcus erysipelas and the Bacillus Prodigiosus). 1909. [PMC free article] [PubMed]
- 111.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 112.Gontero P, et al. The role of bacillus Calmette-Guérin in the treatment of non-muscle-invasive bladder cancer. Eur Urol. 2010;57:410–429. doi: 10.1016/j.eururo.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 113.Huang P, et al. Efficacy of intravesical bacillus Calmette-Guérin therapy against tumor immune escape in an orthotopic model of bladder cancer. Exp Ther Med. 2015;9:162–166. doi: 10.3892/etm.2014.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


