Abstract
Group B streptococci (GBS) are the leading cause of neonatal meningitis and sepsis worldwide. The current treatment strategy is limited to intrapartum antibiotic prophylaxis in pregnant women to prevent early-onset neonatal diseases, but considering the potential for antibiotic resistance, the risk of losing control over the disease is high. To approach this problem, we have developed a bacteriophage (phage) lytic enzyme to remove colonizing GBS. Bacteriophage muralytic enzymes, termed lysins, are highly evolved molecules designed to degrade the cell wall of host bacteria to release phage particles from the bacterial cytoplasm. Several different lysins have been developed to specifically kill bacterial pathogens both on mucosal surfaces and in blood and represent a novel approach to control infection. A lysin cloned from a phage infecting GBS was found to contain two putative catalytic domains and one putative binding domain, which is similar to the domain organization of some staphylococcal phage lysins. The lysin (named PlyGBS) was recombinantly expressed in Escherichia coli, and purified PlyGBS efficiently killed all tested GBS serotypes in vitro. In a mouse model, a single dose of PlyGBS significantly reduced bacterial colonization in both the vagina and oropharynx. As an alternative strategy for intrapartum antibiotic prophylaxis, this approach may be used to reduce vaginal GBS colonization in pregnant women before delivery or to decontaminate newborns, thus reducing the incidence of GBS-associated neonatal meningitis and sepsis.
Group B streptococci (GBS), or Streptococcus agalactiae, are the leading cause of neonatal sepsis and meningitis in the United States and Europe (1). First identified as a pathogen in bovine mastitis, GBS were later found to colonize the human genital and lower gastrointestinal tract, where the organism can be transmitted perinatally to the fetus. In addition to newborn infections, GBS can also cause substantial morbidity and mortality in adults, particularly those who are immunocompromised or elderly (6). GBS are classified into nine serotypes based on differences in the polysaccharide capsule (24). Historically, the most commonly reported serotype associated with diseases is type III; however, recent epidemiological studies found that serotype V now accounts for approximately 30% of nonpregnant adults (11) and 14 to 23% of pregnant women and neonates (30).
The efficacy of antibiotic administration during various stages of pregnancy in order to reduce the rate of early-onset GBS infection in neonates has been tested. Hall et al. (10) demonstrated that treatment during pregnancy (antepartum prophylaxis) reduced maternal GBS colonization transiently, but colonization recurred at delivery. For postpartum prophylaxis, universal administration of penicillin to neonates shortly after birth reduced the early-onset GBS infection by 68%, but it was also associated with a 40% increase in overall mortality and therefore is not recommended (2). The most successful approach to block vertical transmission of GBS from mother to infant is intrapartum prophylaxis (4 h prior to delivery) of colonized women. This method was shown to effectively reduce both GBS neonatal colonization and early-onset infection (4). In one study (21), intrapartum prophylaxis reduced the risk of early-onset infection by 56% in neonates born to women who had risk factors for neonatal disease (premature labor or prolonged rupture of membranes). Therefore, recommendations for intrapartum prophylaxis to prevent perinatal GBS disease were issued by the Centers for Disease Control and Prevention (CDC) in 1996 (4) and were revised in 2002 (5).
In the revised CDC recommendations (5), penicillin remains the first-line agent for intrapartum antibiotic prophylaxis. Erythromycin and clindamycin used to be recommended for penicillin-allergic women; however, this is no longer the case due to the significant number of resistant clinic isolates (7). This may suggest a possible occurrence of more antibiotic resistance in the future. Furthermore, recent clinical trials suggest that antibiotic administration to pregnant women may be associated with adverse neonatal problems, such as necrotizing enterocolitis or the increased need for supplementary oxygen (14, 15). Thus, alternative strategies with fewer side effects are necessary to control and prevent GBS neonatal infection, particularly in situations of increased antibiotic resistance.
Bacteriophage lysins are highly evolved cell wall hydrolases that can kill bacteria by digesting the bacterial cell wall, making cells susceptible to osmotic lysis. Previously, a lysin purified from the streptococcal phage C1 was demonstrated to kill group A streptococci in vitro and to prevent streptococcal colonization in mice (23). Following this study, two other lysins (Pal and PlyG) were shown to be able to eradicate Streptococcus pneumoniae (17) and Bacillus anthracis (27), respectively, from in vivo models. To continue this approach, we explore here the possibility of using a lysin from a GBS bacteriophage as an alternative approach for intrapartum prophylaxis. We cloned a lysin gene from the GBS phage NCTC 11261 and recombinantly expressed and purified the protein, named PlyGBS (for phage lysin for GBS). Evidence presented here demonstrates a significant lytic activity of PlyGBS both in vitro and in vivo.
MATERIALS AND METHODS
Materials.
Restriction endonucleases were obtained from New England Biolabs (Beverly, Mass.). All reagents used were purchased from Sigma (St. Louis, Mo.) unless otherwise indicated. GBS strains and bacteriophage NCTC 11261 (Table 1) were obtained from the National Collection of Type Culture (NCTC; Colindale, London, United Kingdom). All of the GBS strains used in the study are human clinical isolates. Most bacterial strains used in this study were grown in THY media (23), except Lactobacillus acidophilus and Lactobacillus crispatus, which were grown in Lactobacillus MRS broth (Difco) and incubated with 5% CO2. To propagate the bacteriophage, GBS strain NCTC 11237 was grown at 30°C to early log phase, an equal volume of phage lysate was added, and the cells were allowed to incubate at 30°C for 4 h. After centrifugation, the supernatant was passed through a 0.45-μm-pore-size filter (Millipore, Bedford, Mass.) and the lysate was used for further phage propagation. A streptomycin-resistant derivative of NCTC 11237 was generated by growing the NCTC 11237 strain for 18 h in THY broth supplemented with streptomycin increasing stepwise to a final concentration of 200 μg/ml.
TABLE 1.
Bacteria and bacteriophage used in this study
| Bacteria or bacteriophage | Strain | Serotype | Sourcea |
|---|---|---|---|
| Bacteria | |||
| GBS | |||
| NCTC 11234 | IIR | 1 | |
| NCTC 11235 | II/Ic | 1 | |
| NCTC 11237 | IIIR | 1 | |
| NCTC 11244 | Ib | 1 | |
| NCTC 11248 | Ia | 1 | |
| NCTC 11254 | III | 1 | |
| NCTC 11237 derivative | IIIR, Strr | 2 | |
| ATCC BAA-611 | V | 6 | |
| Other Lancefield groups | |||
| Group A streptococcus | D471 | 2 | |
| Group C streptococcus | 2GRP66 | 2 | |
| Group D streptococcus | D76 | 2 | |
| Group D streptococcus | JH2-2 | 2 | |
| Group E streptococcus | K131 | 2 | |
| Group G streptococcus | D166B | 2 | |
| Group L streptococcus | D167A | 2 | |
| Group N streptococcus | C559 | 2 | |
| Oral streptococci | |||
| S. gordonii | PK488 | 4 | |
| FSS2 | 4 | ||
| S. oralis | PK34 | 3 | |
| S. salivarius | ATCC 9222 | 3 | |
| ATCC 27945 | 3 | ||
| S. sobrinus | 6715 | 2 | |
| S. mutans | 10449 | 5 | |
| Other bacteria | |||
| Lactobacillus acidophilus | ATCC 4356 | 6 | |
| Lactobacillus crispatus | ATCC 33197 | 6 | |
| Bacillus cereus | ATCC 4342 | 2 | |
| Staphylococcus aureus | RN6390 | 2 | |
| Bacteriophage | |||
| GBS phage NCTC 11261 | NCTC 11237b | 1 |
1, NCTC, Colindale, London, United Kingdom; 2, The Rockefeller University Collection; 3, Paul Kolenbrander, National Institute of Dental and Craniofacial Research, Bethesda Md.; 4, John Mayo, Department of Biochemistry and Molecular Biology, University of Georgia, Athens; 5, Arnold Bleweis, Department of Dental Science, University of Florida, Gainesville; 6, American Type Culture Collection, Manassas, Va.
Propagating strain.
Identification of the lysin genes and construction of an expression vector.
Genomic DNA of phage NCTC 11261 was isolated with the Lambda Maxi kit (QIAGEN, Valencia, Calif.). The genomic DNA was partially digested with Tsp509I, and 0.5- to 3-kbp DNA fragments were cloned into expression vector pBAD24 (9). The library was transformed into Escherichia coli and screened for lysin activity as previously described (27), using the indicator strain GBS NCTC 11237 as a bacterial overlay. Alternatively, colonies that grew well on Luria-Bertani (LB) ampicillin plates but had no or poor growth on induction plates (containing ampicillin and arabinose) were isolated and their corresponding plasmids were sequenced. DNA sequences were analyzed with the programs BlastX (National Center for Biotechnology Information, Bethesda, Md.) and MacVector (Accelrys, San Diego, Calif.).
To increase the expression level of plyGBS, it was cloned into a high-expression vector, pET28a (Novagen, Madison, Wis.). Two primers (forward, 5′-GAGGAGTCCATGGCAACTTATCAAGAATATAAAAGC; reverse, 5′-GCTACCTCGAGTTATTACATATCTGTTGCGTTAACTAAG) were designed to PCR amplify plyGBS, using genomic DNA of phage NCTC 11261 as the template. The PCR product was end digested with NcoI and XhoI and cloned into pET28a to form pCQJ67-2, which was subsequently transformed into E. coli strain BL21(DE3) for protein expression. DNA sequence analysis was used to confirm that pCQJ67-2 contains no mutation.
PlyGBS overexpression and purification.
The E. coli BL21(DE3)/pCQJ67-2 strain was grown at 37°C overnight in LB broth supplemented with kanamycin (50 μg/ml), subcultured, and grown to an optical density at 600 nm (OD600) of 0.5. Expression of the PlyGBS protein was induced at 30°C for 4 h by the addition of isopropyl-1-thio-β-d-galactopyranoside (IPTG) to a final concentration of 1 mM. Cells were then harvested, resuspended in 50 ml of phosphate-buffered saline plus 500 U of Benzonase (Novagen, Madison, Wis.), and lysed with 10 ml of chloroform for 45 min. After centrifugation, the supernatant was dialyzed against 25 mM Tris-HCl (pH 7.4) at 4°C overnight. The dialyzed lysate was applied to a 40-ml Q-Sepharose column (Amersham Pharmacia, Piscataway, N.J.) and eluted in a linear gradient from 0 to 1 M NaCl in 25 mM Tris-HCl (pH 7.4). Fractions were analyzed for lytic activity as previously described (23). Active fractions were pooled and electrophoresed on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (10% polyacrylamide). Purity of PlyGBS was assessed by scanning densitometry of the Coomassie-stained gel.
Quantification of PlyGBS activity.
Lysin activity was measured as previously described (23), and some modifications were made in this study. Briefly, GBS strain NCTC 11237 was grown to an OD600 of 0.3, centrifuged, and resuspended to a final OD600 of 0.8 in 40 mM phosphate buffer (pH 5.0) plus 200 mM NaCl. Bacterial suspensions (0.1 ml) were added to serial dilutions of purified PlyGBS (0.1 ml) in 96-well plates, and the decrease in OD600 was monitored by spectrophotometer at 37°C. The highest dilution of lysin that decreased the OD600 by 50% in 15 min was determined, and the activity of PlyGBS in units per milliliter was defined as this dilution factor multiplied by 10, taking into account that only 0.1 ml of PlyGBS was used in the assay.
In vitro PlyGBS activity.
To test the in vitro PlyGBS activity on various bacterial strains (Table 1), all strains were inoculated overnight, subcultured (1:100), and grown to an OD600 of 0.3. Cells were centrifuged and resuspended in a 1/10 volume of phosphate buffer (40 mM, pH 5.0). One hundred-microliter aliquots of the bacterial solution (5 × 108 to 5 × 109 CFU/ml) were incubated with indicated amounts of PlyGBS at 37°C for 60 min. A spectrophotometer was used to monitor the lytic activity, measured as the drop in milli-OD600 per minute (−mOD600/min). The initial velocity of this reaction is defined as the rate of lysis. To determine bacterial viability, cells of GBS strain NCTC 11237 from the above assay were centrifuged, diluted, and plated on THY agar plates for cell counts. All experiments were performed in triplicate, and control experiments with the addition of phosphate buffer (pH 5.0) were performed under the same conditions.
Microscopy.
GBS strain NCTC 11237 was grown in THY broth to an OD600 of 0.3. Bacteria were centrifuged and resuspended in 40 mM phosphate buffer (pH 5.0) to an OD600 of 1.0. Three hundred microliters of this bacterial suspension was mixed with 300 μl of PlyGBS (final concentration, 10 U/ml) and incubated at 37°C for 15 min. A buffer control was also included for comparison. For phase-contrast microscopy, samples were viewed at ×1,000 with an Eclipse E400 microscope (Nikon, Tokyo, Japan). For electron microscopy, the samples were prepared as previously described (17) and processed following standard procedures by the Electron Microscopy Service in the Rockefeller University.
In vivo lysin activity on GBS.
Six-week-old BALB/c female mice were purchased from Charles River Laboratories (Wilmington, Mass.). Because bacterial colonization in the mouse vaginal cavity is believed to be more efficient at estrus (20), the estral cycle of all mice was synchronized on day 1 by subcutaneous inoculation of 0.1 mg of β-estradiol valerate. On day 3, 30 mice were anesthetized and inoculated vaginally with ∼106 streptomycin-resistant GBS NCTC 11237 cells with a micropipette (20-μl dose in 40 mM phosphate buffer, pH 5.0). On day 4, mice were treated vaginally with 20 μl of phosphate buffer (pH 5.0) and swabbed with calcium alginate fiber-tipped ultrafine swabs (Fischer, Pittsburgh, Pa.). The surfaces of THY agar plates containing 5% sheep blood and streptomycin (200 μg/ml) were streaked with the wet swabs to determine baseline colonization. Mice were then randomized to be treated vaginally with either 20 μl of phosphate buffer, pH 5.0 (n = 15), or 10 U of PlyGBS lysin (n = 15). At 2 and 4 h posttreatment, all mice were swabbed again for titer determination.
To test if PlyGBS can be used for postpartum treatment of newborns, 38 mice received an upper respiratory challenge of ∼108 Strr GBS NCTC 11237 cells (20 μl orally and 20 μl to each nostril). The next morning, mice were oropharyngeally swabbed and baseline colonization was enumerated as described above. Mice were randomized and administrated orally and nasally with either 20 μl of phosphate buffer, pH 5.0 (n = 18), or 10 U of PlyGBS lysin (n = 20). At 2 and 24 h posttreatment, all mice were oropharyngeally swabbed to determine the bacterial count.
For statistical analysis, MIXED Model (from the SAS Mixed Procedure) was used to compare the colonization status between groups. A P value of <0.05 was considered significant.
Nucleotide sequence accession number.
The identified NCTC 11261 lysin sequence (plyGBS) has been submitted to GenBank under accession no. AY428505.
RESULTS
Cloning and sequence alignment of PlyGBS.
When clones of the GBS phage NCTC 11261 genomic library were screened for a possible lysin gene, no colonies formed clear zones on a GBS NCTC 11237 bacterial overlay. Instead, we identified the partial sequence of a putative lysin gene when sequencing a plasmid from a colony that grew poorly upon induction. Upstream of this partial lysin gene, we discovered a putative holin gene that may have exerted a toxic effect on the cells upon induction (data not shown). The full sequence of the lysin gene (plyGBS) was obtained by sequencing of the phage NCTC 11261 genomic DNA. Similarity searches indicate that, at both the nucleotide and amino acid levels, this gene is over 90% identical to several lysins from various streptococcal species, including GBS phage B30 lysin (GenBank accession no. AAN28166), Streptococcus pyogenes M1 phage-associated lysin (AAK33905), and Streptococcus equi phage-associated protein (AF186180). This suggests that these putative lysin genes may have evolved from an ancestor that was present in all of these streptococcal phages.
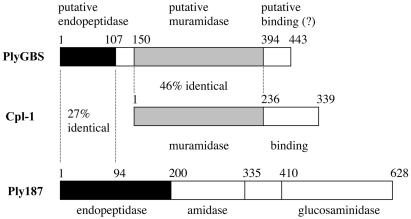
Alignment of the putative PlyGBS protein sequence with pneumococcal phage Cp-1 lysin (Cpl-1) and staphylococcal phage 187 lysin (Ply187) indicates that PlyGBS has three different domains (Fig. 1). The N-terminal 107 amino acids are 27% identical to a domain in Ply187 that functions as an endopeptidase (22). Further experimentation will be required to verify this activity for the PlyGBS N-terminal domain. For amino acids 150 to 394 (central domain), PlyGBS displays 46% identity to the N-terminal muramidase domain of Cpl-1 (8). Two acidic amino acids, Asp and Glu, which have been proposed to be involved in the catalytic activity of Cpl-1 and lysozymes (12), are also present in PlyGBS at positions 158 (Asp) and 185 (Glu). The C terminus of the PlyGBS gene has no similarity to other lytic enzymes. This is not unusual, since it is the location of the cell wall binding domain of most lytic enzymes, which is unique for each enzyme. However, this may not be the case for PlyGBS, and further experimentation is needed to determine the function of its C-terminal domain. The multiple domain organization of PlyGBS indicates that the gene encoding PlyGBS is probably a mosaic gene, composed of conserved muralytic domains rearranged from multiple phage lysin genes.
FIG. 1.
Schematic diagram of domain organization in PlyGBS. Based on sequence analysis, PlyGBS contains two catalytic domains: the N-terminal region has 27% identity to the first 94 amino acids of the endopeptidase domain of staphylococcal phage 187 lysin (Ply187), and the central region of the PlyGBS displays 46% identity to the muramidase domain of pneumococcal phage Cp-1 lysin (Cpl-1). The C terminus of PlyGBS is a putative cell wall binding domain. The domain organization in PlyGBS resembles that of some staphylococcal phage lysins, which have two or more catalytic domains, such as Ply187 containing endopeptidase, amidase, and glucosaminidase domains.
Characterization of PlyGBS.
Based on the deduced amino acid sequence, PlyGBS has a theoretical pI value (isoelectric point) of 4.88. With 25 mM Tris-HCl (pH 7.4) as the elution buffer in ion-exchange chromatography, the protein was negatively charged and bound to a positively charged Q-Sepharose anion exchanger. The enzyme was eluted by an NaCl gradient, and the active fractions were pooled and analyzed by SDS-PAGE. As shown in Fig. 2, the major protein band at ∼50 kDa correlates with the calculated molecular mass for PlyGBS (49.6 kDa). The final preparation, estimated to be >95% pure by scanning densitometry on the Coomassie-stained SDS-PAGE gel, was used for all subsequent experiments.
FIG. 2.
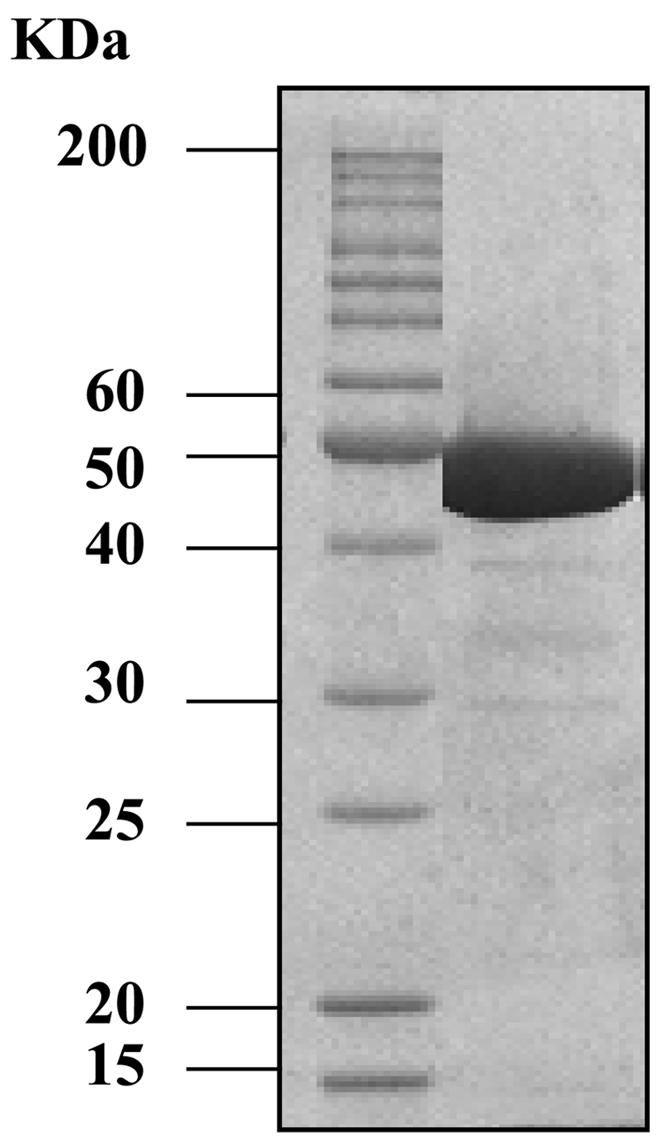
Coomassie blue-stained SDS-PAGE of purified PlyGBS. The molecular mass of the protein ladder is presented in kilodaltons.
The pH optimum of purified PlyGBS was determined to be around 5.0, with an active pH range between 4.0 and 6.0 (data not shown). Gel filtration chromatography of PlyGBS indicates that the protein functions as a monomer (data not shown).
PlyGBS activity in vitro.
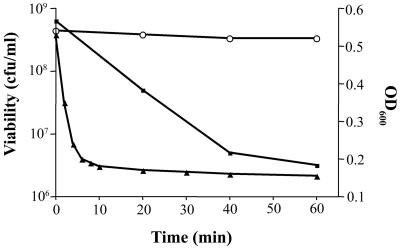
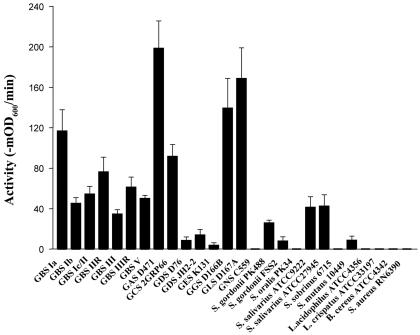
To determine the PlyGBS activity in vitro, GBS cells (NCTC 11237, serotype IIIR) were mixed with 40 U of PlyGBS at 37°C for 60 min. The OD600 dropped to baseline within 10 min, indicating a rapid rate of cell lysis, which is corroborated by the 2-log decrease in viability observed at 60 min (Fig. 3). When multiple strains of GBS (Table 1), representing serotypes Ia, Ib, Ic/II, IIR, and IIIR, as well as the predominant serotypes III and V, were tested for PlyGBS sensitivity based on lytic activity, a similar velocity of lysis (−mOD600/min) was observed with certain strain-to-strain variation (Fig. 4). Because almost all of the GBS strains are encapsulated (24), structural differences between capsules of various serotypes may change the accessibility of PlyGBS to cell wall targets, which contribute to the observed variability of lysin activity.
FIG. 3.
PlyGBS activity against GBS in vitro. GBS NCTC 11237 cells were resuspended in 40 mM phosphate buffer (pH 5.0) and mixed in 96-well microtiter plates with 40 U of PlyGBS. The OD600 (solid triangles) was monitored by a spectrophotometer. To check the cell viability (solid squares), the cells were centrifuged, diluted, and plated on THY agar plates for enumeration. Control experiments (open circles) with the addition of phosphate buffer (pH 5.0) were performed under the same conditions.
FIG. 4.
Lysin activity against different bacteria in vitro. The cells were exposed to 15 U of PlyGBS for 60 min, and the decrease of OD600 was monitored by a spectrophotometer. The activity of lysin was expressed as the initial velocity of the decrease in absorbance over time (−mOD600/min). Error bars show the standard error of the mean.
In addition to GBS, bacterial strains representing a variety of species were analyzed to determine their sensitivity to PlyGBS in vitro (Table 1 and Fig. 4). Of the tested streptococcal strains belonging to different Lancefield groups, PlyGBS had significant lytic activity against group A, C, G, and L streptococci, with little to no activity against group D, E, and N streptococci. This could be explained by the significant similarity between PlyGBS and lysins from S. pyogenes (group A) and S. equi (group C). We also tested the muralytic activity of PlyGBS against non-Lancefield oral streptococcal species, including S. gordonii, S. oralis, S. salivarius, S. sobrinus, and S. mutans. Interestingly, PlyGBS had medium to low activity against S. salivarius, S. gordonii, and S. mutans (Fig. 4) but no activity against the other two commensal species tested. No lytic activity was observed with two nonstreptococcal gram-positive species, Bacillus cereus and Staphylococcus aureus, or two vaginal commensal bacteria, L. acidophilus and L. crispatus.
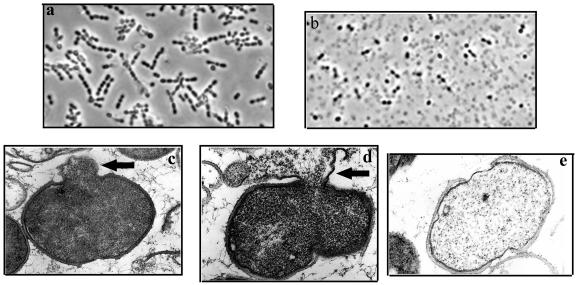
Phase-contrast and electron microscopy were used to visualize the lytic effect of lysin on GBS cells. Normally, intact GBS form short chains, as shown in the buffer control (Fig. 5a). After treatment with lysin (Fig. 5b), the cells lost their cytoplasmic contents and became opaque by light microscopy. As seen with other lysins by electron microscopy, a weakness in the cell wall produced by PlyGBS results in extrusions (Fig. 5c) and rupture (Fig. 5d) of the cytoplasmic membrane, which appears to be more localized within the septum region of the dividing cells. Subsequent loss of cytoplasmic contents transforms the cells into empty “cell ghosts” (Fig. 5e).
FIG. 5.
Visualization of the muralytic effect of lysin on GBS cells by phase-contrast and electron microscopy. (a and b) Phase-contrast microscopy of cells treated with buffer (a) or PlyGBS (b) for 15 min. (c to e) Thin-section transmission electron micrographs (magnification, ×65,000) of cells treated with PlyGBS show the extrusion (c) and rupture (d) of the cytoplasmic membrane which occurred at the septum region, resulting in “ghost” cells (e) after the loss of cytoplasmic contents.
PlyGBS activity in vivo.
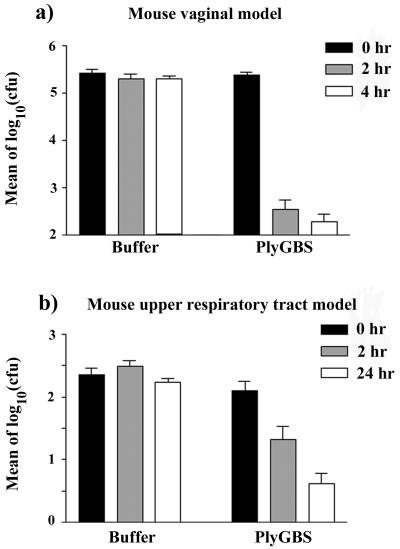
To determine if PlyGBS was able to remove colonizing GBS from the mouse vagina, two groups of mice were challenged vaginally with 106 CFU of Strr GBS cells. Twenty-four hours later, the vaginal cavities were swabbed to determine the initial colonization rate (0-h samples, pretreatment). The mice were then treated vaginally with either buffer (n = 15) or PlyGBS (n = 15) and swabbed 2 and 4 h posttreatment. As shown in Fig. 6a, small effect was observed in the buffer control animals. In contrast, animals treated with a single dose of PlyGBS showed a significant reduction (approximately 3-log drop) in the bacterial load at both the 2- and 4-h intervals compared to the level in the buffer control (P < 0.0001).
FIG. 6.
Control of GBS colonization in mice with PlyGBS. (a) Mice were colonized with GBS vaginally after synchronization with β-estradiol valerate. One day after GBS inoculation, two groups of mice were treated vaginally with either buffer (n = 15) or PlyGBS (n = 15). Each mouse was swabbed vaginally before treatment (0-h samples) and after treatment at 2- to 4-h intervals (2- and 4-h samples). (b) Mice were colonized with GBS in their upper respiratory tract, and 1 day later they were treated with either buffer (n = 18) or PlyGBS (n = 20). Each mouse was swabbed before (0 h) and after treatment (2 and 24 h). After transformed into log10, the colony counts of oropharynx samples were averaged for each time interval in the same group and plotted. Error bars represent the standard error of the mean.
Similarly, two groups of mice were challenged with a total of 108 CFU of Strr GBS delivered orally and nasally to determine if PlyGBS can be used to reduce colonized GBS in the mouse upper respiratory tract. As shown in Fig. 6b, mice treated with a single dose of lysin by the same route exhibited a significant reduction in GBS colonization at both the 2- and 24-h swabbing intervals compared to the level in the buffer control group (P < 0.0001).
DISCUSSION
In this study, we identified a putative lysin gene from a GBS bacteriophage and the recombinantly expressed lysin PlyGBS was purified using ion-exchange chromatography. We demonstrate that PlyGBS is capable of lysing GBS cells effectively in vitro. Using a mouse model, we conclude that the bacterial load of GBS colonizing the vagina and oropharynx of the mice could be reduced significantly by a single treatment with PlyGBS. To our knowledge, this is the first reported GBS phage lysin showing in vivo therapeutic efficacy.
GBS primarily colonize the human vaginal tract along with the normal vaginal flora such as L. acidophilus, which metabolizes glycogen to lactic acid, resulting in the low pH (4.4 to 5.6) observed in the vaginal tract (3). The low pH and the production of hydrogen peroxide (H2O2) produced by vaginal bacteria are important defenses against pathogens. Given the fact that the optimum pH for PlyGBS (around pH 5.0) is within the range normally found in the human vaginal tract, it is reasonable to assume that the effectiveness observed in the mouse model will translate effectively to the human vagina; however, future trials will be necessary to confirm this.
The introduction of intrapartum antibiotic prophylaxis effectively reduces GBS neonatal colonization and early-onset infection (4). However, there are still situations that may make it more desirable to use PlyGBS treatment than antibiotic therapy. In consideration of antibiotic resistance, PlyGBS provides a better choice than antibiotics because of the inability to identify bacterial mutants resistant to phage lysins (18, 27). Additionally, in situations where GBS screening of pregnant women is not a routine practice, universal administration of PlyGBS before delivery would be preferable. It should be realized that any treatment against GBS during labor must be rapidly effective to prevent disease, and this requirement may provide PlyGBS a significant advantage. Furthermore, because of its specificity for GBS and other pathogens and the lack of activity for two common vaginal commensals, L. acidophilus and L. crispatus, PlyGBS is not likely to disrupt the vaginal microflora, a significant problem in antimicrobial therapy (19).
In the mouse vaginal model, only one dose of PlyGBS was delivered to each mouse, resulting in a significant reduction of colonization at 2 and 4 h posttreatment (Fig. 6a). When we determined the vaginal GBS colonization status at 24 h posttreatment, the GBS counts rebounded (data not shown), which was previously seen during antibiotic therapy (10), suggesting that multiple doses at predetermined intervals may be necessary for complete control. Because intrapartum antibiotic prophylaxis is the usual treatment 4 h prior to delivery to prevent vertical transmission of GBS from mother to infant and because PlyGBS is effective vaginally in mice up to 4 h posttreatment, PlyGBS may be used as an alternative approach to replace intrapartum antibiotic prophylaxis prior to delivery. Since phage enzymes are proteins, and thus immunogenic, it is possible that antibodies induced through multiple exposures would interfere with the catalytic action of the enzyme, reducing its potency. However, recent evidence suggests that antibodies directed to PlyGBS, like other phage enzymes (18), do not neutralize the catalytic activity of PlyGBS (data not shown).
GBS can be transmitted to the newborn from a colonized mother during or before delivery. Prospective studies of GBS infections in newborns have shown that GBS can colonize four different body sites (ear, anus, throat, and umbilicus) (25). It is likely, but not proven, that colonization of the oropharynx (throat) is the route by which neonatal meningitis is initiated. Because of this and the fact that this site would be the most difficult to safely decontaminate in a newborn, we chose the oropharynx to determine if PlyGBS treatment can be used to disinfect GBS upper respiratory colonization. Our animal experiments showed that PlyGBS, compared to the buffer control, significantly reduced the bacterial counts of GBS in the oropharynx (Fig. 6b). Thus, PlyGBS may be used to reduce GBS vaginal colonization in pregnant women before delivery and may also provide a novel antibacterial agent to safely decontaminate newborns from GBS and thus further reduce the rate of GBS-associated neonatal meningitis and sepsis without antibiotics.
Our in vitro experiments indicate that PlyGBS has a lower specific activity than several of the other lysins studied in our laboratory (17, 23, 27). A possible explanation is that, distinctive from streptococci in other Lancefield groups, almost all GBS strains isolated from humans are heavily encapsulated (24) and the capsule may limit the access of PlyGBS to the cell wall. Nonetheless, our data show that, despite the low specific activity compared to other lysins, PlyGBS does provide protection in two different mouse models of GBS colonization. Furthermore, a second-generation PlyGBS could be developed through random mutagenesis (16) or directed evolution (28), which may optimize specific activity, and preliminary experiments suggest that this is indeed possible.
In addition to GBS, PlyGBS has lytic activity on a variety of streptococci in the Lancefield grouping system, including groups A, C, G, and L. This is a second bacteriophage lysin studied in our laboratory that has relative broad specificity (29). While group A streptococci are a primary cause of bacterial pharyngitis, streptococci in groups C and G are also important human pathogens associated with bacterial pharyngitis, arthritis, and bacteremia (13, 26). The relatively extensive activity range of PlyGBS may provide a new agent for controlling multiple streptococcal pathogens. The broad lytic activity of PlyGBS also suggests that these streptococci have a common binding receptor for the enzyme, and comparative genomic analysis may be used to identify this receptor. Given that all tested GBS strains representing various serotypes as well as several other streptococcal groups were sensitive to PlyGBS, we suspect that the binding receptor for PlyGBS is located in the cell wall like other lysins, instead of the capsular polysaccharide.
Our results presented here show the potential of applying PlyGBS to control diseases associated with GBS infection. Future research will focus on improving activity and stability as well as developing an efficient method for drug delivery. Considering that bacteriophages are prevalent in the environment and phage lysins are among the most powerful antimicrobial agents identified in nature, the future development of lysins may provide a novel approach to circumvent resistance to control diseases caused by bacterial pathogens.
Acknowledgments
This work was supported by a grant from the Defense Advance Research Project Agency (DARPA).
We particularly thank Bokai Xia and Brian W. Kirk for their assistance in statistical analysis. We are grateful to Helen Shio for her technical support in preparing electron micrographs. We also thank Jutta M. Loeffler and Mattias Collin for valuable discussions and reviewing the manuscript.
REFERENCES
- 1.Baker, C. J., and M. S. Edwards. 2001. Group B streptococcal infections, p. 1091-1156. In J. Remmington and J. O. Klein (ed.), Infectious diseases of the fetus and newborn infants, 5th ed. The W. B. Saunders Co., Philadelphia, Pa.
- 2.Benitz, W. E., J. B. Gould, and M. L. Druzin. 1999. Antimicrobial prevention of early-onset group B streptococcal sepsis: estimates of risk reduction based on a critical literature review. Pediatrics 103:1-13. [DOI] [PubMed] [Google Scholar]
- 3.Boskey, E. R., K. M. Telsch, K. J. Whaley, T. R. Moench, and R. A. Cone. 1999. Acid production by vaginal flora in vitro is consistent with the rate and extent of vaginal acidification. Infect. Immun. 67:5170-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1996. Prevention of perinatal group B streptococcal disease: a public health perspective. Morbid. Mortal. Wkly. Rep. 45:1-24. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2002. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC. Morbid. Mortal. Wkly. Rep. 51:1-22. [PubMed] [Google Scholar]
- 6.Farley, M. M., R. C. Harvey, T. Stull, J. D. Smith, A. Schuchat, J. D. Wenger, and D. S. Stephens. 1993. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. N. Engl. J. Med. 328:1807-1811. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez, M., M. E. Hickman, and C. J. Baker. 1998. Antimicrobial susceptibilities of group B streptococci isolated between 1992 and 1996 from patients with bacteremia or meningitis. Antimicrob. Agents Chemother. 42:1517-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia, J. L., E. Garcia, A. Arrarás, P. Garcia, C. Ronda, and R. López. 1987. Cloning, purification, and biochemical characterization of the pneumococcal bacteriophage Cp-1 lysin. J. Virol. 61:2573-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall, R. T., W. Barnes, W. Krishnan, D. J. Harris, P. G. Rhodes, J. Fayez, and G. L. Miller. 1976. Antibiotic treatment of parturient women colonized with group B streptococci. Am. J. Obstet. Gynecol. 124:630-634. [DOI] [PubMed] [Google Scholar]
- 11.Harrison, L. H., J. Elliott, D. M. Dwyer, J. P. Libonati, P. Ferrieri, L. Billmann, A. Schuchat et al. 1998. Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. J. Infect. Dis. 177:998-1002. [DOI] [PubMed] [Google Scholar]
- 12.Jolles, P., and J. Jolles. 1984. What's new in lysozyme research? Mol. Cell. Biochem. 63:165-189. [DOI] [PubMed] [Google Scholar]
- 13.Kalia, A., and D. E. Bessen. 2003. Presence of streptococcal pyrogenic exotoxin A and C genes in human isolates of group G streptococci. FEMS Microbiol. Lett. 219:291-295. [DOI] [PubMed] [Google Scholar]
- 14.Kenyon, S. L., D. J. Taylor, W. Tarnow-Mordi et al. 2001. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomized trial. Lancet 357:979-988. [DOI] [PubMed] [Google Scholar]
- 15.Kenyon, S. L., D. J. Taylor, W. Tarnow-Mordi et al. 2001. Broad-spectrum antibiotics for spontaneous preterm labor: the ORACLE II randomized trial. Lancet 357:989-994. [DOI] [PubMed] [Google Scholar]
- 16.Kuipers, O. P. 1996. Random mutagenesis by using mixtures of dNTP and dITP in PCR. Methods Mol. Biol. 57:351-356. [DOI] [PubMed] [Google Scholar]
- 17.Loeffler, J. M., D. Nelson, and V. A. Fischetti. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170-2172. [DOI] [PubMed] [Google Scholar]
- 18.Loeffler, J. M., S. Djurkovic, and V. A. Fischetti. 2003. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 71:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLean, N. W., and I. J. Rosenstein. 2000. Characterization and selection of a Lactobacillus species to recolonize the vagina of women with recurrent bacterial vaginosis. J. Med. Microbiol. 49:543-552. [DOI] [PubMed] [Google Scholar]
- 20.Medaglini, D., C. M. Rush, P. Sestini, and G. Pozzi. 1997. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine 15:1330-1337. [DOI] [PubMed] [Google Scholar]
- 21.Mercer, B. M., M. Miodovnik, G. R. Thurnau et al. 1997. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes: a randomized controlled trial. JAMA 278:989-995. [PubMed] [Google Scholar]
- 22.Navarre, W. W., H. Ton-That, K. F. Faull, and O. Schneewind. 1999. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage Ø11. J. Biol. Chem. 274:15847-15856. [DOI] [PubMed] [Google Scholar]
- 23.Nelson, D., L. Loomis, and V. A. Fischetti. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 98:4107-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paoletti, L. C., L. C. Madoff, and D. L. Kasper. 2000. Surface structures of group B Streptococcus important in human immunity, p. 137-153. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 25.Pass, M. A., B. M. Gary, S. Khare, and H. C. Dillon. 1979. Prospective studies of group B streptococcal infections in infants. J. Pediatr. 95:437-443. [DOI] [PubMed] [Google Scholar]
- 26.Schattner, A., and K. L. Vosti. 1998. Bacterial arthritis due to beta-hemolytic streptococci of serogroups A, B, C, F, and G. Analysis of 23 cases and a review of the literature. Medicine (Baltimore) 77:122-139. [DOI] [PubMed] [Google Scholar]
- 27.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]
- 28.Turner, N. J. 2003. Directed evolution of enzymes for applied biocatalysis. Trends Biotechnol. 21:474-478. [DOI] [PubMed] [Google Scholar]
- 29.Yoong, P., R. Schuch, D. Nelson, and V. A. Fischetti. 2004. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J. Bacteriol. 186:4808-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaleznik, D. F., M. A. Rench, S. Hillier, M. A. Krohn, R. Platt, M. L. Lee, A. E. Flores, P. Ferrieri, and C. J. Baker. 2000. Invasive disease due to group B Streptococcus in pregnant women and neonates from diverse population groups. Clin. Infect. Dis. 30:276-281. [DOI] [PubMed] [Google Scholar]