Abstract
The recently emerged chytrid fungus Batrachochytrium salamandrivorans currently causes amphibian population declines. We hypothesized that temperature dictates infection dynamics of B. salamandrivorans, and that therefore heat treatment may be applied to clear animals from infection. We examined the impact of environmental temperature on B. salamandrivorans infection and disease dynamics in fire salamanders (Salamandra salamandra). Colonization of salamanders by B. salamandrivorans occurred at 15°C and 20°C but not at 25°C, with a significantly faster buildup of infection load and associated earlier mortality at 15°C. Exposing B. salamandrivorans infected salamanders to 25°C for 10 days resulted in complete clearance of infection and clinically cured all experimentally infected animals. This treatment protocol was validated in naturally infected wild fire salamanders. In conclusion, we show that B. salamandrivorans infection and disease dynamics are significantly dictated by environmental temperature, and that heat treatment is a viable option for clearing B. salamandrivorans infections.
In the decades following the identification of Batrachochytrium dendrobatidis in 19991 it became apparent that this chytrid fungus was one of the biotic drivers of declines and extinctions of hundreds of amphibian species worldwide2,3,4,5. However, the impact of B. dendrobatidis varies regionally from a dramatic decrease of amphibian diversity to a state of host-pathogen equilibrium. In one such region characterized by the co-existence of B. dendrobatidis with local amphibian communities6, another recently described chytrid fungus, Batrachochytrium salamandrivorans7, caused amphibian population declines. The reason for this obvious difference in disease dynamics between both chytrid fungi is not known. Disease dynamics are dictated by pathogen virulence, host factors and environmental determinants. Virulent strains of both chytrids, as well as susceptible host species are present in the affected regions8,9,10,11. For B. dendrobatidis, temperature is considered a key environmental factor12,13,14. One major difference between both chytrids is their different thermal growth characteristics, which is probably due to differences in host spectrum, B. salamandrivorans being restricted to urodelan hosts15. Knowledge of the infection dynamics of B. salamandrivorans at different temperatures may help to develop treatment protocols16,17,18. These are urgently needed as current therapies developed against B. dendrobatidis19 fail to eliminate B. salamandrivorans from infected amphibians (unpublished results). We hypothesized that temperature dictates infection and disease dynamics of B. salamandrivorans in salamanders, which may be applied to develop a heat treatment protocol to clear infection in animals.
Results & Discussion
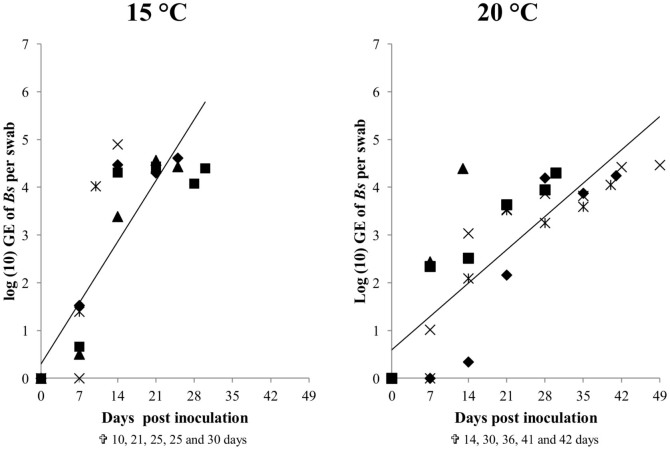
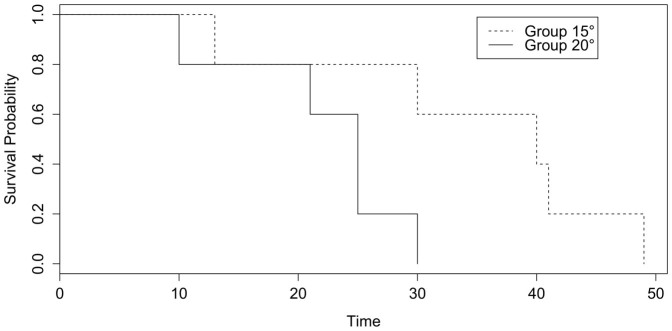
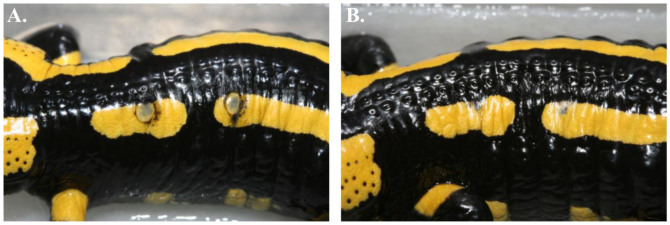
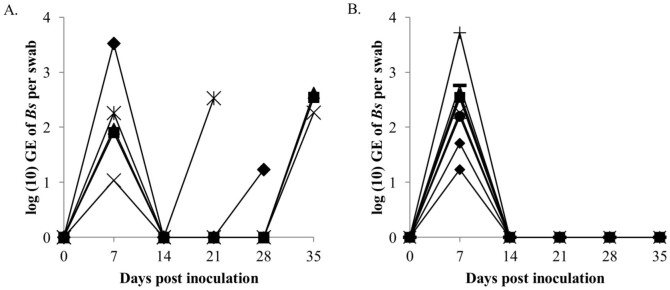
Only after exposure at 15°C or 20°C but not at 25°C, the salamanders were colonized by B. salamandrivorans. If a 10000 GE infection load per swab is considered indicative for a clinical threshold20,21,22, this threshold was reached two times earlier at 15°C than at 20°C (on average 15 ± 4 (SD) days and 31 ± 12 days (SD) respectively, independent t-test p < 0.05) (Fig. 1). The faster buildup of B. salamandrivorans infection loads coincided with earlier mortality at 15°C than at 20°C (22 ± 8 (SD) days and 35 ± 14 (SD) days respectively, Cox regression analysis, χ2 = 3.941, df = 1, p < 0.05) (Fig. 1 and 2). Besides preventing infection of salamanders with B. salamandrivorans, exposure of infected salamanders to a temperature of 25°C during 10 days completely eliminated the infection and resolved B. salamandrivorans lesions from all infected animals (Fig. 3 and 4). However, 7 days exposure at 25°C did not result in fungal clearance since recrudescence of infection was observed in all these salamanders within 1–3 weeks after transferring them to an ambient temperature of 15°C (Fig. 4). This is remarkable since cultures of the fungus are killed in vitro within 5 days of incubation at 25°C7 and shows that B. salamandrivorans is capable to persist in an urodelan host experiencing temperatures that temporarily surpass the fungal thermal maximum for up to one week. Exposure of the relapsing animals to 25°C for 10 days eliminated the infection. Our results reflect B. salamandrivorans growth curves obtained in vitro, with an optimal growth range around 15°C7. In contrast, the pattern of temperature-dependent growth of B. dendrobatidis at 15, 20 and 25°C on frogs was opposite to the pattern of temperature-dependent growth at these temperatures in culture23, and time until death in frogs infected with B. dendrobatidis at 27°C, which is above B. dendrobatidis' thermal preference24, was shorter when compared to time until death of infected frogs kept at lower temperatures14. The suitability of raised ambient temperature as treatment option was validated by keeping 30 wild-caught B. salamandrivorans infected fire salamanders at 25°C during 10 days. Twenty-six animals were cured of B. salamandrivorans infection after this treatment period, 2 died early during treatment, and 2 needed an additional treatment period of 2 days in order to completely clear the infection (Fig. 5). This shows that heat treatment is a viable treatment option for B. salamandrivorans infected amphibians when the clinical condition and the thermal tolerance of the animal is taken into account. In order to completely eliminate B. dendrobatidis infections higher temperatures, composed of short exposure to 37°C16 or extended exposure to 30°C17 are required. These protocols are not suitable for treating salamanders, as these temperatures surpass the upper thermal limit of most urodelans. The 2 animals that died were in poor clinical condition at the start of the treatment period, and probably died due to thermal shock as B. salamandrivorans loads were low at time of death. This points out the narrow margin between the temperature able to eliminate B. salamandrivorans and the upper thermal limit most urodelans tolerate. Furthermore, these results show that the course of infection should be carefully monitored since not all animals tested negative for presence of Bs DNA after 10 days at 25°C. Although we do not think that this is a result of an active infection but explained by presence of residual B. salamandrivorans DNA derived from dead B. salamandrivorans cells, this remains uncertain. This could have been further elucidated by transferring the animals back to 15°C after 10 days but we chose to keep them at 25°C until PCR results became negative. Thermal treatment of B. salamandrivorans infected amphibians would allow large groups of animals to be treated simultaneously at low costs and lacks the possible downsides linked to drug treatment like toxicity or development of acquired antimicrobial resistance.
Figure 1. The course of Batrachochytrium salamandrivorans infection in fire salamanders at 15 and 20°C.
Each symbol represents the course of infection of an individual animal.Time of death of all animals is depicted beneath the graphs. The line represents the average increase in infection intensity in all tested individuals based on a repeated measure regression analysis.
Figure 2. The probability of survival of salamanders housed at 15 or 20°C after infection with Batrachochytrium salamandrivorans.
Survival probability was plotted based on a Cox regression analysis (χ2 = 3.981, df = 1, p < 0.05). Time is displayed in days after initial infection. The dotted line represent survival probability of Batrachochytrium salamandrivorans infected salamanders housed at 15°C and the full line those housed at 20°C.
Figure 3. Heat treatment of amphibians infected with Batrachochytrium salamandrivorans clears infection and resolves associated lesions.
Batrachochytrium salamandrivorans associated skin lesions (A) are clearly reduced after the heat treatment composed of keeping the animals at 25°C during 10 days (B), and will eventually completely resolve.
Figure 4. The effect of exposure to 25°C for 7 and 10 days on the course of Batrachochytrium salamandrivorans infection in fire salamanders.
After establishment of infection fire salamanders were subjected to an ambient temperature of 25°C for 7 days (A), or 10 days (B). Each symbol represents the course of infection of an individual animal.
Figure 5. Heat treatment composed of exposure to 25°C for 10 days of fire salamanders naturally infected with Batrachochytrium salamandrivorans.
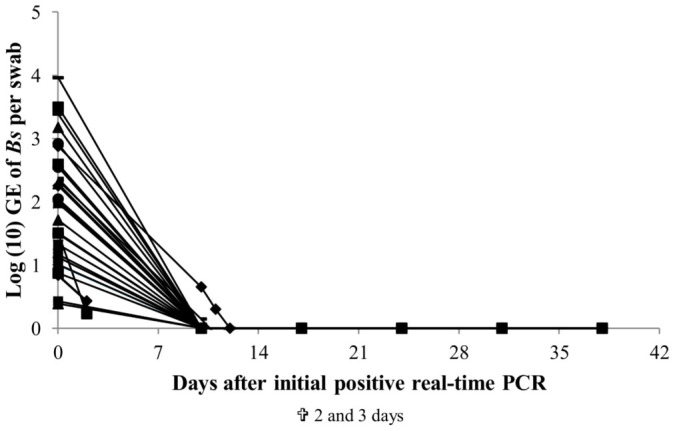
After ascertaining presence of B. salamandrivorans in all animals they were subjected to an ambient temperature of 25°C for 10 days. Each symbol represents the course of infection of an individual animal. Time of death for the 2 deceased animals is displayed beneath the graph.
In conclusion, these results demonstrate that infection and disease dynamics of B. salamandrivorans in urodelans are significantly dictated by environmental temperature. The inability of B. salamandrivorans to survive for more than 10 days at 25°C inside its host, renders temperature treatment of infected urodelans a safe, effective and low-cost treatment option, when taking into account the host thermal tolerance.
Methods
All experiments were performed in accordance with the relevant guidelines and regulations. All experiments with experimental animals were carried out with approval of the ethical committee of the Faculty of Veterinary Medicine, Ghent University.
Batrachochytrium salamandrivorans strain, culture conditions and experimental inoculation
The B. salamandrivorans type strain was grown in TGhL broth (16 g tryptone, 4 g gelatin hydrolysate, 2 g lactose per liter of distilled water) in 25 cm3 cell culture flasks and incubated at 15°C. To obtain B. salamandrivorans zoospores, a 2 ml aliquot of a 5-day-old culture was inoculated on TGhL agar plates (16 g tryptone, 4 g gelatin hydrolysate, 2 g lactose, 10 g bacteriological agar per liter of distilled water) and incubated at 15°C for 5–7 days. Zoospores were collected by flooding the agar plates with 2 ml of distilled water and subsequent collection of the fluid. A hemocytometer was used to count the number of zoospores present in the suspension and the concentration of the zoospore suspension was adjusted to 5 × 103 zoospores per mL. Animals were inoculated with B. salamandrivorans by topically applying one mL of the inoculum on the intact skin.
Animals
Experimental animals
Fire salamanders (Salamandra salamandra) were experimentally infected with B. salamandrivorans to study temperature dependent infection dynamics. The animal experiment was performed with the approval of the ethical committee of the Faculty of Veterinary Medicine (Ghent University, EC2013/87). Twenty-five captive bred fire salamanders were housed individually in plastic containers in a climatized room with an ambient temperature of 15°C. The animals were kept on a moist tissue, with access to a hiding place and water container. Crickets powdered with mineral and vitamin supplement were provided ad libitum as food source. All animals were clinically healthy and free of B. dendrobatidis and B. salamandrivorans as determined by duplex real-time PCR of skin swabs20. An acclimatization period of 1 week was admitted before the start of the experiment.
Field outbreak animals
Heat treatment to clear B. salamandrivorans infections in amphibians was validated on 30 wild fire salamanders found to be infected with B. salamandrivorans as determined by real-time PCR. These animals originated from a population in Robertville Belgium (50°29'58.6″N 6°06'21.9″E) undergoing a B. salamandrivorans outbreak event and were translocated to the research facility with permission (2014/RS/n°23). Housing conditions of these animals were identical to the conditions described for the experimental animals.
Temperature dependency of Batrachochytrium salamandrivorans infection dynamics in salamanders
The experimental animals were randomly assigned to one of the 5 groups (5 animals per group, kept individually). The purpose of the 5 groups was to assess whether B. salamandrivorans was able to colonize the animals at different temperatures (groups 1 to 3), and whether temperature could be applied to clear B. salamandrivorans from colonized animals (groups 4 and 5). In group 1, animals were inoculated and subsequently kept at 15°C, in group 2 kept at 20°C and in group 3 kept at 25°C (the animals kept at 20 and 25°C were placed in incubators set at the corresponding temperature). The animals in group 4 and 5 were inoculated at 15°C and put at 25°C for 7 or 10 days respectively, after B. salamandrivorans infection was established (determined as an increase in infection load between two subsequent samplings). To determine whether the infection would recrudesce after the 25°C exposure, salamanders of groups 4 and 5 were put back at 15°C afterwards and were followed up for another 3 weeks. In case of recrudescence of infection, the animals were put back at 25°C for 10 days. During the experiment, all animals were checked daily for the presence of clinical signs. Skin swabs for B. salamandrivorans real-time PCR analysis were collected once every 7 days and/or at the time of death of the animals. An animal was considered negative for B. salamandrivorans infection after 4 consecutive negative real-time PCR results. Real-time PCR's were performed on a CFX96 Real Time System (Biorad, Hercules, California, USA) with amplification conditions, primer, and probe concentrations as described elsewhere20. Infection loads are presented as genomic equivalents (GE) of B. salamandrivorans zoospores. Results were analyzed by means of independent t-test and Cox regression analysis using respectively the mass25 and survival library in R26. The censored response variable for the Cox regression analysis was time until death with temperature (15 or 20°C) as explanatory variable.
Thermal treatment of Batrachochytrium salamandrivorans infected salamanders
Based on the results of the thermal infection experiments, the B. salamandrivorans infected field outbreak animals were treated by putting them at 25°C for 10 days. Skin swabs for B. salamandrivorans real-time PCR analysis were collected after 10 days and subsequently every 7 days or at the time of death of the animals. Animals that remained positive after the heat treatment at 25°C during 10 days were kept at 25°C and swabbed daily to follow up remaining infection intensities until the first negative real-time PCR result and subsequently every 7 days. An animal was considered negative for B. salamandrivorans infection after 4 consecutive negative real-time PCR results. Real-time PCR's were performed as described above.
Footnotes
The authors declare no competing financial interests.
Author Contributions M.B., A.M. and F.P. designed the experiments. M.B. carried out the experiments. M.B., A.M., F.H., F.V., D.B. and F.P. contributed in writing and reviewing the manuscript.
References
- Longcore J. E., Pessier A. P. & Nichols D. K. Batrachochytrium dendrobatidis gen et sp nov, a chytrid pathogenic to amphibians. Mycologia 91, 219–227 (1999). [Google Scholar]
- Skerratt L. F. et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4, 125–134 (2007). [Google Scholar]
- Daszak P. et al. Emerging infectious diseases and amphibian population declines. Emerg. Infect. Dis. 5, 735–748 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake D. B. & Vredenburg V. T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. P. Natl. Acad. Sci. USA 105, 11466–11473 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. C. et al. Emerging fungal threats to animal, plant and ecosystem health. Nature 484 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzen-van der Sluijs A. et al. Environmental determinants of recent endemism of Batrachochytrium dendrobatidis infections in amphibian assemblages in the absence of disease outbreaks. Conserv. Biol. B28, 1302–1311 (2014). [DOI] [PubMed] [Google Scholar]
- Martel A. et al. Batrachochytrium salamandrivorans sp nov causes lethal chytridiomycosis in amphibians. P. Natl. Acad. Sci. USA 110, 15325–15329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasmans F. et al. Chytridiomycosis related mortality in a midwife toad (Alytes obstetricans) in Belgium. Vlaams Diergen. Tijds. 79, 460–462 (2010). [Google Scholar]
- Farrer R. A. et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. P. Natl. Acad. Sci. USA 108, 18732–187368 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner T. W. J. et al. Chytrid fungus in Europe. Emerg. Infect. Dis. 11, 1639–1641 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel A. et al. The Absence of zoonotic agents in invasive bullfrogs (Lithobates catesbeianus) in Belgium and The Netherlands. EcoHealth 10, 344–347 (2013). [DOI] [PubMed] [Google Scholar]
- Kriger K. M. & Hero J. M. Large-scale seasonal variation in the prevalence and severity of chytridiomycosis. J. Zool. 271, 352–359 (2007). [Google Scholar]
- Bosch J., Carrascal L. M., Duran L., Walker S. & Fisher M. C. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? P. Roy. Soc. B-Biol. Sci. 274 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger L. et al. Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust. Vet. J. 82, 434–439 (2004). [DOI] [PubMed] [Google Scholar]
- Martel A. et al. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346, 630–631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams D. C., Alford R. A. & Marantelli G. Emerging disease of amphibians cured by elevated body temperature. Dis. Aquat. Organ. 55, 65–67 (2003). [DOI] [PubMed] [Google Scholar]
- Chatfield M. W. H. & Richards-Zawacki C. L. Elevated temperature as a treatment for Batrachochytrium dendrobatidis infection in captive frogs. Dis. Aquat. Organ. 94, 235–238 (2011). [DOI] [PubMed] [Google Scholar]
- Geiger C. C., Kupfer E., Schar S., Wolf S. & Schmidt B. R. Elevated temperature clears chytrid fungus infections from tadpoles of the midwife toad, Alytes obstetricans. Amphibia-Reptilia 32, 276–280 (2011). [Google Scholar]
- Martel A. et al. Developing a safe antifungal treatment protocol to eliminate Batrachochytrium dendrobatidis from amphibians. Med. Myc. 49, 143–149 (2011). [DOI] [PubMed] [Google Scholar]
- Blooi M. et al. Duplex real-time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J. Clin. Microbiol. 51, 4173–4177 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vredenburg V. T., Knapp R. A., Tunstall T. S. & Briggs C. J. Dynamics of an emerging disease drive large-scale amphibian population extinctions. P. Natl. Acad. Sci. USA 107, 9689–9694 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney V. C., Heemeyer J. L., Pessier A. P. & Lannoo M. J. Seasonal Pattern of Batrachochytrium dendrobatidis infection and mortality in Lithobates areolatus: affirmation of Vredenburg's “10,000 zoospore rule”. Plos One 6, 1–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffel T. R. et al. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 3, 146–151 (2013). [Google Scholar]
- Piotrowski J. S., Annis S. L. & Longcore J. E. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96, 9–15 (2004). [PubMed] [Google Scholar]
- Venables W. N. & Ripley B. D. Modern applied statistics with S. 4 edn (Springer, New York, 2002). [Google Scholar]
- Therneau T. M. Package 'survival' CRAN repository available at: http://r-forge.r-project.org/ last accessed at the 10th of November 2014. [Google Scholar]