Abstract
In cryptococcal infection, phenotypic switching from a smooth to a mucoid variant can occur in vivo, producing variants with enhanced virulence that are subsequently selected and affect the outcome of infection. Here, we demonstrate that antifungal treatment of the chronically infected host can promote this phenomenon. Amphotericin B treatment reduces fungal burden less effectively in mucoid variant-infected than in smooth variant-infected mice. Consequently, amphotericin B treatment resulted in a more pronounced prolongation of survival in smooth variant-infected than in mucoid variant-infected mice (20 versus 42 days; P < 0.05). Administration of anticapsular monoclonal antibody mediated better protection in smooth variant-infected than in mucoid variant-infected mice, although a protective effect was not consistently observed at all doses. Most interestingly, both antifungal drug therapy and administration of anticapsular monoclonal antibody promoted the selection of mucoid variants in smooth variant-infected mice, a phenomenon manifested by a statistically higher percentage of mucoid colonies in smooth variant-infected mice than in nontreated control mice. This finding suggests that both chemotherapeutic and immunological antifungal interventions may promote the selection of the more virulent mucoid variant, which could affect the outcome of infection in chronically infected hosts.
Cryptococcus neoformans is an encapsulated yeast that causes disease primarily in patients with impaired immunity. Cryptococcosis is a common complication of end-stage human immunodeficiency virus (HIV) infection and can affect 2 to 30% of patients with advanced HIV infection (12, 33, 34). Clinically, C. neoformans infections manifest themselves as cases of chronic meningoencephalitis that have a high tendency to relapse after cessation of antifungal therapy (46). Treatment failures are generally not the result of acquired drug resistance but appear to represent persistence of the initial strain despite therapy (3, 4, 43). On the one hand, treatment failure may reflect the inability of an impaired immune system to eradicate the infection despite the assistance of antifungal therapy. On the other hand, the ability of C. neoformans to undergo microevolution during chronic infection could contribute to persistence of infection by challenging the already compromised host with variants that escape ongoing immune responses.
Microevolution produces phenotypic variability that can alter the virulence of the pathogen. Several observations support the concept that microevolution of C. neoformans occurs during the course of mammalian infection. First, prolonged in vitro and in vivo passage of C. neoformans isolates can result in phenotypic changes associated with differences in virulence, as demonstrated in murine infection models (2, 6, 8, 11, 17). Second, serial isolates from chronically infected patients can exhibit differences in virulence (18). Third, C. neoformans can switch in vitro reversibly to produce switch variants that differ in colony morphology and virulence (19, 24). We define and use the phrase “phenotypic switching” to describe the process by which colony variants arise in a C. neoformans population that can revert to their original appearance at a switching frequency that is greater than the background mutation rate. Similar phenomena have been also described for Candida species (1, 30, 52, 55, 56).
One advantage of C. neoformans as a model pathogen is that phenotypic switching occurs in vivo in a murine model of infection. More importantly, the occurrence of phenotypic switching in vivo was associated with an increased likelihood of lethal outcome for the host. The mucoid (MC) variant produces a different capsular polysaccharide, which renders it more resistant to phagocytosis by alveolar macrophages. In contrast, the smooth (SM) variant elicits an effective lymphocyte response whereas the MC variant fails to elicit a protective immune response and instead elicits a macrophage-dominated inflammatory response associated with extensive lung damage and rapid demise (20). The objective of this study was to investigate whether antifungal interventions in the form of drugs and specific antibodies altered the likelihood of recovering phenotypic switch variants in chronically infected mice. Our results indicate that both chemotherapeutic and immunological interventions can promote the emergence of MC variants in chronically SM variant-infected mice, an event that could influence the outcome of therapy and infection.
MATERIALS AND METHODS
C. neoformans strain.
Isolate RC-2 was previously described and is a variant of serotype D strain 24067 originally obtained from the American Tissue Type Collection (Rockville, Md.) (17, 20). The strain was streaked to single colonies and maintained on Sabouraud dextrose agar (SDA) plates (Difco, Detroit, Mich.). Isolate RC-2 produces two colony morphologies known as SM and MC, both of which are characteristic of C. neoformans colonies. C. neoformans cells were grown in Sabouraud dextrose broth at 30°C overnight with moderate shaking (150 rpm). Yeast cells were washed three times in phosphate-buffered saline (PBS), suspended in PBS, and counted using a hemocytometer.
Antifungal susceptibility testing.
Antifungal susceptibility assays were performed by the broth macrodilution method proposed by the National Committee for Clinical Laboratory Standards to determine the MICs of amphotericin B (AMB) (Boehringer Mannheim) and fluconazole (Flu) (Roerig-Pfizer, New York, N.Y.) (42). MIC determination was performed in RPMI 1640 medium (Sigma) supplemented with l-glutamine, without bicarbonate, and buffered to pH 7.0 with 0.165 M MOPS (morpholinepropanesulfonic acid) (Sigma) as well as in AM3 medium (Difco) with and without supplementation of 2% glucose as previously described (10). Inocula (103 or 105 cells/ml) in 0.9 ml of medium were added to polystyrene plastic tubes containing 0.1-ml aliquots of each drug at 10 times the final concentration. Final drug concentrations ranged from 0.03 to 256 μg/ml for Flu and from 0.03 to 2 μg/ml for AMB. For visual MIC determination, endpoints were detected as the lowest concentration of AMB or FLU that completely inhibited the growth of the strain. The MICs were recorded after incubation at 35°C for 72 h. For time-kill (TK) assays, yeast cells were suspended in PBS at a density of 2.2 × 103 cells/ml with increasing concentrations of antifungal drugs. After incubation at 35°C for 1 and 4 h, survival rates as measured by CFU were determined by plating on SDA. As an endpoint, the drug concentration was determined at which one 1-log reduction of CFU relative to the CFU of the untreated control was observed.
The monoclonal antibody (MAb) 2H1 was chosen for this study, because it has been used extensively in prior studies of antibody-mediated protection (37). The concentration of 2H1 MAb relative to isotype-matched standards of known concentrations was determined by enzyme-linked immunosorbent assay (ELISA). Immunofluorescence binding patterns of 2H1 MAb on SM and MC cells were determined as follows. Yeast cells from an overnight culture were incubated with MAb 2H1 at 10 μg/ml, and an isotype-specific, fluorescein isothiocyanate-conjugated secondary antibody was used for detection, as previously described (9). Glucuronoxylomannan (GXM) was isolated from the supernatant of SM and MC colonies and binding of MAb 2H1 to the isolated polysaccharide was tested by ELISA, as previously described (5, 7).
Oxidative burst assay.
Oxidative burst production by J774.16 macrophage-like cells was measured through oxidation of the cyclic hydrazide 5-amino-2,3-dihydro-1,4-phthalazinedione (luminol) by reactive oxygen species. Luminol-dependent chemiluminescence provides a convenient assay for phagocytic function as well as opsonization efficiency. The J774.16 cells were prepared for assaying by incubating cells with 0.5 mM EDTA-PBS for 30 min at 37°C. The detached cells were then collected by centrifugation and suspended at a density of 106 cells/ml in Hanks balanced salt solution containing 50 M luminol (sodium salt; Sigma-Aldrich). C. neoformans cells (5 × 106 cells) and MAb (10 μg/ml) were incubated for 1 h at room temperature. Cuvettes (BD Pharmingen) (12 by 75 mm) containing 1 ml of macrophage suspension were prewarmed at 37°C for 5 min, mixed with 100 μl of MAb-coated C. neoformans at an effector-to-target cell ratio of 1:5, and placed immediately in a luminometer (Moonlight 2010; Analytical Luminescence Laboratory, San Diego, Calif.). Chemiluminescence was measured for 2 h at 10-min intervals.
Animal studies.
BALB/c mice (male, 6 to 12 weeks of age) were obtained from the National Cancer Institute (Bethesda, Md.) and infected intratracheally (i.t.) for the majority of experiments. In selected experiments to test the efficacy of antibody, mice were infected intraperitoneally (i.p.). For i.t. infection, mice were anaesthetized and infected by i.t. inoculation of 104 or 106 C. neoformans cells in 50 μl of sterile nonpyrogenic PBS by use of a 26-gauge needle as described previously (26). The inoculum was confirmed by counting CFU on SDA plates. Mice were observed daily for signs of disease. Mice were killed by cervical dislocation after anesthesia, and organ fungal burden was determined by homogenizing lung and brain tissue in 10 ml of PBS and plating 100 μl of different dilutions of the homogenate on SDA plates. Colonies were counted after 72 to 96 h (1 colony = 1 CFU). The lower limit of detection is <log 2 when organ homogenates are plated undiluted.
For AMB treatment experiments, mice were infected i.t. with various doses of SM and MC cells. Treatment of mice with AMB was started 14 days after infection. Mice were treated with 0.5 mg of AMB dissolved in PBS per kg of body weight and injected for 10 days. Control mice were injected with PBS. Two separate experiments were performed, and the results were pooled. For MAb protection experiments, unpurified ascites with MAb 2H1 (0.5 to 1 mg) were given i.p. prior to infection in 0.5 ml of PBS. Control mice were treated with equivalent amounts of NSO ascites, which was made by injecting mice with the myeloma cell partner.
Determination of switching of the SM to the MC phenotype.
The occurrence of switching from the SM to the MC colony type was determined by visually scoring the colonies derived from homogenized organs after growth on SDA plates. For calculation of switching frequencies, 200 to 300 SM cells were plated on SDA plates, and approximately 104 colonies were scored. The percentage of colonies of the total CFU with MC morphology relative to those with SM morphology was determined. To determine whether a therapeutic intervention promoted the recovery of MC variants from an SM variant-infected mouse, we visually screened the colonies that were recovered from infected mice. A minimum of 1,000 colonies was screened, which allowed detection of 0.1% MC colonies. This number is still 20-fold above the background rate of in vitro switching, which is about 0.005% (20). One primary isolate from a patient with cryptococcosis was analyzed by directly plating dilutions of spinal fluid on SDA plates. These samples were analyzed before the patient had received antifungal therapy.
Statistical analysis.
Standard statistical analysis, including Kaplan-Meyer analysis, log rank regression analysis, t tests, and Kruskal-Wallis tests, were performed using the programs Primer of Biostatistics (version 3.01), SPSS (version 8.0), and Excel (Microsoft).
RESULTS
In vitro susceptibility of switch variants to antifungal drugs.
To determine the susceptibility of SM and MC switch variants to the commonly used antifungal agents Flu and AMB, we employed a growth-dependent standard test that determines the MIC of the antifungal and a growth-independent TK assay that determines the microbicidal drug concentration (41, 45, 47, 61). The MICs were comparable for SM and MC cells under various conditions; minor increased sensitivity to FLU was observed for both SM and MC cells in glucose-supplemented AM3 medium. We also determined the MIC of SM and MC isolates derived from mice after completion of antifungal therapy (day 28) and from MC isolates that had switched in vivo and were derived from SM variant-infected mice; we found no change in MIC for SM and MC cells, respectively (data not shown). The TK assay was done because the MC variant grows in vitro slightly more slowly than the SM variant (doubling time, 2.4 versus 2.7 h), which could affect MIC determination. For the TK assay, the concentrations of antifungals were comparable for SM and MC cells at 1 and 4 h. Both tests determined comparable levels of sensitivity to Flu and AMB for the SM and MC switch variants (Table 1). Of note is that the in vitro switching rates of the SM and MC phenotypes were not affected by exposure to antifungal drugs (data not shown).
TABLE 1.
MICs of the SM and MC switch variants
| Growth condition and antifungal treatment | SM MIC (μg/ml) | MC MIC (μg/ml) |
|---|---|---|
| Flu MIC AM3 103a | 2 | 2 |
| Flu MIC AM3 103 + glucose | 1 | 1 |
| Flu MIC AM3 105 | 4 | 4 |
| Flu MIC AM3 105 + glucose | 4 | 4 |
| AMB MIC AM3 103 | 0.25 | 0.25 |
| AMB MIC AM3 103 + glucose | 0.25 | 0.25 |
| AMB MIC AM3 105 | 0.25 | 0.125 |
| AMB MIC AM3 105 + glucose | 0.25 | 0.125 |
| Flu MIC RPMI 103 | 4 | 4 |
| Flu MIC RPMI 103 + glucose | 4 | 4 |
| Flu MIC RPMI 105 | 8 | 8 |
| Flu MIC RPMI 105 + glucose | 8 | 8 |
| AMB MIC RPMI 103 | 0.25 | 0.25 |
| AMB MIC RPMI 103 + glucose | 0.5 | 0.5 |
| AMB MIC RPMI 105 | 0.25 | 0.5 |
| AMB MIC RPMI 105 + glucose | 0.5 | 0.5 |
| 1 h TK assay 1 log reduction after Flub | NIc | NIc |
| 4 h TK assay 1 log reduction after Flub | 8 | 8 |
| 1 h TK assay 1 log reduction after AMB | 1.0 | 0.5 |
| 4 h TK assay 1 log reduction after AMB | 1.0 | 0.5 |
MIC of Flu determined in medium AM3 with a starting inoculum of 103 per ml.
Time-kill assay to determine the MIC of Flu that leads to a 1-log reduction of control CFU after 1 or 4 h of coincubation.
NI, no inhibition by Flu after 1 h at concentrations up to 256 μg/ml.
Oxidative burst in response to antibody-mediated phagocytosis.
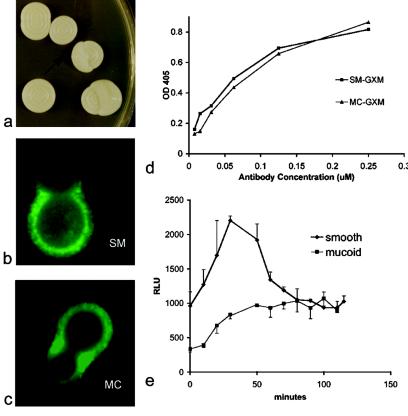
SM and MC cells derived from switching colonies (Fig. 1a) were further analyzed. Immunofluorescence revealed comparable results of binding by MAb 2H1 to the SM and MC polysaccharide capsules (Fig. 1b and c). Similarly, the results of MAb 2H1 binding to SM and MC GXM were comparable when measured by ELISA (Fig. 1d). Since antibody-mediated phagocytosis is associated with an oxidative burst in macrophages, we used chemiluminescence to measure the generation of oxygen radicals in J774 macrophage-like cells incubated with MAb 2H1-opsonized SM and MC cells. Under these conditions, oxidative burst production was more pronounced in macrophages exposed to SM cells than in those exposed to MC cells (Fig. 1e). Again, in vitro switching rates of the SM and MC phenotype were not affected by exposure to anticapsular antibody (data not shown).
FIG. 1.
(a) Switching SM colony with MC and SM colony morphology. (b and c) Immunofluorescence patterns of the SM and MC variants stained with the MAb 2H1 reveal demonstrates comparable binding patterns. (d) Results of binding of MAb 2H1 to SM and MC variant GXM were comparable by ELISA. OD, optical density. (e) Production of oxygen radicals in macrophages exposed to the opsonized MC variant was depressed compared to that seen with macrophages exposed to the opsonized SM variant. RLU, relative light units.
Efficacy of antifungal drugs in a murine infection model with SM and MC.
In vitro antifungal drug susceptibility can be a poor predictor of in vivo response, and therapeutic failure is well described for several pathogenic fungi (23, 31, 48). Therefore, we tested the efficacy of mice infected i.t. with 104 SM or MC cells. Mice (n = 4 per group in two separate experiments; data pooled) were treated at day 14 of infection, when the infection was fully established. At that time, SM variant- and MC variant-infected mice had comparable lung CFU results (log 7.0 versus 7.1; P = 0.6). Mice were treated with 10 i.p. doses of AMB (0.5 mg/kg of body weight). A decrease in the lung fungal burden relative to that in untreated mice was observed after completion of treatment. AMB treatment reduced lung fungal burden significantly more in SM variant-infected mice than in MC variant-infected mice (P = 0.004 by Kruskal-Wallis test). For SM variant-infected mice, the lung CFU decreased 100-fold (from log 6.1 ± 0.6 to log 4.1 ± 1.1) in treated mice and in two of eight mice the fungal infection was cleared from the lung. In contrast, lung CFU in MC variant-infected mice only decreased 10-fold (from log 6.49 ± 0.62 to log 5.3 ± 0.8) after AMB treatment. Similar trends were seen for brain fungal burden after AMB treatment. In SM variant-infected mice, the brain CFU was detectable in five of eight control mice and decreased after treatment from log 4.5 ± 0.7 to log 2.3 (and was undetectable in seven of eight mice). In MC variant-infected mice, brain CFU was detectable in five of eight control mice and decreased from log 4.6 ± 2.0 to log 4.1 ± 1.3 (and was undetectable in three of eight mice).
In this infection model, AMB treatment was started relatively late and did not eradicate the infection in either the SM variant- or MC variant-infected mice. Survival in mice infected with both SM and MC variants was nevertheless significantly prolonged by AMB treatment (P < 0.001; log rank). The results with respect to prolongation of survival in low-dose infection (104 cells administered i.t.) were comparable at 27 days for the SM variant (from a median of 74 to 101 days) and 32 days for the MC variant (from a median of 28 to 60 days). After high-dose (106 cells) infection, the prolongation in median survival resulting from therapy was significantly (P < 0.05) shorter in MC variant-infected mice than in SM variant-infected mice at 20 days versus 42 days (for MC variant infection from 30 to 50 days and for SM variant infection from 40 to 82 days). In summary, although in vitro MICs of AMB did not differ for the SM and MC variants, in vivo experiments demonstrated a more pronounced CFU reduction after AMB treatment in SM variant-infected than in MC variant-infected mice. Consistent with this finding, a significant increase in prolongation of survival by AMB treatment was also documented for SM variant-infected mice.
Effects of AMB treatment in the setting of phenotypic switching.
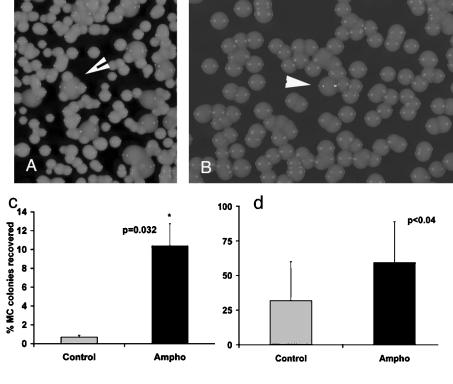
In mice chronically infected with the SM variant, phenotypic switching to the MC variant can occur in vivo (20). This can also be observed in AMB-treated mice. The MC switch variant is then selected and can be recovered from organ homogenates of SM variant-infected mice (Fig. 2A). In addition, careful examination of colony morphology in primary spinal fluid isolates of one patient revealed a heterogeneity of colony morphology. The majority of the colonies in that isolate were smooth, but a small fraction (0.3%) exhibited a MC colony phenotype. The two colony morphologies were similar to the SM and MC colony morphologies in ATCC strain 24067, which can undergo phenotypic switching (Fig. 2B). Hence, selection of MCs could also occur in human infection under the appropriate pressure (Fig. 2B). Therefore, we investigated in a separate i.t. infection experiment (dose, 5 × 105 cells administered i.t.) whether phenotypic switching of the SM variant and MC variant strains was affected by AMB treatment. Colonies recovered from MC variant-infected mice consistently exhibited a MC phenotype, a finding which implies that SM variants are not selected in the host, assuming that phenotypic switching from MC to SM occurred in vivo.
FIG. 2.
(A) Recovery of MC colonies from SM variant-infected mice treated with AMB (arrowhead, MC colony). (B) MC colonies were also detected in patient cerebrospinal fluid isolates that were plated prior to initiation of therapy (arrowhead, shiny MC colony). (c) Recovery of MC switch variants was significantly increased in mice infected i.t. with the SM variant that were treated with 10 doses of AMB (Ampho) compared to control mouse results (P = 0.032 by Kruskal-Wallis test; error bar reflects standard error) (n = 16 per group). (d) The percentages of MC colonies are shown for mice that were infected with a mixed inoculum of SM variant/MC variant at a ratio of 999:1 and then treated with AMB (P = 0.04 [t test]; error bar reflects standard deviation).
In contrast, analysis of colony phenotypes recovered from SM variant-infected mice (n = 16 per group; dose, 5 × 105 cells administered i.t.) revealed a higher percentage of MC colonies in AMB-treated mice than in control mice (Fig. 2c). Recovery of MC colonies in this infection model depends on the natural switching rate, which in vitro is determined to be approximately 1 switch in 20,000 colonies screened. We further investigated the hypothesis that MC was selected in vivo by performing infections with air inoculum that contained both SM and MC cells followed by antifungal therapy. We infected mice i.t. (n = 20 per group) with various ratios of SM and MC cells, specifically, 90:10, 99:1, and 999:1, and treated the mice at day 14 with 10 doses of AMB i.p. (0.5 mg/kg once a day). At the end of the treatment course, the mice were sacrificed and the percentage of MC colonies was determined and compared to that seen with nontreated mice. At a 90:10 ratio, we found that MC cells outgrew the SM cells by day 28 in both AMB-treated and control mice such that 90 to 100% of cells recovered were MC. With mice infected with SM variant/MC variant ratios of 99:1 and 999:1, we found a higher percentage of MC colonies in treated mice than in control mice for the ratio of 99:1 at 20.3% ± 17% for treated versus 12.05% ± 2.6% for control mice (difference not significant). For the 999:1 ratio, the percentages of MC were 59.3% ± 29% for AMB-treated mice versus 31.05% ± 28% for control mice (P = 0.04) (Fig. 2d). These results demonstrate that treatment with AMB can promote the selection of MC variants in mice that are infected with both the SM and MC variants.
Effects of MAb administration in the setting of phenotypic switching.
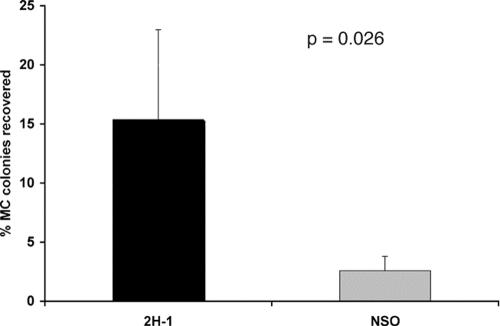
To determine whether phenotypic switching could interfere with MAb protection, we administered MAb to mice before infection with the SM and MC switch variants. Antibody-mediated protection was more evident in SM variant-infected than in MC variant-infected mice, especially in the i.p. infection model, although the protective effect was not seen at all doses (Table 2). We further investigated the impact of MAb administration on the recovery of the MC switch variant in mice infected with the SM variant by determining the percentages of MC colonies in MAb variant-treated compared to control-treated mice (NSO). A total of 140 mice were infected with 104 SM cells (n = 70 per group) and analyzed either at the time of death or at specific times after infection (the results from day 53 and day 80 were pooled). Quantitative analysis of CFU response after antibody administration in SM variant-infected mice demonstrated that fungal burden results at day 14 did not differ between MAb-treated mice and control mice (log 6.0 ± 0.5 for SM variant-infected mice with MAb treatment versus log 6.1 ± 0.3 for control mice). Recovery of MC colonies was inconsistent in this large experiment and was observed in only 50% of the mice. However, in mice where MC switch variants could be detected, a significantly (P = 0.026 by Kruskal-Wallis test) higher percentage of MC colonies were recovered in MAb-treated mice than in control mice (Fig. 3). The same trend was observed for all mice that were analyzed at the time of death (16.2% versus 9.6%; n = 30 and 28 per group), but the difference did not achieve statistical significance, possibly because of insufficient power. In summary, our results suggest that treatment with MAb 2H1 can also promote the selection of the MC switch variant antibody-treated mice infected with SM variant cells.
TABLE 2.
MAb treatment of mice infected with SM and MC switch variants
| Infection and treatment regimen | Median days of survivala
|
||||
|---|---|---|---|---|---|
| SM control | SM + MAb | MC control | MC + MAb | P valueb | |
| 104 cells i.t. + 1 mg of MAb | 99 | 129 | 32 | 48 | 0.0018 |
| 105 cells i.t. + 1 mg of MAb | 53 | 53 | 29 | 32 | No difference |
| 106 cells i.t. + 1 mg of MAb | 59 | 76 | 31 | 38 | 0.03 |
| 5 × 106 cells i.p. + 0.5 mg of MAb | 3 | 10 | 4 | 3 | 0.02 |
| 105 cells i.p. + 0.5 mg of MAb | 39 | 45 | 4 | 5 | No difference |
n = 10 mice per treatment group.
Log rank denotes difference in survival.
FIG. 3.
Recovery of MC switch variants was significantly increased in SM variant-infected mice (i.t.) treated with 1 mg of MAb 2H1 i.p. prior to infection (P = 0.026 [Kruskal-Wallis test]; error bar represents standard error).
Treatment may alter virulence by promoting selection of MC switch variants.
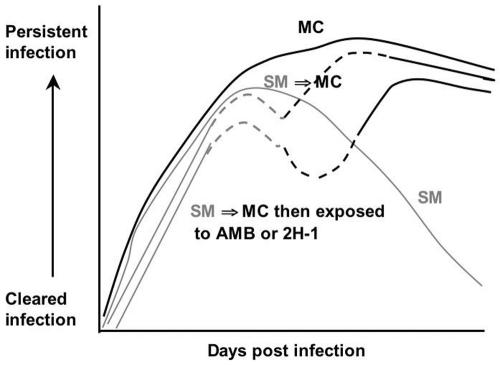
Figure 4 presents a model based on interpretation of the data whereby antifungal treatment can promote the selection of the MC switch variant in the setting of chronic infection with a C. neoformans strain that can undergo phenotypic switching. Our data indicate that under conditions of high fungal burden and/or protracted clearance of fungal infection, as often seen with an immunocompromised host, treatment may not eradicate the fungus sufficiently and through selection may eventually shift the pathogen population to a different variant, which could complicate treatment efforts.
FIG. 4.
Model of how antifungal therapy may affect the selection of phenotypic switch variants and the persistence of C. neoformans infection. In MC infection, fungal burden is never cleared because this variant fails to elicit a protective immune response. In SM infection, fungal burden rose initially but then the immune response effectively reduced the CFU. When SM to MC switching occurs, infection persists and the MC variant dominates the pathogen population. When SM variant-infected mice are treated and switching occurs, then treatment of the SM variant is more effective and hence promotes selection of the MC variant.
DISCUSSION
Failure of antifungal therapy occurs in up to 30% of cases of cryptococcosis in immunocompromised hosts (51). In a manner analogous to chronic viral infection (where microevolution leads to emergence of so-called “quasi species”), microevolution in pathogenic fungi may produce new variants with increased fitness in the host (22, 24, 25, 57). Most of the systematic research on the dynamics of microbial microevolution stems from the systematic analysis of HIV variants from chronically infected patients. In chronic viral infections, microevolution becomes more evident with the exposure of infected patients to treatment, which represents a selection pressure that promotes the emergence of more-fit variants such as drug-resistant clones (32, 58). It is, however, important to emphasize that antimicrobial resistance is not the only reason why infections persist and treatment fails (29). In that respect, the emergence of HIV variants with alterations in surface receptor structures can result in a shift of tropisms of the HIV viral population, leading to evasion of the host's immune response (27, 54).
It is often difficult to investigate whether microevolution in a microbial population contributes to therapeutic failure, because microevolution is inherently difficult to identify and quantify. Nevertheless, careful analysis of microbial populations can often detect significant diversification of the population in vivo as a result of microevolution (28). In contrast, the emergence of drug resistance is relatively easy to detect and microevolution of microbial populations yielding variants with differences in drug susceptibility has also been described for chronic bacterial, fungal, and parasitic infections (15, 39, 49, 58). In chronic cryptococcosis treatment, failure in immunocompromised patients is common but this outcome is not usually associated with the emergence of drug resistance (3, 4). The discovery that C. neoformans was capable of phenotypic switching resulting in emergence of variants with different virulence characteristics raised the possibility that its occurrence in vivo could contribute to persistent infections. To investigate this problem we used an animal model whereby a chronic infection can be achieved with low levels of inocula and the microevolution can be monitored by observation of plated organ cultures for differences in colony morphology. The baseline in vitro phenotypic switching rate of the RC-2 strain appeared to be very stable. Neither exposure to antifungal drugs nor to MAb 2H1 enhanced the switching rate in vitro. In contrast to results obtained with other microbial switching systems (57), we have not identified conditions that alter the rate at which new colony morphologies arise. Hence the emergence of MC variants in chronically infected mice treated with AMB or MAb 2H1 is more likely to reflect selection rather than a change of the actual switching rate. We have not identified differences in antifungal drug susceptibility or MAb binding between SM variant and MC variant strains that could explain the preferential selection of MC. The most plausible reason is that successful antifungal therapy is often only effective in conjunction with an effective immune response, which differs greatly for SM and MC variant infections (20, 40, 44).
Because chronic cryptococcosis is difficult to cure in severely immunocompromised patients, adjunctive therapeutic immunological intervention has been investigated over the past decade. Several investigators have demonstrated that administration of MAb to the polysaccharide capsule prior to infection can prolong survival (13, 14, 16, 35, 36, 53). The efficacy of antibody protection can be variable and is dependent on the cryptococcal strain, the inoculum dose, the antibody isotype, and the dose of the MAb (38, 59). Our data suggest that the efficacy of antibody protection may also differ for switch variants and that MAb treatment can promote selection of the MC switch variant in the setting of a switching strain. As phenotypic switching is known to change the polysaccharide capsule, this may also alter the optimal MAb dose to achieve protection (60). In MC variant infection the MC polysaccharide capsule inhibits MAb-mediated phagocytosis (20).
The results of this study now also demonstrate a reduced ability for MAb 2H1-opsonized MC cells to trigger a strong oxidative burst in macrophages, suggesting that this mechanism may reduce the ability of antibodies to protect against MC variant infection. We note that the protection against MC and SM variants observed with MAb 2H1 was more variable and less consistent than has been observed historically for the parent strain 24067. Given that antibody efficacy can depend on the genetic background of the strain (38), we attribute this finding to changes that accumulate in the RC-2 strain as a consequence of in vitro microevolution. Our findings underscore previous findings that the efficacy of antibody protection may differ for individual C. neoformans strains, raising the concern that certain strains can undergo phenotypic switching, change their polysaccharide capsules, and decrease the efficacy of MAb protection (38). This observation may have clinical implications given the ongoing efforts to develop vaccines and antibody-based therapies for cryptococcosis.
The MC switch variant spontaneously emerges in cryptococcal strain RC-2 in 2 × 104 colonies screened. The chance that a switch event can occur is dependent on the length of infection and fungal burden (20). Many patients that present with chronic cryptococcosis exhibit high fungal burden and are immunocompromised, with impaired immune systems that cannot efficiently assist antifungal therapy. Therefore, these patients often exhibit for a prolonged time high fungal burdens that promote the selection of switch variants with enhanced virulence which could ultimately result in treatment failure. Several studies have now shown that cryptococcal isolates can exhibit heterogeneous phenotypes with respect to colony morphology, polysaccharide structure, and cellular morphology (2, 8, 21, 50). These studies suggest that most clinical strains have the ability to vary their phenotypes. In addition, our analysis of primary isolates from patients indicates that different colony phenotypes can be observed in cerebrospinal fluid isolates even before the start of therapy.
In summary, the results of this study support the concept that treatment intervention in chronic cryptococcosis could select for new variants that escape the immune response and complicate therapy. We present evidence that this phenomenon can follow both chemotherapeutic and immunological antifungal interventions. We propose that fungal microevolution may contribute to treatment failure in patients with cryptococcosis. The rates at which an individual C. neoformans strain can undergo phenotypic switching may differ for the individual strains, and this may explain why some patients are more prone to fail therapy than others. Our data suggest that careful monitoring of pathogen phenotypes should be encouraged, especially if new regimens such as passive antibody treatment and vaccination are introduced.
Acknowledgments
We thank Aaron Mednick for technical help.
B.C.F. is supported by grant AI059681. A.C. is supported by grants AI033142, AI033774, and HL059842.
REFERENCES
- 1.Anderson, J. M., and D. R. Soll. 1987. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J. Bacteriol. 169:5579-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasi, E., A. Brozzetti, D. Francisci, R. Neglia, G. Cardinali, F. Bistoni, V. Vidotto, and F. Baldelli. 2001. Evidence of microevolution in a clinical case of recurrent Cryptococcus neoformans meningoencephalitis. Eur. J. Clin. Microbiol. Infect. Dis. 20:535-543. [DOI] [PubMed] [Google Scholar]
- 3.Brandt, M. E., M. A. Pfaller, R. A. Hajjeh, E. A. Graviss, J. Rees, E. D. Spitzer, R. W. Pinner, L. W. Mayer, and the Cryptococcal Disease Active Surveillance Group. 1996. Molecular subtypes and antifungal susceptibilities of serial Cryptococcus neoformans isolates in human immunodeficiency virus-associated cryptococcosis. J. Infect. Dis. 174:812-820. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, M. E., M. A. Pfaller, R. A. Hajjeh, R. J. Hamill, P. G. Pappas, A. L. Reingold, D. Rimland, and D. W. Warnock. 2001. Trends in antifungal drug susceptibility of Cryptococcus neoformans isolates in the United States: 1992 to 1994 and 1996 to 1998. Antimicrob. Agents Chemother. 45:3065-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall, A., J. Mukherjee, and M. D. Scharff. 1992. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J. Immunol. Methods 154:27-35. [DOI] [PubMed] [Google Scholar]
- 6.Chen, L. C., and A. Casadevall. 1999. Variants of a Cryptococcus neoformans strain elicit different inflammatory responses in mice. Clin. Diagn. Lab. Immunol. 6:266-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherniak, R., L. Morris, B. Anderson, and S. Meyer. 1991. Facilitated isolation purification and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect. Immun. 59:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherniak, R., L. C. Morris, T. Belay, E. D. Spitzer, and A. Casadevall. 1995. Variation in the structure of glucuronoxylomannan in isolates from patients with recurrent cryptococcal meningitis. Infect. Immun. 63:1899-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleare, W., R. Cherniak, and A. Casadevall. 1999. In vitro and in vivo stability of a Cryptococcus neoformans [corrected] glucuronoxylomannan epitope that elicits protective antibodies. Infect. Immun. 67:3096-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuenca-Estrella, M., T. M. Diaz-Guerra, E. Mellado, and J. L. Rodriguez-Tudela. 2001. Detection of resistance to amphotericin B in Candida isolates by using Iso-Sensitest broth. Antimicrob. Agents Chemother. 45:2070-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Currie, B., H. Sanati, A. S. Ibrahim, J. E. Edwards, Jr., A. Casadevall, and M. A. Ghannoum. 1995. Sterol compositions and susceptibilities to amphotericin B of environmental Cryptococcus neoformans isolates are changed by murine passage. Antimicrob. Agents Chemother. 39:1934-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Currie, B. P., and A. Casadevall. 1994. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin. Infect. Dis. 19:1029-1033. [DOI] [PubMed] [Google Scholar]
- 13.Dromer, F., and J. Charreire. 1991. Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: study during murine cryptococcosis and mechanisms of action. J. Infect. Dis. 163:1114-1120. [DOI] [PubMed] [Google Scholar]
- 14.Dromer, F., J. Charreire, A. Contrepois, C. Carbon, and P. Yeni. 1987. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55:749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feil, E. J. 2004. Small change: keeping pace with microevolution. Nat. Rev. Microbiol. 2:483-495. [DOI] [PubMed] [Google Scholar]
- 16.Feldmesser, M., and A. Casadevall. 1997. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J. Immunol. 158:790-799. [PubMed] [Google Scholar]
- 17.Franzot, S. P., J. Mukherjee, R. Cherniak, L. C. Chen, J. S. Hamdan, and A. Casadevall. 1998. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect. Immun. 66:89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fries, B., and A. Casadevall. 1998. Serial isolates of Cryptococcus neoformans from patients with AIDS differ in virulence for mice. J. Infect. Dis. 178:1761-1766. [DOI] [PubMed] [Google Scholar]
- 19.Fries, B. C., D. L. Goldman, R. Cherniak, R. Ju, and A. Casadevall. 1999. Phenotypic switching in Cryptococcus neoformans results in changes in cellular morphology and glucuronoxylomannan structure. Infect. Immun. 67:6076-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fries, B. C., C. P. Taborda, E. Serfass, and A. Casadevall. 2001. Phenotypic switching of Cryptococcus neoformans occurs in vivo and influences the outcome of infection. J. Clin. Investig. 108:1639-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Hermoso, D., F. Dromer, and G. Janbon. 2004. Cryptococcus neoformans capsule structure evolution in vitro and during murine infection. Infect. Immun. 72:3359-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gee, S. F., S. Joly, D. R. Soll, J. F. Meis, P. E. Verweij, I. Polacheck, D. J. Sullivan, and D. C. Coleman. 2002. Identification of four distinct genotypes of Candida dubliniensis and detection of microevolution in vitro and in vivo. J. Clin. Microbiol. 40:556-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghannoum, M. A. 1997. Susceptibility testing of fungi and correlation with clinical outcome. J. Chemother. 9(Suppl. 1):19-24. [PubMed] [Google Scholar]
- 24.Goldman, D. L., B. C. Fries, S. P. Franzot, L. Montella, and A. Casadevall. 1998. Phenotypic switching in the human pathogenic fungus Cryptococcus neoformans is associated with changes in virulence and pulmonary inflammatory response in rodents. Proc. Natl. Acad. Sci. USA 95:14967-14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellstein, J., H. Vawter-Hugart, P. Fotos, J. Schmid, and D. R. Soll. 1993. Genetic similarity and phenotypic diversity of commensal and pathogenic strains of Candida albicans isolated from the oral cavity. J. Clin. Microbiol. 31:3190-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huffnagle, G. B., M. F. Lipscomb, J. A. Lovchik, K. A. Hoag, and N. E. Street. 1994. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 55:35-42. [DOI] [PubMed] [Google Scholar]
- 27.Imamichi, H., K. A. Crandall, V. Natarajan, M. K. Jiang, R. L. Dewar, S. Berg, A. Gaddam, M. Bosche, J. A. Metcalf, R. T. Davey, Jr., and H. C. Lane. 2001. Human immunodeficiency virus type 1 quasi species that rebound after discontinuation of highly active antiretroviral therapy are similar to the viral quasi species present before initiation of therapy. J Infect. Dis. 183:36-50. [DOI] [PubMed] [Google Scholar]
- 28.Israel, D. A., N. Salama, U. Krishna, U. M. Rieger, J. C. Atherton, S. Falkow, and R. M. Peek, Jr. 2001. Helicobacter pylori genetic diversity within the gastric niche of a single human host. Proc. Natl. Acad. Sci. USA 98:14625-14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, W. E., and R. C. Desrosiers. 2002. Viral persistence: HIV's strategies of immune system evasion. Annu. Rev. Med. 53:499-518. [DOI] [PubMed] [Google Scholar]
- 30.Lachke, S. A., T. Srikantha, L. K. Tsai, K. Daniels, and D. R. Soll. 2000. Phenotypic switching in Candida glabrata involves phase-specific regulation of the metallothionein gene MT-II and the newly discovered hemolysin gene HLP. Infect. Immun. 68:884-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lortholary, O., M. Nicolas, S. Soreda, L. Improvisi, O. Ronin, O. Petitjean, B. Dupont, M. Tod, and F. Dromer. 1999. Fluconazole, with or without dexamethasone for experimental cryptococcosis: impact of treatment timing. J. Antimicrob. Chemother. 43:817-824. [DOI] [PubMed] [Google Scholar]
- 32.Mansky, L. M. 2002. HIV mutagenesis and the evolution of antiretroviral drug resistance. Drug Resist. Updates 5:219-223. [DOI] [PubMed] [Google Scholar]
- 33.Mirza, S. A., M. Phelan, D. Rimland, E. Graviss, R. Hamill, M. E. Brandt, T. Gardner, M. Sattah, G. P. de Leon, W. Baughman, and R. A. Hajjeh. 2003. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992-2000. Clin. Infect. Dis. 36:789-794. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell, T., and J. Perfect. 1995. Cryptococcosis in the Era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8:515-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee, J., A. Casadevall, and M. D. Scharff. 1993. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J. Exp. Med. 177:1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee, J., L. A. Pirofski, M. D. Scharff, and A. Casadevall. 1993. Antibody-mediated protection in mice with lethal intracerebral Cryptococcus neoformans infection. Proc. Natl. Acad. Sci. USA 90:3636-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1995. Variable efficacy of passive antibody administration against diverse Cryptococcus neoformans strains. Infect. Immun. 63:3353-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadler, S. A. 1995. Microevolution and the genetic structure of parasite populations. J. Parasitol. 81:395-403. [PubMed] [Google Scholar]
- 40.Perfect, J. R., B. Wong, Y. C. Chang, K. J. Kwon-Chung, and P. R. Williamson. 1998. Cryptococcus neoformans: virulence and host defences. Med. Mycol. 36:79-86. [PubMed] [Google Scholar]
- 41.Pfaller, M. A., M. Bale, B. Buschelman, M. Lancaster, A. Espinel-Ingroff, J. H. Rex, M. G. Rinaldi, C. R. Cooper, and M. R. McGinnis. 1995. Quality control guidelines for National Committee for Clinical Laboratory Standards recommended broth macrodilution testing of amphotericin B, fluconazole, and flucytosine. J. Clin. Microbiol. 33:1104-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaller, M. A., M. G. Rinaldi, J. N. Galgiani, M. S. Bartlett, B. A. Body, A. Espinel-Ingroff, R. A. Fromtling, G. S. Hall, C. E. Hughes, F. C. Odds, et al. 1990. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob. Agents Chemother 34:1648-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaller, M. A., J. Zhang, S. A. Messer, M. E. Brandt, R. A. Hajjeh, C. J. Jessup, M. Tumberland, E. K. Mbidde, and M. A. Ghannoum. 1999. In vitro activities of voriconazole, fluconazole, and itraconazole against 566 clinical isolates of Cryptococcus neoformans from the United States and Africa. Antimicrob. Agents Chemother. 43:169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pietrella, D., B. Fries, P. Lupo, F. Bistoni, A. Casadevall, and A. Vecchiarelli. 2003. Phenotypic switching of Cryptococcus neoformans can influence the outcome of the human immune response. Cell Microbiol. 5:513-522. [DOI] [PubMed] [Google Scholar]
- 45.Pietrella, D., T. R. Kozel, C. Monari, F. Bistoni, and A. Vecchiarelli. 2001. Interleukin-12 counterbalances the deleterious effect of human immunodeficiency virus type 1 envelope glycoprotein gp120 on the immune response to Cryptococcus neoformans. J. Infect. Dis. 183:51-58. [DOI] [PubMed] [Google Scholar]
- 46.Powderly, W. G. 2000. Current approach to the acute management of cryptococcal infections. J. Infect. 41:18-22. [DOI] [PubMed] [Google Scholar]
- 47.Ramage, G., K. VandeWalle, S. P. Bachmann, B. L. Wickes, and J. L. Lopez-Ribot. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rex, J. H., and M. A. Pfaller. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35:982-989. [DOI] [PubMed] [Google Scholar]
- 49.Rico-Hesse, R. 2003. Microevolution and virulence of dengue viruses. Adv. Virus Res. 59:315-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivera, J., M. Feldmesser, M. Cammer, and A. Casadevall. 1998. Organ-dependent variation of capsule thickness in Cryptococcus neoformans during experimental murine infection. Infect. Immun. 66:5027-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saag, M. S., R. J. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, W. E. Dismukes and the Infectious Diseases Society of America. 2000. Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 52.Samaranayake, Y. H., L. P. Samaranayake, J. Y. Yau, R. S. Dassanayake, T. K. Li, and S. Anil. 2003. Phenotypic diversity of oral C. albicans isolated on single and sequential visits in an HIV-infected Chinese cohort. APMIS 111:329-337. [DOI] [PubMed] [Google Scholar]
- 53.Sanford, J. E., D. M. Lupan, A. M. Schlageter, and T. R. Kozel. 1990. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect. Immun. 58:1919-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sankale, J. L., R. S. De La Tour, R. G. Marlink, R. Scheib, S. Mboup, M. E. Essex, and P. J. Kanki. 1996. Distinct quasi-species in the blood and the brain of an HIV-2-infected individual. Virology 226:418-423. [DOI] [PubMed] [Google Scholar]
- 55.Slutsky, B., J. Buffo, and D. R. Soll. 1985. High-frequency switching of colony morphology in Candida albicans. Science 230:666-669. [DOI] [PubMed] [Google Scholar]
- 56.Slutsky, B., M. Staebell, J. Anderson, L. Risen, M. Pfaller, and D. R. Soll. 1987. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J. Bacteriol. 169:189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soll, D. R., B. Morrow, and T. Srikantha. 1993. High-frequency phenotypic switching in Candida albicans. Trends Genet. 9:61-65. [DOI] [PubMed] [Google Scholar]
- 58.Spira, S., M. A. Wainberg, H. Loemba, D. Turner, and B. G. Brenner. 2003. Impact of clade diversity on HIV-1 virulence, antiretroviral drug sensitivity and drug resistance. J. Antimicrob. Chemother. 51:229-240. [DOI] [PubMed] [Google Scholar]
- 59.Taborda, C. P., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 60.Taborda, C. P., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 170:3621-3630. [DOI] [PubMed] [Google Scholar]
- 61.van Duin, D., A. Casadevall, and J. D. Nosanchuk. 2002. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 46:3394-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]