Abstract
Tigecycline, a novel glycylcycline antibiotic, exhibits strong activity against gram-positive, gram-negative, aerobic, anaerobic, and atypical bacterial species, including many resistant pathogens, i.e., vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumoniae. The safety and tolerability of tigecycline administered as single or multiple doses or at various infusion rates were explored in three phase 1, randomized, double-blind, placebo-controlled studies in healthy subjects. Full pharmacokinetic profiles of tigecycline were determined in two of these studies. Subjects in the single-dose study received 12.5 to 300 mg of tigecycline, which differed with respect to the duration of infusion, subjects' feeding status, and ondansetron pretreatment. Subjects in the ascending multiple-dose study received 25 to 100-mg doses of tigecycline as a 1-h infusion every 12 h. The variable volume and infusion rate study consisted of administration of 100-mg loading dose of tigecycline, followed by 50 mg every 12 h for 5 days. Serum samples were analyzed for tigecycline by validated high-pressure liquid chromatography or liquid chromatography/tandem mass spectrometry methods. Systemic clearance ranged from 0.2 to 0.3 liters/h/kg, and the tigecycline half-life ranged from 37 to 67 h. Tigecycline had a large volume of distribution (7 to 10 liters/kg), indicating extensive distribution into the tissues. Food increased the maximum tolerated single-dose from 100 to 200 mg, but the duration of infusion did not affect tolerability. Side effects, mainly nausea and vomiting, which are common to the tetracycline class of antimicrobial agents, were seen in these studies. Tigecycline exhibits linear pharmacokinetics and is safe and well tolerated in the dose ranges examined.
Tigecycline, a novel, first-in-class glycylcycline (3, 17, 22, 26), has shown in vitro activity against gram-positive, gram-negative, aerobic, anaerobic, and atypical bacterial species, including antibiotic-resistant strains. In studies with clinical isolates, tigecycline exhibits activity against tetracycline-resistant bacteria such as methicillin-susceptible Staphylococcus aureus, methicillin-resistant S. aureus, and glycopeptide-intermediate S. aureus. Penicillin-susceptible and -resistant Streptococcus pneumoniae (10) and vancomycin-resistant enterococci are also susceptible to tigecycline. In addition, tigecycline is active against most gram-negative pathogens, including Enterobacteriaceae, Acinetobacter spp., Stenotrophomonas maltophilia (1, 9, 13), Haemophilus influenzae, and Neisseria gonorrhoeae (5). Tigecycline's expanded broad-spectrum activity is further evidenced by its activity against Legionella pneumophila (6), Chlamydia (20), rapidly growing nontuberculosis mycobacteria (25), and anaerobes (18). A few reports on the pharmacokinetics of tigecycline in animals are documented in the literature. After administration of 14C-labeled tigecycline to rats, tigecycline tissue levels, with the highest concentrations in bone, liver, spleen, and kidney, exceeded those in plasma and persisted longer (23). In the same study, tigecycline exhibited a long terminal-phase disposition half-life (t1/2) of 36 h in plasma, 208 h in bone, 128 h in thyroid, and 77 h in kidney (23). Tigecycline mean serum t1/2 in a rabbit model of enterococcal endocarditis ranged from 3.3 to 3.6 h (11). Pharmacokinetic data obtained by using a murine thigh infection model in neutropenic mice receiving tigecycline doses in the range of 3 to 48 mg/kg demonstrated a dose-dependent t1/2 in the range 1.05 to 2.34 h, a peak concentration in serum (Cmax) in the range 0.42 to 11.1 μg/ml, and a serum protein binding of ca. 60% (24).
The ascending single-dose study in healthy subjects demonstrated that after ascending single doses of tigecycline up to 300 mg, the tigecycline Cmax and area under the serum concentration-time curve (AUC) increased in a dose-related manner. The pharmacokinetics of tigecycline after single ascending doses of 25 to 150 mg in Japanese men were similar to those seen in the study involving a predominantly Caucasian population (21). Nearly 15% of tigecycline was excreted in urine as unchanged drug.
In an open-label, single-dose study, in which tigecycline was administered to men and women belonging to various age groups, mean Cmax and AUC values, which were between 0.9 to 1.1 μg/ml and 4.2 to 5.5 μg h/ml, respectively, were similar across ages and sex (14). As seen in the animal studies, tigecycline was extensively distributed into the tissues (Vss was 5.6 to 6.1 liters/kg in women and 5.5 to 7.1 liters/kg in men).
Full studies on tigecycline clinical safety and pharmacokinetics in humans have not been presented to date. This report describes the safety, tolerability, and pharmacokinetics of tigecycline after administration of single and multiple ascending intravenous (i.v.) doses in healthy subjects. In addition, the safety and tolerability of tigecycline given by various volumes and infusion rates in healthy subjects are also documented.
(The ascending single-dose study was presented previously [G. Muralidharan, J. Getsy, P. Mayer, et al., poster at the 39th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, 26 to 29 September 1999].)
MATERIALS AND METHODS
Study design.
This report includes data from 3 phase 1 studies: an ascending single-dose (SAD), an ascending multiple-dose (MAD), and a variable volumes and infusion rate (VVIR) study, all of which were randomized, double-blind, single-center, and placebo-controlled studies. The primary objectives of the SAD and MAD trials were to assess the safety and tolerability of ascending i.v. doses of tigecycline given as single or multiple doses, respectively. Assessment of tigecycline pharmacokinetics data was the secondary objective of the SAD and the MAD studies. The primary objective of the VVIR study was to evaluate the safety and tolerability of various infusion rates of tigecycline. A few blood samples were taken during the infusion phase of the VVIR study in order to correlate the serum concentrations to any potential adverse events.
In the SAD trial, seven dose levels of tigecycline (12.5, 25, 50, 75, 100, 200, and 300 mg) were administered to healthy subjects in 11 dose groups (Table 1). Of the eight subjects in each group, six received tigecycline and two received a placebo. Subjects received tigecycline doses from 12.5 mg to 200 mg (groups 1 to 6) as a 1-h i.v. infusion in the fasting condition. Another cohort of subjects (group 7) received 200 mg of the test product as a 4-h i.v. infusion in the fasting condition. The 200-mg dose of tigecycline was also repeated in a different cohort (group 8) as a 1-h i.v. infusion after the subjects were given a standard medium-fat breakfast in order to compare the tolerability of tigecycline in the fed and fasting states (group 8 versus group 6, respectively). In two different groups of subjects, 300 mg of tigecycline was administered after breakfast as a 1-h (group 9) and a 4-h (group 10) i.v. infusion. Subjects in group 11 received ondansetron (32 mg administered i.v. over 30 min) before 200 mg of tigecycline was infused i.v. over 1 h in the fasting condition.
TABLE 1.
Tigecycline regimen description
| Study | Cohort | No. active/no. placebo | Dosage regimen | Infusion duration (h) | Infusion vol (ml) | Fasted or fed | Antiemetic medication |
|---|---|---|---|---|---|---|---|
| SAD | 1 | 6/2 | 12.5 mg | 1 | 250 | Fasted | None |
| 2 | 6/2 | 25 mg | 1 | 250 | Fasted | None | |
| 3 | 6/2 | 50 mg | 1 | 250 | Fasted | None | |
| 4 | 6/2 | 75 mg | 1 | 250 | Fasted | None | |
| 5 | 6/2 | 100 mg | 1 | 250 | Fasted | None | |
| 6 | 6/2 | 200 mg | 1 | 250 | Fasted | None | |
| 7 | 6/2 | 200 mg | 4 | 250 | Fasted | None | |
| 8 | 6/2 | 200 mg | 1 | 250 | Fed | None | |
| 9 | 6/2 | 300 mg | 1 | 250 | Fed | None | |
| 10 | 6/2 | 300 mg | 4 | 250 | Fed | None | |
| 11 | 6/2 | 200 mg | 1 | 250 | Fasted | Ondansetron | |
| MAD | 1 | 6/2 | 25 mg q12h | 1 | 250 | Fed | None |
| 2 | 6/2 | 50 mg q12h | 1 | 250 | Fed | None | |
| 3 | 6/2 | 100 mg q12h | 1 | 250 | Fed | None | |
| 4 | 6/2 | 75 mg q12h | 1 | 250 | Fed | None | |
| VVIR | 1 | 6/3 | 100 mg + 50 mg q12h | 1 | 100 | Fed | None |
| 2 | 7/3 | 100 mg + 50 mg q12h | 0.5 | 100 | Fed | None | |
| 3 | 6/3 | 100 mg + 50 mg q12h | 1 | 250 | Fed | None |
In the MAD study, subjects received 25, 50, or 100 mg of tigecycline as 60-min i.v. infusions every 12 h (q12h) for 9 consecutive days, followed by a single dose on the morning of day 10 (total of 19 doses). A fourth cohort of subjects receiving tigecycline 75 mg q12h was enrolled, but this cohort discontinued on day 5 due to poor tolerability. In each cohort, eight subjects were randomly assigned to each dose group; six subjects received tigecycline, and two subjects received placebo. Because of the tolerability issues (increased frequency of gastrointestinal [GI] adverse events) with tigecycline at the 100-mg q12h dose level, subjects in this group received tigecycline for only 8 consecutive days, followed by a single dose on the morning of day 9 (total of 17 doses).
Subjects in the VVIR study received a 100-mg loading dose, followed by nine doses of 50 mg of tigecycline (six or seven subjects) or placebo (three subjects) q12h over 5 days. The durations and rates of the i.v. infusions for administration of the dose were 60 min at 100 ml/h, 30 min at 200 ml/h, and 60 min at 250 ml/h, respectively, to separate cohorts of subjects. Because of the hypothesized relationship between serotonin release and nausea observed after tigecycline administration, urinary excretion of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) was measured in all subjects (4). Subject diets on days 1 through 5 were modified so that foods containing serotonin precursors were limited.
For all three studies, tigecycline lyophilized powder was supplied in 5-ml open-label vials containing 100 mg of drug. Normal saline (0.9% NaCl in water) was used as the placebo.
Subjects.
Healthy men aged 18 to 50 years were recruited in all three studies. No clinically abnormal findings on medical history, physical examination, 12-lead electrocardiogram (ECG), or clinical laboratory evaluation were allowed. Subjects who had not taken any over-the-counter, investigational, or prescription drug 14 days before the study or did not have any condition known to interfere with the absorption, distribution, metabolism, or excretion of drugs were included in the study. Subjects who tested positive for human immunodeficiency virus antibodies, hepatitis B surface antigen, and/or hepatitis C antibodies or who had a history or presence of any allergic condition or any major organ or systemic disease were excluded from the study.
All subjects gave written informed consent, and all studies were performed in accordance with the Declaration of Helsinki and its amendments and in accordance with local laws and guidelines. Each of the study protocols was reviewed and approved by an institutional review board or independent ethics committee.
Bioanalytical methods.
In the SAD and MAD studies, tigecycline was assayed in serum by a high-performance liquid chromatography method with calibrators in the range of 25 to 12,500 ng/ml. Briefly, the method consisted of protein precipitation of the serum samples by addition of acetonitrile, separation of tigecycline on a Supelco LC-18 DB column (150 by 4.6 mm [internal diameter], 3-μm particle size) and subsequent UV detection of tigecycline at 350 nm. The extraction efficiency was higher than 76%, and the lower limit of quantitation was 25 ng/ml. The intrabatch and interbatch coefficients of variation (CV) were <13% at all concentrations.
Tigecycline in urine was quantified in the SAD study by a high-pressure liquid chromatography method with UV detection (350 nm) with a lower limit of detection of 2 μg/ml. Briefly, the urine samples were diluted to 1:10, and tigecycline was chromatographed on a C18 column (150 by 4.6 mm [inner diameter], 3-μm particle size). The intraday CV and bias for the low quality control (QC) samples (6 μg/ml) were 4.6 and −1.5%, respectively. The interday CV and bias values at 6 μg/ml were 7.0 and −1.5%, respectively. The interday (n = 25) precision (CV) values for the middle QC (12 and 36 μg/ml) and the high QC (72 μg/ml) samples were between 5.1 and 9.0%, and the interday bias ranged from −7.9 to −0.6%. The precision (CV) and accuracy (expressed as bias) of tigecycline calibration standards in the lower-tier curve were between 1 and 3.5% and between −2 and 2.3%, respectively, and in the upper-tier curve were between 0.9 and 3.0% and between −5.5 and 3.3%, respectively.
In the VVIR study, tigecycline concentrations in serum were quantified by using a validated methodology that used the API 3000 LC/MS/MS system. The lower limit of quantitation was 10 ng/ml, and the upper limit of quantitation was 2,000 ng/ml. QC samples of tigecycline prepared in human serum at concentrations of 1,500 ng/ml (high), 200 ng/ml (medium), and 25 ng/ml (low) were analyzed, along with the subject samples. The overall precision and accuracy for the standards and the QC samples were in the range of 0.9 to 12% and 93 to 110%, respectively.
Pharmacokinetic methods.
Pharmacokinetic parameters based on serum data for tigecycline were estimated by noncompartmental methods with a validated SAS (versions 6.12 and 8.02) program. The AUC from 0 h to the last quantifiable concentration was estimated by using the linear-trapezoidal rule for increasing concentrations and log-trapezoidal rule for decreasing concentrations. The trough serum concentrations of tigecycline (Cmin) and Cmax were obtained directly from observed data. Systemic tigecycline clearance (CL) was normalized to body weight by using the dose/AUC/body weight. The apparent volume of distribution at steady-state (Vss) was calculated based on the following formula: CL × [(AUMC/AUC) − T/2], where AUMC is the area under the first moment curve and T is the duration of the i.v. infusion. The apparent terminal-phase disposition rate constant (λz) was estimated by regression of the terminal log-linear concentration time points. The apparent t1/2 was estimated as the ln(2)/λz. In the SAD study, the percentage of tigecycline excreted unchanged in urine (fe [%]), the amount of tigecycline excreted in urine over 48 h (Ae0-48), and renal clearance (CLR = Ae0-48/AUC0-48) were also determined.
Statistical methods.
Arithmetic means, standard deviations, and CVs were determined for all pharmacokinetic parameters by using the SAS software. The use of the word “significant” refers to statistically significant results at the α level of 0.05. All tests of hypotheses were two sided.
In the SAD study, potential differences among dose groups in concentrations in plasma and pharmacokinetic parameters of tigecycline were assessed by using a one-factor analysis of variance (ANOVA) after normalization of the dose-dependent pharmacokinetic parameters (e.g., Cmax and AUC) to the 100-mg dose. The pairwise comparisons among dose groups were made by using the Tukey multiple comparison test.
In the MAD study, tigecycline concentrations in serum and pharmacokinetic parameters on days 1 and 10 were compared by using a one-factor ANOVA and the Tukey multiple comparison test. All Cmax and AUC values were normalized to the 100-mg dose before ANOVA was performed. In addition, the dose-normalized serum trough concentrations of tigecycline were compared across dose groups by using ANOVA.
Safety.
In all three studies, safety was evaluated on the basis of results from scheduled physical examinations, vital sign measurements, ECG, and clinical laboratory evaluations (hematology, blood chemistry, and urinalysis). Adverse events were recorded throughout the study. In the VVIR study, excretion of 5-HIAA and creatinine in urine was determined.
RESULTS
Subjects.
Healthy subjects aged 18 to 44 years participated in the SAD study. Body weights ranged from 51 to 95 kg. Subjects with similar demographics completed the MAD and VVIR studies. Altogether, 66 subjects in the SAD study were analyzed for pharmacokinetic characteristics of tigecycline. In the SAD study, two subjects were excluded from the pharmacokinetic analysis due to improper dosing (injection site reaction in one subject and infusion pump malfunction in another subject), but these subjects were included in the safety evaluation.
Safety.
The maximum tolerated single dose for fasting subjects was 100 mg. The 200-mg dose was not tolerated by fasting subjects but was well tolerated by fed subjects. Ondansetron appeared to partially improve the tolerability of tigecycline for fasting subjects. GI adverse events were dose limiting at 300 mg, and dose escalation was halted at this dose. Overall, the most common adverse events were nausea (33 of 68 tigecycline recipients [48.5%]) and vomiting (20 of 68 tigecycline recipients [29.4%]), and both of these adverse events tended to increase in frequency with increasing dose (e.g., for fasting administration of 1-h infusions, zero of six subjects experienced nausea at 12.5 mg compared to three of six subjects at 100 mg and five of six subjects at 200 mg). In addition, there were no clinically relevant changes in clinical laboratory parameters, blood pressure, or ECG intervals.
In the MAD study, the dose administration schedule was well tolerated by subjects in the tigecycline 25- and 50-mg dose groups, although GI intolerance occurred in all six tigecycline-treated subjects in the 100-mg dose group, and treatment in that dose group was discontinued after 9 days (total of 17 doses). After the 100-mg dose group completed, an additional 75-mg dose group was enrolled, but this group was discontinued after only 5 days (total of nine doses) due to GI intolerance. Twelve (12) subjects withdrew from the study: one subject (25-mg dose group) developed a rash after receiving 12 doses of tigecycline; ten subjects (one in the 50-mg dose group, six in the 75-mg dose group, and three in the 100-mg dose group) discontinued because of nausea and/or vomiting; and one patient receiving placebo discontinued because of thrombophlebitis. The incidence of nausea increased with increasing dose (four of six subjects, five of six subjects, six of six subjects, and six of six subjects for 25, 50, 75, and 100 mg q12h, respectively). In addition, there were no clinically relevant changes in clinical laboratory parameters, blood pressure, or ECG intervals.
In the VVIR study, the incidence of adverse events did not vary greatly among the treatment groups and the incidence of local irritations, specifically injection site phlebitis, was similar in placebo-treated and tigecycline-treated subjects. Six subjects withdrew from the study because of adverse events: three subjects in the 100-ml/60-min group with reasons that included the presence of gonorrhea before enrollment in the study, as well as diarrhea or vomiting; one subject each in the 100-ml/30-min and 250-ml/60-min groups because of vomiting; and one subject who received placebo in the 250-ml/60-min group because of pruritus and urticaria. Among subjects treated with tigecycline, the incidence of nausea and vomiting was lowest in the 100-ml/30-min treatment group. Subjects given tigecycline at 250 ml/60 min had fewer infusion-related adverse events. However, the incidence of other adverse events, such as nausea and vomiting, was similar to that of the other treatment groups, suggesting that there is little benefit to infusing the drug more slowly and in a larger volume. No deaths and no serious adverse events occurred in any of the three studies.
Tigecycline is most likely excreted in bile, and it is hypothesized that tigecycline may irritate the GI tract, releasing serotonin, which then causes nausea (4). The serotonin metabolite 5-HIAA is commonly measured in urine as a marker of serotonin release. In the VVIR study, there was no definite relationship between the occurrence of nausea and the excretion of 5-HIAA in the urine of subjects (data not shown), suggesting that the nausea and vomiting associated with tigecycline administration are not associated with increased serotonin production.
Concomitant medications.
The most commonly administered concomitant medications were given for nausea and vomiting in all three studies. In the SAD study, the most commonly administered concomitant medication was the propulsive agent, metoclopramide used to treat nausea and vomiting in groups 6 through 11. No adverse events were attributed to ondansetron (32 mg) administration. In the MAD study, concomitant medications were not permitted during the study with the exception of antiemetics such as prochlorperazine, trimethobenzamide, or ondansetron. In the VVIR study, the most frequently used concomitant medications were sucralfate and prochlorperazine edisylate—both given for nausea and vomiting.
Pharmacokinetics. (i) SAD Trial.
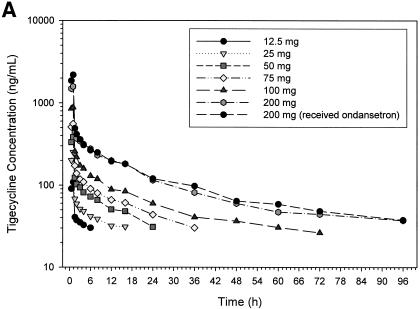
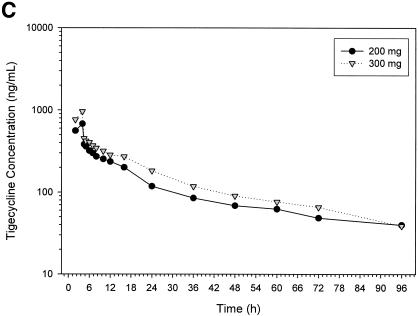
The mean concentration in serum versus time profiles at each of the seven-dose levels used in the study are shown in Fig. 1A. No appreciable differences were seen in tigecycline concentrations in serum after a 200-mg dose, regardless of whether tigecycline was given with or without food (see Fig. 1B). In addition, the concentrations of tigecycline (200 mg given as a 1-h infusion) in serum were not affected by pretreatment with ondansetron (Fig. 1B). Except for the anticipated delay in tmax and the reduction in Cmax values due to the slower rate of infusion, the mean pharmacokinetic profiles of subjects receiving 200 and 300 mg as a 4-h infusion (Fig. 1C) were not markedly different from those receiving identical doses as a 1-h infusion (Fig. 1A).
FIG. 1.
Mean tigecycline concentrations in serum in the single ascending dose study in fasting subjects as 1-h infusions (A), as 1-h infusions in the 200-mg dose group (B), and as 4-h infusions (C).
Mean tigecycline Cmax after a 1-h infusion ranged from ca. 109 ng/ml after a single dose of 12.5 mg to a 2,817 ng/ml after a dose of 300 mg (Table 2). As expected, mean serum Cmax values in the 200-mg (680 ng/ml) and 300-mg (960 ng/ml) dose groups, where the length of the infusion was 4 h, were lower than the values seen after a 1-h infusion of similar doses. Mean values for Cmax obtained in the fed (1,528 ng/ml) and fasting (1,643 ng/ml) groups receiving 200-mg doses of tigecycline were not markedly different. Although statistically not significant, the mean Cmax values observed in the 200-mg dose group, in which subjects were pretreated with ondansetron, was slightly higher (2,189 ng/ml) than in the other 200-mg dose groups. Differences in the dose-normalized values for Cmax were significant (P < 0.05) when examined across all dose groups. However, such differences in dose-normalized Cmax values did not exist within the dose groups receiving 1-h infusions or between the two dose groups receiving 4-h tigecycline infusions.
TABLE 2.
Mean pharmacokinetic parameters (%CV) of tigecycline after various single i.v. doses in SAD study
| Parameter | Mean valuea (% CV) after:
|
P | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-h infusion (group no., tigecycline [mg], fasting or fed)
|
4-h infusion (group no., tigecycline [mg], fasting or fed)
|
|||||||||||
| 1, 12.5 fasting | 2, 25, fasting | 3, 50, fasting | 4, 75, fasting | 5, 100, fasting | 6, 200, fasting | 8, 200, fed | 11, 200, fastingb | 9, 300, fed | 7, 200, fasting | 10, 300, fed | ||
| Cmax (ng/ml) | 108.5 (10) | 252 (25) | 383 (17) | 566 (14) | 911 (29) | 1,643 (18) | 1,528 (22) | 2,189 (30) | 2,817 (17) | 680 (22) | 960 (10) | 0.001 |
| AUC (ng h/ml) | 753 (68) | 2,255 (45) | 2,558 (21) | 3,658 (27) | 6,396 (10) | 12,426 (23) | 11,719 (19) | 14,462 (17) | 17,856 (10) | 14,237 (22) | 16,732 (16) | 0.06 |
| CL (l/h/kg) | 0.29 (67) | 0.20 (50) | 0.28 (14) | 0.29 (16) | 0.20 (13) | 0.25 (22) | 0.22 (12) | 0.20 (19) | 0.25 (11) | 0.24 (11) | 0.26 (13) | 0.27 |
| Vss (l/kg) | 2.8* (34) | 6.4* (20) | 6.4* (31) | 7.5* (10) | 8.6 (18) | 11 (25) | 13 (18) | 12 (43) | 12 (20) | 14 (11) | 12 (24) | 0.001 |
| t1/2 (h) | 11* (84) | 32* (64) | 18* (21) | 21* (25) | 38 (14) | 42 (28) | 54 (28) | 53 (20) | 46 (13) | 58 (14) | 42 (23) | 0.001 |
| CLR (l/h) | ND | ND | ND | ND | 2.6 (26) | 3 (50) | 2.2 (22) | 1.8 (24) | 2.7 (23) | NA | 2 (28) | 0.13 |
| fe (%) | 2.1 (113) | 6.5 (39) | 1.9 (72) | 8 (37) | 11 (25) | 12 (31) | 7.8 (25) | 8.5 (38) | 10.4 (22) | NA | 8 (39) | 0.001 |
ND, could not be determined accurately because of low concentrations in urine and serum; NA, not available. Values marked with an asterisk may not be reliably estimated due to the limit of quantification.
Received ondansetron.
Similarly, the mean AUC ranged from 753 ng h/ml after a 12.5-mg dose to 17,856 ng h/ml after a 300-mg dose, when the doses were given as a 1-h infusion. Dose-normalized AUCs were not significantly different among dose groups (P > 0.05, Table 2).
Mean CL was not significantly different among dose levels (P > 0.2, Table 2) and ranged from 0.2 to 0.3 liter/h/kg across dose groups. Because of the limitations in sensitivity of the assay that was used to quantify tigecycline, the t1/2 of tigecycline could be characterized accurately only after single doses of 100 mg or higher. The mean t1/2 was ca. 40 to 60 h in these groups. Tigecycline was well distributed into various tissues as shown by the mean Vss, in excess of 8 liter/kg, at all dose levels at which the t1/2 could be accurately characterized (≥100 mg, Table 2). Less than 13% of tigecycline was excreted in urine as unchanged drug. In addition, no significant differences in tigecycline pharmacokinetics were seen between subjects who received tigecycline in the fasting state and those who were given tigecycline in the fed state.
(ii) MAD trial.
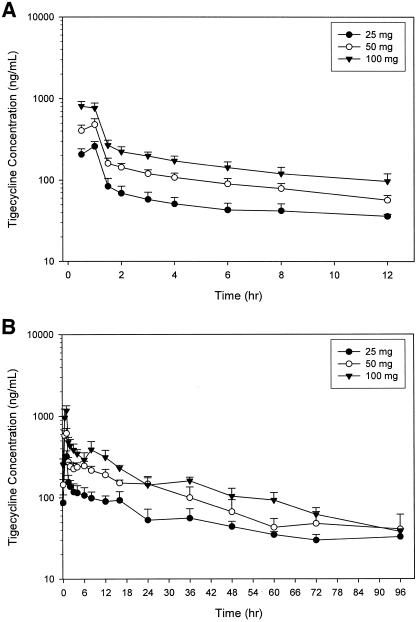
Pharmacokinetics determined in the MAD study are presented in Table 3 and concentration levels in serum on days 1 and 10 are presented in Fig. 2A and 2B, respectively. The mean AUC0-τ values on day 1 ranged from 796 ng h/ml in the tigecycline 25-mg dose group to 2,389 ng h/ml in the tigecycline 100-mg dose group. None of the steady-state pharmacokinetic parameter values (obtained on day 10) were significantly different among dose groups (P > 0.05). In all dose groups, tigecycline had a mean CL value of 0.2 liter/h/kg. Mean t1/2 of tigecycline obtained on day 10 ranged from 36.9 h in the 50-mg dose group to 66.5 h in the 100-mg dose group. High intersubject variability (CV > 30%) was seen in the t1/2 of tigecycline in each dose group examined.
TABLE 3.
Mean pharmacokinetic parameters of tigecycline after various i.v. doses infused over a 1-h period q12h in the MAD study
| Tigecycline dose group (mg) | Mean Value (%CV) at:
|
||||||
|---|---|---|---|---|---|---|---|
| Day 1
|
Day 10a
|
||||||
| Cmax (ng/ml) | AUC0-τ (ng h/ml) | Cmax (ng/ml) | AUCss (ng h/ml) | t1/2 (h) | CL (l/h/kg) | Vss (l/kg) | |
| 25 | 261 (14) | 796 (8) | 324 (17) | 1,482 (18) | 49.3 (72) | 0.20 (17) | 8.6 (23) |
| 50 | 487 (17) | 1,440 (14) | 621 (15) | 3,069 (12) | 36.9 (32) | 0.20 (9) | 7.2 (7) |
| 100 | 816 (15) | 2,389 (13) | 1,173 (15) | 4,980 (19) | 66.5 (34) | 0.24 (20) | 9.1 (32) |
Day 10 values for the 100-mg tigecycline dose group are actually day 9 values.
FIG. 2.
Tigecycline concentrations (mean plus the standard deviation) serum in multiple ascending dose study on day 1 (A) and day 10 (B).
Trough concentrations of tigecycline in serum were compared across days 7, 8, 9, and 10 for the 25-, 50-, and 100-mg q12h dose groups (Table 4). The differences among days were not significant (P > 0.05) for the 25- and 100-mg dose groups. In the 50-mg dose group, the trough tigecycline concentrations obtained on days 8 and 9 were higher (P < 0.05) than those obtained on days 7 and 10, but the day 7 trough concentrations in this group were not significantly different from those obtained on day 10 (P > 0.05). In addition, differences in trough tigecycline concentrations in serum (normalized to 100-mg dose) among dose groups on each of the days 7, 8, 9, and 10 were not significant (P > 0.05, Table 4).
TABLE 4.
Trough concentrations of tigecycline among various dose groups after multiple doses of tigecycline given as a 1-h infusion in the MAD study
| Day | Mean (% cv) concn (ng/ml)a at:
|
P | ||
|---|---|---|---|---|
| 25 mg | 50 mg | 100 mg | ||
| 7 | 86 (28) | 153 (13) | 214 (13) | 0.07 |
| 8 | 81 (34) | 195 (15) | 255 (16) | 0.12 |
| 9 | 83 (28) | 204 (17) | 257 (17) | 0.06 |
| 10 | 87 (26) | 145 (16) | NA | 0.23 |
For comparison among dose groups, concentrations were dose normalized to the 100-mg dose before statistical analysis. For the 25-, 50-, and 100-mg dose groups, the P values were 0.98, 0.01, and 0.37, respectively. NA, not available.
(iii) VVIR trial.
Only limited pharmacokinetic data were collected in the VVIR trial. The mean tigecycline single-dose Cmax (%CV) was 642 ng/ml (24%) for the 100-ml/60-min regimen, 668 ng/ml (3%) for the 250-ml/60-min regimen, and 969 ng/ml (15%) for the 100-ml/30-min regimen. Thus, as expected, the tigecycline Cmax was higher for the 30-min infusion than for the 60-min infusion regimens, and the infusion volume did not affect the mean Cmax value.
Dose proportionality.
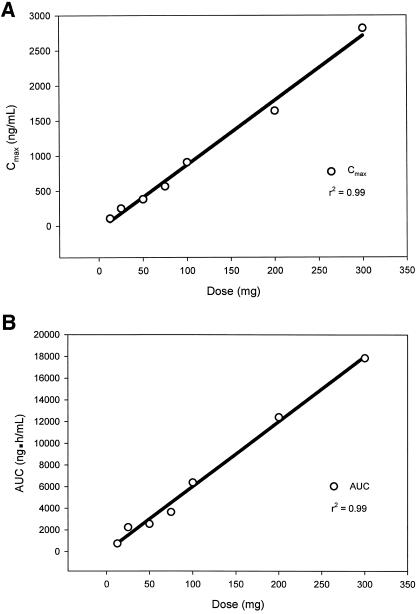
In the SAD study tigecycline Cmax and AUC increased proportionally to the dose in the range of 12.5 to 300 mg. In fact, a linear relationship was seen between Cmax and dose (r2 = 0.99, Fig. 3A) and also between AUC and dose (r2 = 0.99, Fig. 3B).
FIG. 3.
Relationship between tigecycline dose in the single ascending dose study and Cmax (A) and AUC (B).
In the MAD study, dose-normalized values for Cmax and AUC on day 1 were not significantly different among the dose groups, and Cmax ranged from 261 ng/ml in the 25-mg dose group to 816 ng/ml in the 100-mg dose group (Table 3). The mean Cmax values obtained at steady state ranged from 324 ng/ml in the 25-mg dose group to 1,173 ng/ml in the 100-mg dose group. The mean AUCss values increased proportionately from 1,482 ng h/ml to 4,980 ng h/ml with an increase in dose from 25 to 100 mg.
DISCUSSION
Tigecycline, a first-in-class glycylcycline with broad-spectrum activity against many pathogens, is being developed for treatment of skin and skin structure infections and intra-abdominal infections (15, 19).
No serious adverse events were reported in any of the three studies. The most common adverse events reported in all studies that appeared to be dose-related were nausea and vomiting, which are common to the tetracycline class of antibiotics. At the higher doses in the SAD study (200 and 300 mg), prolonging the infusion duration to 4 h did not improve the incidence or severity of nausea in this small study (n = 6 per group), indicating that the nausea is not directly related to the drug's Cmax in serum. Also, the nausea and vomiting diminished when the 200-mg dose was administered to subjects who had been fed compared to those who had fasted. In phase 2 trials with tigecycline in patients with skin and skin structure infections (tigecycline at 25 or 50 mg q12h) (19) or in patients with intra-abdominal infections (tigecycline at 50 mg q12h) (15), the incidence of nausea and vomiting was lower than was observed in these healthy subjects.
Tigecycline is likely excreted in bile in humans. It has been hypothesized that tigecycline might irritate the GI tract, releasing serotonin, which then causes nausea. In the VVIR study, the urinary recovery of 5-HIAA on study day 1 was 5.57 ± 1.23 mg in the nine tigecycline-treated subjects who experienced nausea or vomiting on day 1, 5.86 ± 2.94 mg in the nine tigecycline-treated subjects who did not experience nausea or vomiting on day 1, and 5.56 ± 0.83 mg in the placebo-treated subjects, none of whom experienced nausea on day 1. Therefore, the urinary recovery of 5-HIAA did not correlate with the occurrence of nausea or vomiting, suggesting that the nausea and vomiting associated with tigecycline administration may not be caused by increased serotonin release in the GI tract.
The data obtained from the VVIR study showed that there were no clinically important differences in safety among the three groups with various infusion rates. The incidence of adverse events such as nausea and vomiting was similar among all three groups, with slightly less nausea and vomiting with the shorter 30-min infusion compared to the longer infusions. Adjusting the infusion of tigecycline more rapidly (30 min) and in a smaller volume (100 ml) can be accomplished without significant safety issues. It is important to note that the 30-min infusion was also not associated with an increase in the incidence of local injection site reactions compared to the 60-min infusion. These results indicate that tigecycline could be safely administered as a 30-min infusion.
The serum concentration-time profile of tigecycline after ascending single or multiple doses shows that the decline in drug concentrations after the end of tigecycline infusion follows a polyphasic pattern (Fig. 1 and 2). The steep decrease in tigecycline concentrations in serum at the end of infusion demonstrates the rapid distribution of the drug from the central compartment into the various tissues. This is followed by a long terminal-phase t1/2 of ca. 40 h for tigecycline. The long t1/2 of tigecycline and the extended postantibiotic effect demonstrated against certain microorganisms (16, 18) are among the reasons for the anticipated efficacy of tigecycline in patients.
The CLR of tigecycline (0.03 liters/h/kg) accounts for ca. 10 to 15% of tigecycline's total systemic CL (ranging from 0.2 to 0.3 liter/h/kg in all SAD and MAD dose groups), and only 10 to 15% of the administered dose is recovered unchanged in urine. Therefore, the renal elimination of tigecycline is a secondary pathway of tigecycline's elimination. Unlike minocycline, which undergoes extensive metabolism (12), no major metabolites of tigecycline have been identified to date, indicating that metabolic elimination is likely to be a minor elimination pathway. In addition, tigecycline exhibits a high degree of biliary excretion in rats (23). Therefore, the major component of tigecycline systemic CL in humans might be biliary secretion, GI secretion across the gut walls, or both.
In separate experiments, the in vitro protein binding of tigecycline was measured by ultrafiltration and by ultracentrifugation at 37°C (data on file at Wyeth Research). For ultrafiltration, the in vitro protein binding ranged from 71% at 0.1 μg/ml to 87% at 1.0 μg/ml, and for ultracentrifugation the in vitro protein binding ranged from 73% at 0.1 μg/ml to 79% at 1.0 μg/ml. The mechanism for the atypical pattern of increased protein binding at higher concentrations is unknown, but it may be related to the ability of tigecycline to form metal ion complexes. The formation of such complexes by tetracycline has been shown to result in complex interactions with serum proteins that affect diffusion rates across semipermeable membranes and binding to cellular proteins (2, 7, 8).
Tigecycline is extensively distributed into the tissues, as shown by its high volume of distribution in both SAD and MAD studies. In fact, radiolabeled 14C studies in rats have confirmed that tigecycline distributes extensively to various tissues, including lung, skin, liver, heart, and bone (23). The ratio of tigecycline in skin and lungs in rats were nearly three- to fourfold higher than that in plasma. This suggests that for drugs such as tigecycline, concentrations in serum may significantly underestimate the concentration of the drug in various tissues.
After i.v. administration, tigecycline exhibited linear pharmacokinetics after single doses in the range 12.5 to 300 mg and multiple doses of 25 to 100 mg q12h. In fact, the linearity of tigecycline pharmacokinetics is evident by the absence of significant differences in systemic CL among the various dose groups after single and multiple doses. In addition, for dose groups with sufficiently high concentrations in serum to provide a reliable estimate of t1/2 (i.e., doses of >75-mg [single dose]), the Vss did not differ significantly among the dose groups. Trough tigecycline concentrations in serum obtained on days 7, 8, 9, and 10 in all dose groups indicate that tigecycline attained steady-state levels in serum by day 7.
As predicted by the t1/2 of 40 to 60 h obtained in these studies, some accumulation of tigecycline is seen after multiple doses, and the observed accumulations (R = C12 h,multiple-dose/C12 h,single-dose) were 2.5, 3.4, and 3.2 for the 25-, 50-, and 100-mg q12h regimens, respectively. Interestingly, the observed accumulation is lower than the theoretical accumulation of 5.3 to 7.7 for a t1/2 of 40 to 60 h [R = 1/(1 − e−λτ), assuming the trough concentration is measured in the terminal disposition phase of the curve]. Another method to evaluate the observed and theoretical accumulation is to compare the single-dose AUC0-∞ and the multiple-dose AUC0-τ for the same dose. The design of the MAD study did not allow a reliable estimate of the single-dose AUC0-∞ because the concentrations in serum were sampled for only 12 h before the second dose was administered. Comparison of the single-dose AUC0-∞ from the SAD study and the multiple-dose AUC0-τ from the MAD study showed that the single-dose AUC0-∞ underpredicted the multiple-dose AUC0-τ by ca. 20% for the 50-mg dose and overpredicted the multiple-dose AUC0-τ by ca. 20% for the 100-mg dose. Therefore, the accumulation of tigecycline after multiple-dose administration is approximately linear, but these comparisons should be interpreted cautiously because the AUCs were measured in different populations and the comparisons are based on a small number of subjects (n = 6 per group).
This article establishes the pharmacokinetic profile and dose proportionality of tigecycline when given as single or multiple twice-daily doses to healthy subjects; it also shows that the infusion duration can be varied without significant safety concerns. These characteristics, along with the in vitro activity and extended postantibiotic effect of tigecycline against several strains of microorganisms, make tigecycline a promising antibiotic agent.
Acknowledgments
We thank the investigators (Henri Caplain and Richard Fruncillo) and the subjects involved in the trials. Medical writing support was provided by Donna Simcoe of Wyeth Research. We thank Wyeth employees Jay Getsy, Parviz Mojaverian, Isabelle Paty, and Barbara Wester for contributions to this study.
This study was supported by Wyeth Research, Collegeville, Pa. (as study drug and grants to investigational sites).
REFERENCES
- 1.Betriu, C., I. Rodriguez-Avial, B. A. Sanchez, M. Gomez, J. Alvarez, J. J. Picazo, et al. 2002. In vitro activities of tigecycline (GAR-936) against recently isolated clinical bacteria in Spain. Antimicrob. Agents Chemother. 46:892-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin, T., and J. L. Lach. 1975. Drug diffusion and bioavailability: tetracycline metallic chelation. Am. J. Hosp. Pharm. 32:625-629. [PubMed] [Google Scholar]
- 3.Chopra, I. 2002. New developments in tetracycline antibiotics: glycylcyclines and tetracycline efflux pump inhibitors. Drug Resist. Update 5:119-125. [DOI] [PubMed] [Google Scholar]
- 4.Cubeddu, L. X., I. S. Hoffmann, N. T. Fuenmayor, and A. L. Finn. 1990. Efficacy of ondansetron (GR 38032F) and the role of serotonin in cisplatin-induced nausea and vomiting. N. Engl. J. Med. 322:810-816. [DOI] [PubMed] [Google Scholar]
- 5.Deshpande, L. M., A. C. Gales, and R. N. Jones. 2001. GAR-936 (9-t-butylglycylamido-minocycline) susceptibility test development for streptococci, Haemophilus influenzae and Neisseria gonorrhoeae: preliminary guidelines and interpretive criteria. Int. J. Antimicrob. Agents 18:29-35. [DOI] [PubMed] [Google Scholar]
- 6.Edelstein, P. H., W. J. Weiss, and M. A. Edelstein. 2003. Activities of tigecycline (GAR-936) against Legionella pneumophila in vitro and in guinea pigs with L. pneumophila pneumonia. Antimicrob. Agents Chemother. 47:533-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fey, G., M. Reiss, and H. Kersten. 1973. Interaction of tetracyclines with ribosomal subunits from Escherichia coli: a fluorometric investigation. Biochemistry 12:1160-1164. [DOI] [PubMed] [Google Scholar]
- 8.Gabler, W. L. 1991. Fluxes and accumulation of tetracyclines by human blood cells. Res. Commun. Chem. Pathol. Pharmacol. 72:39-51. [PubMed] [Google Scholar]
- 9.Gales, A. C., and R. N. Jones. 2000. Antimicrobial activity and spectrum of the new glycylcycline, GAR-936, tested against 1,203 recent clinical bacterial isolates. Diagn. Microbiol. Infect. Dis. 36:19-36. [DOI] [PubMed] [Google Scholar]
- 10.Hoellman, D. B., G. A. Pankuch, M. R. Jacobs, and P. C. Appelbaum. 2000. Antipneumococcal activities of GAR-936 (a new glycylcycline) compared to those of nine other agents against penicillin-susceptible and -resistant pneumococci. Antimicrob. Agents Chemother. 44:1085-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefort, A., M. Lafaurie, L. Massias, Y. Petegnief, A. Saleh-Mghir, C. Muller-Serieys, D. Le Guludec, and B. Fantin. 2003. Activity and diffusion of tigecycline (GAR-936) in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 47:216-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macdonald, H., R. G. Kelly, E. S. Allen, J. F. Noble, and L. A. Kanegis. 1973. Pharmacokinetic studies on minocycline in man. Clin. Pharmacol. Ther. 14:852-861. [DOI] [PubMed] [Google Scholar]
- 13.Milatovic, D., F. J. Schmitz, J. Verhoef, and A. C. Fluit. 2003. Activities of the glycylcycline tigecycline (GAR-936) against 1,924 recent European clinical bacterial isolates. Antimicrob. Agents Chemother. 47:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muralidharan, G., P. Mojaverian, M. Micalizzi, J. Speth, S. Tse, R. Stroshane, J. Getsy, and P. Mayer. 2000. The effects of age and gender on the pharmacokinetics, safety and tolerability of GAR-936, a novel glycylcycline antibiotic, in healthy subjects. Program Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 500.
- 15.Murray, J., S. Wilson, S. Klein, A. Yellin, and E. Loh. 2003. The clinical response to tigecycline in the treatment of complicated intra-abdominal infections in hospitalized patients: a phase 2 clinical trial. Program Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-739.
- 16.Petersen, P. J., H. E. Hartman, T. Z. Wang, R. G. Dushin, and P. A. Bradford. 2002. Time kill kinetics and postiantibiotic (PAE) effects of the AC98 novel semisynthetic cyclic glycopeptide antibiotic derivative WAY-176446. Program Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-354.
- 17.Petersen, P. J., N. V. Jacobus, W. J. Weiss, P. E. Sum, and R. T. Testa. 1999. In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t-butylglycylamido derivative of minocycline (GAR-936). Antimicrob. Agents Chemother. 43:738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen, P. J., W. J. Weiss, P. Labthavikul, and B. P. A. 1998. The post-antibiotic effect and time-kill kinetics of the glycylcyclines, GAR-936 (TBG-MINO) and (PAM-MINO). Program Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-132.
- 19.Postier, R. G., S. L. Green, S. R. Klein, E. J. Ellis-Grosse, E. Loh, et al. 2004. Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients. Clin. Ther. 26:704-714. [DOI] [PubMed] [Google Scholar]
- 20.Roblin, P. M., and M. R. Hammerschlag. 2000. In vitro activity of GAR-936 against Chlamydia pneumoniae and Chlamydia trachomatis. Int. J. Antimicrob. Agents 16:61-63. [DOI] [PubMed] [Google Scholar]
- 21.Sesoko, S., K. Umemura, and M. Nakashima. 2002. Pharmacokinetics (PK), safety, and tolerability of tigecycline (GAR-936) in healthy Japanese males. Program Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1403.
- 22.Testa, R. T., P. J. Petersen, N. V. Jacobus, P. E. Sum, V. J. Lee, and F. P. Tally. 1993. In vitro and in vivo antibacterial activities of the glycylcyclines, a new class of semisynthetic tetracyclines. Antimicrob. Agents Chemother. 37:2270-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tombs, N. L. 1999. Tissue distribution of Gar-936, a broad-spectrum antibiotic, in male rats. Program Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 413.
- 24.van Ogtrop, M. L., D. Andes, T. J. Stamstad, B. Conklin, W. J. Weiss, W. A. Craig, and O. Vesga. 2000. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 44:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace, R. J., Jr., B. A. Brown-Elliott, C. J. Crist, L. Mann, and R. W. Wilson. 2002. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob. Agents Chemother. 46:3164-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhanel, G. G., K. Homenuik, K. Nichol, A. Noreddin, L. Vercaigne, J. Embil, A. Gin, J. A. Karlowsky, and D. J. Hoban. 2004. The glycylcyclines: a comparative review with the tetracyclines. Drugs 64:63-88. [DOI] [PubMed] [Google Scholar]