Abstract
Fourteen fluoroquinolone-resistant fusobacterial strains, originating from cats or dogs, were characterized by sequencing of the 16S-23S and 16S rRNA genes and DNA-DNA hybridization and were described as a new species, Fusobacterium canifelinum. All of the strains are intrinsically resistant (MIC, >4 g/ml) to levofloxacin and other fluoroquinolones. Compared to the quinolone resistance-determining region (gyrA) of the susceptible relative F. nucleatum, we found that Ser79 was replaced with leucine and Gly83 was replaced with arginine.
Fusobacteria are obligately anaerobic, non-spore-forming, nonmotile, pleomorphic, rod-shaped bacilli. Although they stain gram negatively, they are allied with the gram-positive phylum.
During studies of the microflora of infected cat and dog bite wounds in humans, 25 isolates were identified as Fusobacterium nucleatum. Nine of 16 isolates that were tested for fluoroquinolone susceptibility were found to be resistant (levofloxacin MIC, >4 μg/ml), unlike human strains, which are typically susceptible (5, 7, 9, 11). In addition, four strains of F. nucleatum-like organisms from cat and dog infections, as well as one isolate from an upper left leg soft tissue infection of a diabetic patient with a pet dog that licked his legs, were also found to be resistant. These quinolone-resistant strains were further characterized, and all 14 strains were identical to each other at the 99 to 100% level by internal transcribed spacer and 16S data and at the 75 to 92% level by DNA-DNA hybridization but distinct from all other currently known fusobacterial species (4); hence, the name Fusobacterium canifelinum was proposed (3) and published in the List of Bacterial Names with Standing in Nomenclature, no. 100.
Bacterial strains and culture media.
All 14 strains (RMA 1036T [ATCC BAA 689T, DSM 15542T], RMA 1072, RMA 1079, RMA 7631, RMA 7654, RMA 7723, RMA 7782, RMA 7897, RMA 7903, RMA 11693, RMA 12701 [ATCC BAA 690, DSM 15543], RMA 12702, RMA 12703, and RMA 12708) were phenotypically characterized on the basis of the description in reference 15. Strains were cultivated at 37°C on supplemented Brucella agar (Anaerobe Systems) in an anaerobic chamber.
Quinolone susceptibility and phenotypic characterization of efflux mechanism.
Levofloxacin, gatifloxacin, moxifloxacin, and gemifloxacin were obtained from their respective manufacturers. Susceptibility tests were performed by the agar dilution method as described in the NCCLS M11-A6 document (17). Briefly, the test medium was Brucella agar supplemented with vitamin K1, hemin, and laked blood. The inoculum was prepared directly from 48-h plates and applied to plates containing serial twofold dilutions of the drugs at a final concentration of 105 CFU/spot. After 48 h of incubation at 37°C under anaerobic conditions, the plates were examined for growth. The MIC was defined as the lowest concentration of a drug that significantly reduced growth compared to that on the drug-free growth control plate.
To investigate the presence of an active efflux mechanism, the MICs of the four quinolones were also determined, in duplicate, with the addition of carbonyl cyanide m-chlorophenylhydrazone (CCCP) or reserpine (Sigma Chemical Co., St. Louis, Mo.) at 20 μg/ml in the agar dilution test. An efflux mechanism is inferred to be present when the quinolone MIC in the presence of CCCP and/or reserpine is at least fourfold less (2 doubling dilutions) than the corresponding MIC in the absence of these compounds. All MICs (with and without addition of reserpine) are given in Table 1.
TABLE 1.
In vitro activities of moxifloxacin, levofloxacin, gatifloxacin, and gemifloxacin, with and without 20 μg of reserpine per ml, against 14 strains of F. canifelinum
| Antimicrobial agent | Addition of reserpine | MIC (μg/ml)a
|
||
|---|---|---|---|---|
| Range | 50% of strains | 90% of strains | ||
| Moxifloxacin | + | 16-64 | 32 | 32 |
| Moxifloxacin | − | 16-64 | 32 | 32 |
| Levofloxacin | + | 16-64 | 32 | 64 |
| Levofloxacin | − | 16-64 | 32 | 64 |
| Gatifloxacin | + | 32-64 | 64 | 64 |
| Gatifloxacin | − | 32-64 | 64 | 64 |
| Gemifloxacin | + | 8-16 | 8 | 8 |
| Gemifloxacin | − | 4-16 | 8 | 8 |
For contrast, MICs of fluoroquinolone susceptible fusobacterial species are between 0.015 and a maximum of 4 (8).
Amplification of gyrA and gyrB.
Two topoisomerase class IIa enzymes are the principal targets for the antibacterial activity of quinolones: topoisomerase IV (genes parC and parE, which are not present in the fusobacterial genome according to GenBank information) and DNA gyrase (genes gyrA and gyrB).
By comparing the gyrA- and gyrB-containing genome fragments of F. nucleatum subsp. nucleatum ATCC 25586 (GenBank accession no. AE010515) and F. nucleatum subsp. vincentii ATCC 49256 (GenBank accession no. AABF1000051), we deduced F. nucleatum group gyrA- and gyrB-specific PCR primers fusoGyrAF1 (5′ TCT TAC TTG GAT TAC TCA ATG AGT G 3′) and fusoGyrAR1 (5′ ACC AAA GAA AGC ATT ATA AC 3′), amplifying the first 960 bp of gyrA, and primers fusoGyrBF1 (5′ AAA AGG CTA TAA TTC TGC TGT AAA 3′) and fusoGyrBR1 (5′ CTC CAA CAT TAT CTT GTT CAG ATG 3′) and primers fusoGyrBF2 (= fusoGyrBR1′) (5′ CAT CTG AAC AAG ATA ATG TTG GAG 3′) and fusoGyrBR2(5′ ACC TTA TAT AGT GGA GGA CAA GCA AT 3′), amplifying the first 860 and the subsequent 866 bp of gyrB, which were combined. According to the literature (2, 5, 23) the 5′ ends and quinolone resistance-determining regions (QRDRs) of gyrA and gyrB were covered by this approach. The PCR was carried out in a 50-μl reaction mixture containing 200 μM each deoxynucleoside triphosphate, 100 ng of total bacterial DNA, 3 μl (5 pmol) of each primer (primers fusoGyrAF1 and fusoGyrAR1, primers fusoGyrBF1 and fusoGyrBR1, or primers fusoGyrBF2 and fusoGyrBR2), 5 μl of PCR buffer (Roche Diagnostics GmbH, Mannheim, Germany), and 2 U of Taq polymerase (Roche). Thermocycling was denaturation at 95°C for 5 min, followed by 30 cycles of 95°C for 30 s and 50°C for 30 s and elongation at 72°C for 2 min. DNA fragments of 960, 860, and 866 bp, respectively, were produced. The nucleotide sequences of these fragments were determined completely for reference strains RMA 1036T (ATCC BAA 689T) and RMA 12701 (ATCC BAA 690) and partially (QRDR only) for all of the other strains listed above by using the amplification primers for sequencing.
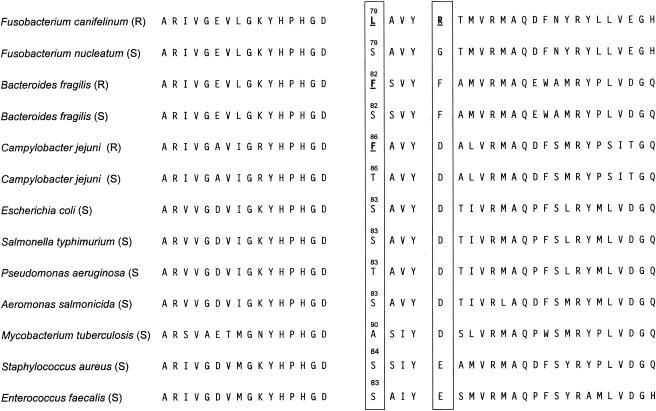
In comparison with the gyrA QRDR sequence of its fluoroquinolone-susceptible relatives F. nucleatum subsp. nucleatum and vincentii, we found five single nucleotide mutations in all of the strains of F. canifelinum sequenced (representative results are shown in Fig. 1), with three being silent (CTA to TTA [both coding for L], GCA to GCT [both coding for A], and GTA to GTT [both coding for V]). Relevant for the level of resistance is the TCA-to-TTA mutation replacing Ser79 (polar amino acid, equivalent to Escherichia coli Ser83 and Bacteroides fragilis Ser82) with leucine, a hydrophobic and aliphatic amino acid (16, 21-23). In addition, by mutation of GGA to AGA, Gly83 (hydrophobic, equivalent to Asp87 in quinolone-susceptible E. coli strains) in F. nucleatum subsp. nucleatum and vincentii is replaced with polar and charged arginine in F. canifelinum. Both positions are hot spots of relevant gyrA mutations found in a lot of other bacterial species of various taxa (Fig. 2) (2, 22, 23). Alterations in the QRDR of gyrB are also related to quinolone resistance (12, 25) but are less frequently implicated and were not found in F. canifelinum RMA 1036T or RMA 12701 (GenBank accession no. AY660894 and AY660895) or in any of the other strains partially sequenced. DNA topoisomerase IV has been demonstrated to be an alternative target for quinolones in gram-negative or gram-positive bacteria. By screening the proteins derived from the genome sequences of F. nucleatum subsp. nucleatum ATCC 25586 (GenBank accession no. AE010515 [complete]) and F. nucleatum subsp. vincentii ATCC 49256 (GenBank accession no. AABF1000051 [almost complete]), we found that topoisomerase IV was absent. As gyrase and topoisomerase IV have the same function, this situation is likely to occur, and by further screening of completed and annotated bacterial genome projects, we found the same situation in a few other species, for example, in Aquifex aeolicus VF5, Deinococcus radiodurans R1, Leptospira interrogans serovar Lai 56691, and Thermus thermophilus HB27.
FIG. 1.
Comparison of the QRDRs (5′ end of gyrA) of resistant strains F. canifelinum RMA 1036T (ATCC BAA 689T) and RMA 12701 (ATCC BAA 690) and closely related susceptible strains F. nucleatum subsp. nucleatum ATCC 25586T and F. nucleatum subsp. vincentii ATCC 49256. Functional mutations, conserved on the species level, are boxed with solid lines, and silent mutations are boxed with broken lines. The program GeneDoc (18) was used for alignment.
FIG. 2.
QRDRs (amino acid sequences) of F. canifelinum (resistant), F. nucleatum (susceptible, for contrast), and nine representatives of distantly related species (22, 23). The hot spots of mutations are boxed. R, resistant; S, susceptible.
Reserpine at 20 μg/ml did not affect the fluoroquinolone MICs; however, CCCP at 20 μg/ml inhibited the growth of all of the fusobacterial strains tested.
Several recently developed fluoroquinolones have good activity against a broad range of aerobic and anaerobic bacteria and are thus candidates for the treatment of serious mixed infections (5-7, 10, 11, 13, 14, 19, 24). However, resistance to quinolones among aerobic and anaerobic bacteria is increasing worldwide. Two main mechanisms are associated with resistance: (i) alteration of target enzymes (gyrase and topoisomerase IV) caused by single or stepwise chromosomal mutations in encoding genes and (ii) reduced intracellular accumulation due to increased efflux of the drug (20). The increasing emergence of resistance among anaerobes, namely, B. fragilis and Clostridium difficile, may be a consequence of previous widespread use of quinolones, which may have enriched first-step mutants in the intestinal tract (1, 21). Quinolone resistance in the B. fragilis group strains is strongly correlated with amino acid substitutions at positions 82 and 86 in GyrA (equivalent to positions 83 and 87 of E. coli). The study of Miyamae at al. indicated that B. fragilis group strains also possess efflux pump systems that actively expel quinolones, contributing to resistance (16). DNA gyrase seems also to be the primary target for quinolones in C. difficile, since amino acid substitutions in GyrA and GyrB have been detected in resistant strains (20).
This report represents the first description of the mechanism of fluoroquinolone resistance in a fusobacterial species, caused by alterations in gyrA, most likely intrinsic in our strains, as isolates RMA 1036, RMA 1072, and RMA 1079 were isolated in 1977 and 1984, before the extensive use of (fluoro)quinolones.
Nucleotide sequence accession numbers.
The gyrA and gyrB sequences of strains RMA 1036T and RMA 12701 have been deposited in the GenBank database under accession no. AY660893 to AY660896.
Acknowledgments
We thank Helen T. Fernandez, Hans-Peter Horz, Spencer Jang, Vreni Merriam, Kerin L. Tyrrell, Yumi Warren, and Ilse Seyfarth for various forms of assistance.
REFERENCES
- 1.Ackermann, G., Y. J. Tang-Feldman, R. Schaumann, J. P. Henderson, A. C. Rodloff, J. Silva, and S. H. Cohen. 2003. Antecedent use of fluoroquinolones is associated with resistance to moxifloxacin in Clostridium difficile. Clin. Microbiol. Infect. 9:526-530. [DOI] [PubMed] [Google Scholar]
- 2.Bearden, D. T., and L. H. Danziger. 2001. Mechanism of action of and resistance to quinolones. Pharmacotherapy 21:224S-232S. [DOI] [PubMed] [Google Scholar]
- 3.Conrads, G., D. Citron, R. Mutters, S. Jang, and E. Goldstein. 2004. Fusobacterium canifelinum sp. nov., from the oral cavity of cats and dogs. Syst. Appl. Microbiol. 27:407-413. [DOI] [PubMed] [Google Scholar]
- 4.Conrads, G., M. C. Claros, D. M. Citron, K. L. Tyrrell, V. Merriam, and E. J. Goldstein. 2002. 16S-23S rDNA internal transcribed spacer sequences for analysis of the phylogenetic relationships among species of the genus Fusobacterium. Int. J. Syst. Evol. Microbiol. 52:493-499. [DOI] [PubMed] [Google Scholar]
- 5.Dalhoff, A., and F. J. Schmitz. 2003. In vitro antibacterial activity and pharmacodynamics of new quinolones. Eur. J. Clin. Microbiol. Infect. Dis. 22:203-221. [DOI] [PubMed] [Google Scholar]
- 6.Eguchi, T., Y. Shimizu, K. Furuhata, and M. Fukuyama. 2002. Antibacterial activity of new-quinolone and macrolide antibiotics against oral bacteria. Kansenshogaku Zasshi 76:939-945. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein, E. J., D. M. Citron, M. Hudspeth, S. Hunt Gerardo, and C. V. Merriam. 1998. Trovafloxacin compared with levofloxacin, ofloxacin, ciprofloxacin, azithromycin and clarithromycin against unusual aerobic and anaerobic human and animal bite-wound pathogens. J. Antimicrob. Chemother. 41:391-396. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein, E. J., D. M. Citron, C. V. Merriam, Y. A. Warren, K. L. Tyrrell, and H. Fernandez. 2002. In vitro activities of the des-fluoro(6) quinolone BMS-284756 against aerobic and anaerobic pathogens isolated from skin and soft tissue animal and human bite wound infections. Antimicrob. Agents Chemother. 46:866-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goumas, P. D., S. S. Naxakis, D. A. Papavasiliou, E. D. Moschovakis, S. J. Tsintsos, and A. Skoutelis. 1997. Periapical abscesses: causal bacteria and antibiotic sensitivity. J. Chemother. 9:415-419. [DOI] [PubMed] [Google Scholar]
- 10.Hecht, D. W., and J. R. Osmolski. 2003. Activities of garenoxacin (BMS-284756) and other agents against anaerobic clinical isolates. Antimicrob. Agents Chemother. 47:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht, D. W., and H. M. Wexler. 1996. In vitro susceptibility of anaerobes to quinolones in the United States. Clin. Infect. Dis. 23(Suppl. 1):S2-S8. [DOI] [PubMed] [Google Scholar]
- 12.Ito, H., H. Yoshida, M. Bogaki-Shonai, T. Niga, H. Hattori, and S. Nakamura. 1994. Quinolone resistance mutations in the DNA gyrase gyrA and gyrB genes of Staphylococcus aureus. Antimicrob. Agents Chemother. 38:2014-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King, A., J. Downes, C. E. Nord, and I. Phillips. 1999. Antimicrobial susceptibility of non-Bacteroides fragilis group anaerobic gram-negative bacilli in Europe. Clin. Microbiol. Infect. 5:404-416. [DOI] [PubMed] [Google Scholar]
- 14.Kleinkauf, N., G. Ackermann, R. Schaumann, and A. C. Rodloff. 2001. Comparative in vitro activities of gemifloxacin, other quinolones, and nonquinolone antimicrobials against obligately anaerobic bacteria. Antimicrob. Agents Chemother. 45:1896-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krug, N. R., and J. G. Holt (ed.). 1984. Bergey's manual of systematic bacteriology. Williams & Wilkins, Baltimore, Md.
- 16.Miyamae, S., H. Nikaido, Y. Tanaka, and F. Yoshimura. 1998. Active efflux of norfloxacin by Bacteroides fragilis. Antimicrob. Agents Chemother. 42:2119-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2000. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standard, 5th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Nicholas, K. B., and H. B. J. Nicholas. 1997. GeneDoc: a tool for editing and annotation multiple sequence alignments. www.psc.edu/biomed/genedoc.
- 19.Nord, C. E. 1996. In vitro activity of quinolones and other antimicrobial agents against anaerobic bacteria. Clin. Infect. Dis. 23(Suppl. 1):S15-S18. [DOI] [PubMed] [Google Scholar]
- 20.Oh, H., and C. Edlund. 2003. Mechanism of quinolone resistance in anaerobic bacteria. Clin. Microbiol. Infect. 9:512-517. [DOI] [PubMed] [Google Scholar]
- 21.Oh, H., N. El Amin, T. Davies, P. C. Appelbaum, and C. Edlund. 2001. gyrA mutations associated with quinolone resistance in Bacteroides fragilis group strains. Antimicrob. Agents Chemother. 45:1977-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onodera, Y., and K. Sato. 1999. Molecular cloning of the gyrA and gyrB genes of Bacteroides fragilis encoding DNA gyrase. Antimicrob. Agents Chemother. 43:2423-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piddock, L. J. 1999. Mechanisms of fluoroquinolone resistance: an update 1994-1998. Drugs 58(Suppl. 2):11-18. [DOI] [PubMed] [Google Scholar]
- 24.Stein, G. E., S. Schooley, K. L. Tyrrell, D. M. Citron, and E. J. Goldstein. 2003. Bactericidal activities of methoxyfluoroquinolones gatifloxacin and moxifloxacin against aerobic and anaerobic respiratory pathogens in serum. Antimicrob. Agents Chemother. 47:1308-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida, H., M. Bogaki, M. Nakamura, L. M. Yamanaka, and S. Nakamura. 1991. Quinolone resistance-determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob. Agents Chemother. 35:1647-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]