Abstract
Curvularia a dematiaceous fungus is ubiquitously found in soil around the world. We report an epidural abscess due to Curvularia lunata in a 48 years male farmer who underwent decompressive laminectomy as primary modality of treatment followed by isolation, identification and confirmation of the isolate from tissue by ITS sequencing. Antifungal therapy with voriconazole and amphotericin B for 3 and 2 weeks respectively improved patient's condition and is presently on regular follow up with no sequelae since last 7 months.
Keywords: Curvularia lunata, Central India, Epidural abscesss, Melanized fungus, Molecular identification, Phaeohyphomycosis
1. Introduction
CNS infections by melanized fungi have increasingly been reported in recent years. Primary cerebral infections are predominantly caused by Exophiala dermatitidis, Cladophialophora bantiana, Ramichloridium mackenzie with occassional Ochroconis gallopava. [1] Secondary cerebral infections are usually an extension from chronic sinusitis and are due to grass-inhabiting species under genera the Bipolaris, Dissitimurus, Exserohilum, Curvularia lunata, Cladosporium cladosporioides, Nodulisporium species. [1] The infection is encountered in apparently immunocompetent hosts with chronic sinusitis and the agents are commonly airborne saprobes. [1].
Phaeohyphomycosis (PH) are a rare group of heterogenous dematiaceous (phaeoid) brown pigment producing fungi, which cause superficial, cutaneous, and subcutaneous infections in the immunocompetent and systemic illness, especially brain abscesses in the immunosuppressed.[2] A myriad of fungal species causes this unique infection, which includes Exophiala, Phialophora, Cladosporium, Wangiella, Fonsacaea, Alternaria, Bipolaris,and Curvularia species. The usual source of infection is usually exogenous, following pricks with thorns or wood splinters. [2].
The pathogenesis of primary CNS phaeohyphomycosis is poorly understood. Melanin, found in the cell walls of dematiaceous fungi, is a known virulence factor and may play an important role in the host immune system evasion of these fungi. Most of the CNS infections are thought to be secondary to extension from paranasal sinuses; however, some infections appear to have resulted from hematogenous dissemination, direct inoculation from penetrating head trauma, and from contaminated wounds. [3].
Curvularia is a filamentous, dematiaceous fungus characterized by melanin pigmentation in the cell walls of its hyphae. It is a mold, ubiquitously found in soil around the world, with preference to the tropical and subtropical regions. It was first documented as a human pathogen in 1959 in Africa, isolated from lung mycetomas. [3] The first central nervous system (CNS) Curvularia infection was described in 1977 by Lampert et al. Since then, only eight CNS Curvularia infections have been documented in the literature. [3] The ninth case was Curvularia infection of brain stem documented by Branko Skovrlj et al. [3].
Curvularia received its current name in 1933 and related to the sexual teleomorph Cochliobolus typified by C. lunata and morphologically characterized by the production of sympodial conidiophores with tretic, terminal and intercalary conidiogenous cells and elongate, transversely septate conidia with a dark basal scar [4].
Frequently, diagnosis is understandably delayed in cases of spinal epidural abscess because the initial presentation may be only nonspecific back pain. One half of cases are estimated to be misdiagnosed or have a delayed diagnosis [5]. The classic presentation of spinal epidural abscess involves the triad of spine pain, fever, and neurologic deficit [6].
We present our case, which is the 10th case so far, for documentation and future reference; as melanized fungal infections of CNS are difficult to diagnose and treat.
2. Case
A 48 years male farmer from central India came to neurosurgery Out Patient Department of AIIMS Bhopal on 4th April 2016 with chief complaint of low backache since 3years which was insidious in onset, gradually progressive, radiating to bilateral lower limb, associated with numbness and paraesthesia, inability to walk since 15 months and decreased urinary sensation. The patient was habituated to tobacco chewing and smoking.
On clinical examination patient was afebrile, oriented, had inability to walk without support. There was loss of sensation bilaterally in L3, L4, L5, S1 region. An atrophic change over palmar muscles due to chronic use of crutches was observed. There was no motor or sensory deficit of upper limb. Rest of the examination was normal.
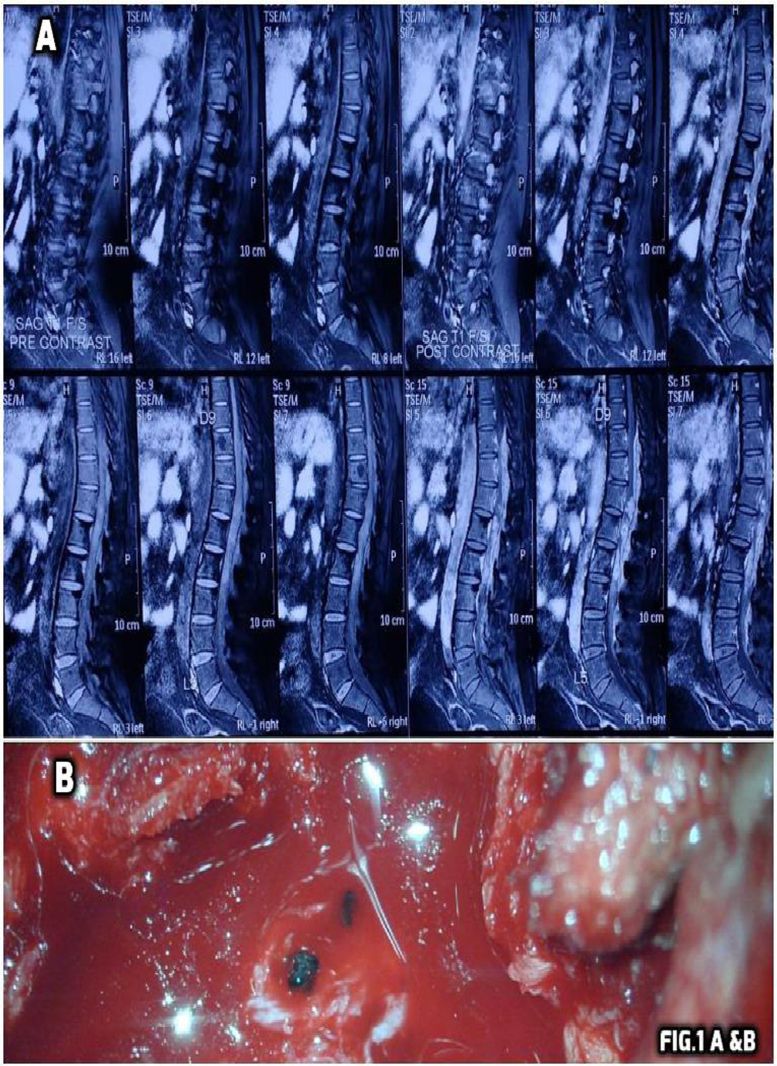
His routine investigations of blood heamoglobin, total and differential leucocyte count, Erythrocyte sedimentation rate, platelet count, C reactive protein, random blood sugar, serum creatinine kinase, Blood Na+, K+ and renal function tests were normal. Negative for urine Bence–Jones proteins and normal prostate specific antigen. Electrocardiogram was normal. His HIV status was negative. Magnetic resonance imaging of dorsolumbar spine showed intensely enhancing posterior epidural and intrathecal space occupying lesions extending from D10 to L4 levels causing spinal canal stenosis and compression of lower spinal cord & cauda equina nerve roots with mild erosion of adjoining vertebral bodies (Fig. 1A).
Fig. 1.
Shows contrast enhanced MRI of dorsolumbar spine in sagittal plane. Long segment heterogenous intensity lesions are seen in the spinal canal and posterior epidural space extending from D10 to L4 levels appearing hypointense and intense in post contrast enhancement (A). Bottom shows black coloured rounded structures with pus pockets in epidural space during decompressive laminectomy (B).
Patient was admitted on day of first hospital visit (4th April 2016) which is Day 0 (D0) for our case study.
2.1. Primary treatment
As Primary treatment modality surgery was preferred and a D10- L4 decompressive laminectomy was performed on D8 (12th April 2016). The lesion showed inflammation with fibrosis, pus pockets and several black coloured rounded structures. (Fig. 1B).
The tissue from D10-L4 epidural space occupying lesion was sent for squash cytology, histopathology, and clinical microbiology comment and diagnosis. Also a portion of the tissue was sent for tuberculosis diagnosis.
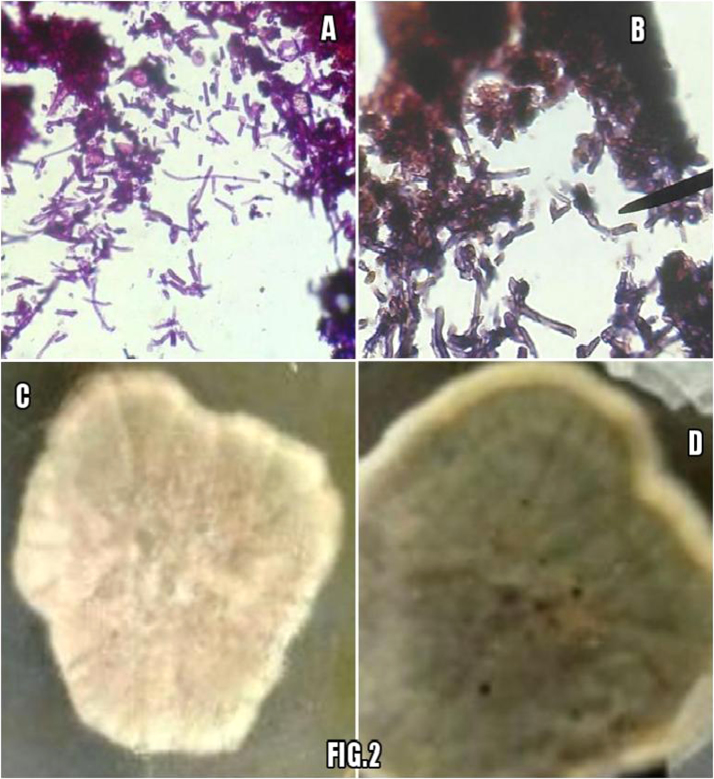
Squash cytology smears showed mixed inflammatory infiltrate comprising of predominantly lymphocytes, plasma cells, histiocytes and polymorphs along with many capillary fragments and fibroblasts in a granular background suggestive of inflammatory granulation tissue. 2 weeks after surgery on D23 histopathology showed fibrocollagenous tissue infiltrated by inflammatory infiltrate comprising of lymphocytes, plasma cells, polymorphs and histiocytes along with fungal hyphae (Fig. 2A). Periodic acid schiff (PAS) stained section showed clusters of PAS positive septate branching dark pigmented fungal hyphae (Fig. 2B).
Fig. 2.
A & B show tissue sections with inflammatory infiltrate and several fungal fragments appearing pink on H&E stained and dark pink on PAS stained sections. Bottom shows growth on SDA appearing whitish gray in 4days (C) followed by brownish black appearance on obverse and reverse black pigment (D).
In microbiology laboratory the tissue was subjected to Gram Staining, Ziehl Neelsen staining, 10% Potassium hydroxide (KOH) mount, bacterial culture and fungal culture. Gram stain showed only pus cells and no organisms, Ziehl Neelsen staining was negative for acid fast bacilli and bacterial culture was sterile after 48hrs of aerobic infection.
Potassium hydroxide mount of the tissue for fungal elements showed no hyphae or spores. Fungus culture on Saboraud dextrose agar (SDA) at 25 °C and 37 °C showed woolly, white to pinkish gray coloured colonies on D16; which turned olive brown and black later with reverse showing black pigment. (Fig. 2C & D).
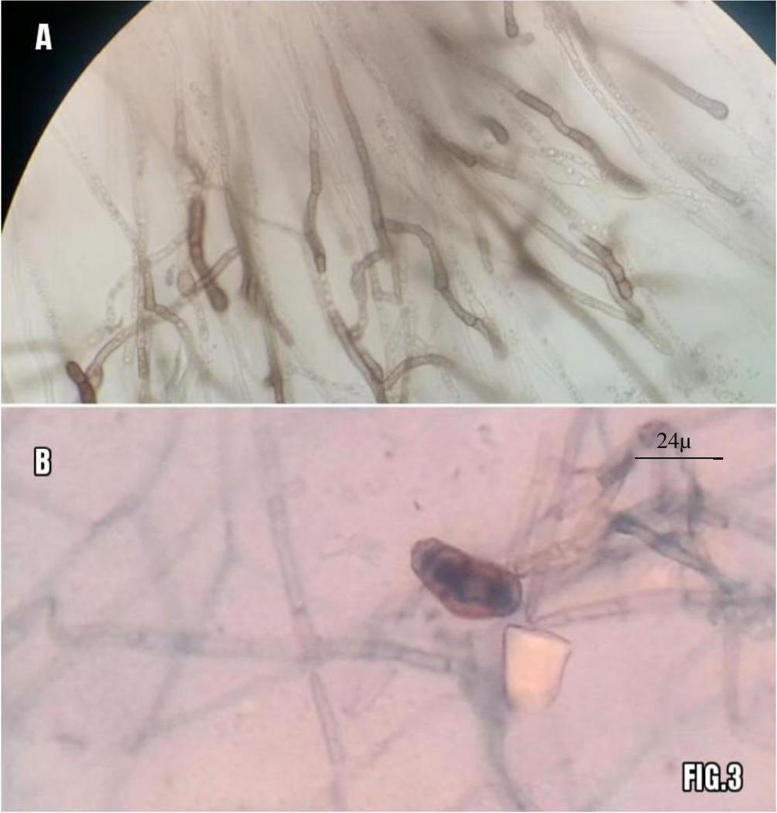
Lactophenol cotton blue mount (LCB) of the colonies on SDA showed septate hyphae, brown conidiophores, and conidia. The conidia were 3 distoseptate, ellipsoidal, disproportionately enlarged at the third cell 26 µ×12.5 µ and smooth. Conidia were sparse in culture. Based on the morphology on slide culture, the organism was presumptively identified as Curvularia lunata. (Fig. 3A&B).
Fig. 3.
shows slide culture appearance after 5days on top with brownish hyphae and conidiophores (A). LCB mount from culture in bottom shows with single brownish 3-distoseptate conidia and several brownish hyphae seen (B).
Identity of fungal isolate was confirmed on D45, by molecular testing at advanced reference centre for Mycology PGIMER Chandigarh. The product of amplification was 588 bp. Sequencing of internal transcribed spacer (ITS) region specific for Curvularia sp. was done by using ITS4 and ITS5 primers. The sequencing data aligned with C. lunata rRNA in NCBI gene bank (KX6103221) and resulted in a 98% identity match.
2.2. Differential diagnosis
In an adult, major differential diagnosis of low backache with paravertebral swelling closely mimic muscoloskeletal (degenerative facet joint disease, prolapsed intervertebral disk, vertebral fracture or collapse), malignancy (vertebral metastasis, myeloma), disseminated tuberculosis, granulomatous and/or abscess producing lesions of the spine.
Among spinal infections in addition to mycobacterial tubercular infection and brucellosis; fungal infections caused by coccidioidomycosis and blastomycosis are also important differentials. This above mentioned case was immunocompetent, relatively young and previously well, so malignancy was thought to be unlikely.
2.3. Postoperative treatment
Postoperatively the patient was not given any antibiotics. His symptoms were improving with partial regain of sensations. As the urinary incontinence continued the patient was catheterized. Patient was started physiotherapy from 9th postoperative day on D17. On same day culture report confirmed fungal isolate morphologically suggesting Curvularia lunata. So from D17 ( 9th postoperative day) the patient was started on voriconazole 400 mg stat followed by 200 mg daily for 3 weeks. Also liposomal amphotericin B was started, loading dose 35 mg IV over 4 h and maintenance dose of 15 mg IV over 2hrs daily for 2 weeks.
2.4. Outcome and follow up
On D48 patient was symptomatically better with improvement in gait and discharged. Patient presented with no sequelae, complications or recurrence during 7 month follow up period.
3. Discussion
Our case with an immunocompetent farmer coming from remote village of central India, presenting only backache as the predominant symptom leading to morbidity and affecting his livelihood; is very important as it emphasizes the need for thorough clinical and laboratory work up.
Spinal epidural abscess is a devastating disease with high morbidity and mortality. When identifying and treating patients presenting with acute or sub-acute back pain, we need to entertain the diagnosis of epidural abscess, particularly in the setting of any significant risk factors, and ruthlessly pursue proper work-up even when the classic triad of fever, spine pain, and neurologic deficit is not present. [6] Three ubiquitous species of Curvularia have been recovered from human infections. They are Curvularia lunata, Curvularia pallescens and Curvularia geniculata. Of these, Curvularia lunata is more commonly found in immunocompromised individuals. They are spread via inhalational or dermal inoculation routes. Thus, they are seen after corneal perforation or surgery, presence of peritoneal and venous catheters and in IV drug abusers. [7].
A fatal case of cerebral Curvularia infection in which there was no known history of immunocompromise or prior respiratory tract or sinus infection in the patient was reported by Carter E and Boudreaux C in 2004. Interestingly, infections with Curvularia do not appear to require an immunosuppressed host. [8] In our case also the patient was immunocompetent and had no prior infections. Most probable mode of infection could have been traumatic implantation as our patient is a farmer.
Curvularia and other dematiaceous fungi are seen in tissue sections as 2- to 6-um wide hyphal forms which differ greatly in length and may be fragmented. Vesicular swellings of hyphae producing bizarre hyphal outlines are common. Pigmentation of these classically brown hyphae may be variable in tissue sections. The fungal organisms may be associated with abscess cavities or with intense granulomatous inflammation. [8] Squash cytology and histopathology reports in our case showed similar findings.
Due to the ubiquitous nature of melanized fungi, examination of direct specimens is critical and the finding of fungal elements within tissue is required to document a black mold as the etiologic agent when recovered in culture. Conversely, recovery in culture without visualization in tissue should be interpreted with caution. Isolation of the same organism multiple times or from multiple sites also supports its role in disease when microscopic evidence is lacking. [9] In our case direct KOH mount showed no fungal elements whereas the haematoxylin & eosin (H&E) and PAS stain of the tissue showed pigmented fungal hyphae. Also culture isolation was same from all inoculated tubes and plates of SDA.
No standardized antifungal regimen exists in the treatment of CNS phaeohyphomycosis. Surgical resection in combination with amphotericin B and an azole, such as voriconazole, achieves good CNS penetration. [7], [10], [11] Limited experiences with flucytosine, terbinafine, and echinocandins have also been reported in several cases reports.
In an interesting case of 1979 a young immunocompetent footballer with presumptive traumatic cutaneous inoculation of Curvularia lunata causing leg ulcers progressed to disseminated infection as deep, soft tissue abscesses, pulmonary suppuration, paravertebral abscess and cerebral abscess. Patient's delay in receiving treatment lead to paravertebral–mediastinal–pleural-cutaneous fistula. Infection was arrested only by surgery emphasizing role of prompt and aggressive surgical drainage. [12].
Their study also showed Amphotericin B (1 mg/kg/day) as clearly beneficial but only after effective surgical drainage. Compliance and follow up of patient is equally important as their patient stopped taking medications and was bedridden with recurrence of infection. [12].
Our patient also responded to surgical and combination antifungal therapy of amphotericin B and voriconazole. Even after hospital discharge patient complied with regular follow up and did not present any sequelae or recurrence.
Conflict of interest
There are none.
Acknowledgements
Authors thank Dr. Arunaloke Chakrabarti, Professor and In-Charge, Center of Advance Research in Medical Mycology (WHO Collaborating Center for Reference and Research of Fungi of Medical Importance) PGIMER Chandigarh, India for his kind help in the final identification of the organism.
References
- 1.Chakrabarti Arunaloke. Epidemiology of central nervous system mycoses. Neurol. India. 2007;55(3) doi: 10.4103/0028-3886.35679. [DOI] [PubMed] [Google Scholar]
- 2.Gopinathan N.K., Sukumaran P.N. Phaeohyphomycosis presenting as a solitary nodulocystic lesion in a renal transplant patient. Indian Dermatol. Online J. 2015;6(5):359–361. doi: 10.4103/2229-5178.164468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skovrlj Branko, Haghighi Maryam. Curvularia abscess of the brainstem. World Neurosurg. 2014;82 doi: 10.1016/j.wneu.2013.07.014. (1/2:241) [DOI] [PubMed] [Google Scholar]
- 4.Madrid H. Novel Curvularia species from clinical specimens. Pers.: Mol. Ph. Evol. Fungi. 2014;33:48–60. doi: 10.3767/003158514X683538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darouiche R.O. Spinal epidural abscess. N. Engl. J. Med. 2006;355(19):2012–2020. doi: 10.1056/NEJMra055111. [DOI] [PubMed] [Google Scholar]
- 6.Haas Brian M., Yu Yen-Hua, Kim Jongoh. Morbidity and mortality Reports: delay in diagnosis of spinal epidural abscess. Neurol. Bull. 2011;3:18–24. [Google Scholar]
- 7.Deepu Alex, Dongmei Li, et.al. Identification of Curvularia lunata by polymerase chain reaction in a case of fungal endophthalmitis. Medical Mycology Case Reports, 2, 2013, 137–140. [DOI] [PMC free article] [PubMed]
- 8.Carter E., Boudreaux C. Fatal cerebral phaeohyphomycosis due to Curvularia lunata in an immunocompetent patient. J. Clin. Microbiol. 2004;42(11):5419–5423. doi: 10.1128/JCM.42.11.5419-5423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Revankar Sanjay G., Sutton Deanna A. Melanized fungi in human disease. Clin. Microbiol. Rev. 2010;23(4):884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.G.M. Cox, C.A. Kauffman, Central nervous system infections due to dematiaceous fungi (cerebral phaeohyphomycosis). UpToDate, 2012.
- 11.Johnson L.B., Kauffman C.A. Voriconazole: a new triazole antifungal agent. Clin. Inf. Dis. 2003;36:630–637. doi: 10.1086/367933. [DOI] [PubMed] [Google Scholar]
- 12.Rohwedder Disseminated Curvularia infection. Arch. Intern Med. 1979;139:940–941. [PubMed] [Google Scholar]