Abstract
Indifference or moderate antagonism of linezolid combined with other antibiotics in vitro and in vivo have mainly been reported in the literature. We have assessed the in vitro activities of linezolid, alone or in combination with imipenem, against methicillin-resistant Staphylococcus aureus (MRSA) strains using the dynamic checkerboard and time-kill curve methods. Linezolid and low concentrations of imipenem had a synergistic effect, leading us to evaluate the in vivo antibacterial activity of the combination using the rabbit endocarditis experimental model. Two MRSA strains were used for in vivo experiments: one was a heterogeneous glycopeptide-intermediate clinical S. aureus strain isolated from blood cultures, and the other was the S. aureus COL reference strain. Animals infected with one of two MRSA strains were randomly assigned to one of the following treatments: no treatment (controls), linezolid (simulating a dose in humans of 10 mg/kg of body weight every 12 h), a constant intravenous infusion of imipenem (which allowed the steady-state concentration of about 1/32 the MIC of imipenem for each strain to be reached in serum), or the combination of both treatments. Linezolid and imipenem as monotherapies exhibited no bactericidal activity against either strain. The combination of linezolid plus imipenem showed in vivo bactericidal activity that corresponded to a decrease of at least 4.5 log CFU/g of vegetation compared to the counts for the controls. In conclusion, the combination exhibited synergistic and bactericidal activities against two MRSA strains after 5 days of treatment. The combination of linezolid plus imipenem appears to be promising for the treatment of severe MRSA infections and merits further investigations to explore the mechanism underlying the synergy between the two drugs.
The prevalence of bacterial pathogens resistant to the available antibiotics has been increasing over the past several decades. This situation constitutes a major challenge for clinicians and microbiologists and is particularly acute for the treatment of infections caused by gram-positive organisms. Methicillin-resistant Staphylococcus aureus (MRSA) is an increasingly common cause of nosocomial infections, and the reduced susceptibilities of MRSA strains to glycopeptides emphasize the need for new therapeutic options for clinical practice (17).
Linezolid is an antimicrobial agent from the oxazolidinone class with potent activity against multidrug-resistant gram-positive pathogens, such as vancomycin-resistant Enterococcus faecalis and Enterococcus faecium, penicillin-resistant Streptococcus pneumoniae, and MRSA strains (46). Oxazolidinones are bacterial protein synthesis inhibitors that act by preventing the formation of the translation initiation complex (2, 38, 39). This mechanism of action is unique to this class of antimicrobials, with the benefit that cross-resistance with other antimicrobial agents has not yet been observed. Linezolid displays nonbactericidal, time-dependent activity in vitro against staphylococci (22, 36).
Antimicrobial combination therapy may be used to provide broad-spectrum coverage, prevent the emergence of resistant mutants, and obtain a synergy between both antimicrobial agents (10). Because bactericidal activity is considered important in the treatment of severe infections, such as endocarditis, the use of linezolid as monotherapy appears to be problematic and the use of combinations is recommended. Recently published studies have investigated the in vitro activities of linezolid in combination with different antimicrobial agents (15, 16, 18). From the results of those in vitro studies, no synergistic combination that included linezolid in combination with gentamicin, vancomycin, rifampin, ciprofloxacin, fusidic acid, or fosfomycin was observed. In fact, a trend for antagonism was noted when linezolid was combined with gentamicin (with the effect mainly being on the early bactericidal activity of the aminoglycoside), vancomycin, ciprofloxacin, and fosfomycin. In this context, new investigations into the effects of drugs in combination with linezolid may be worthwhile.
Imipenem is a broad-spectrum β-lactam antibiotic active against gram-positive and gram-negative bacteria. This drug is resistant to hydrolysis by most β-lactamases and exhibits a postantibiotic effect against gram-positive bacteria (27, 30). Although imipenem does not exhibit bactericidal activity against MRSA strains, many studies have reported on its efficacy when it is used in combination with other antimicrobial agents, including fosfomycin (11, 28), vancomycin (4, 35, 41), and cephalosporins (43). In this context, the use of unconventional combinations of drugs may prove to be an effective strategy for the management of MRSA infections (23).
The purposes of this study were (i) to evaluate the in vitro activities of linezolid combined with imipenem against MRSA strains and (ii) to determine the in vivo antibacterial effects of the combination by using the rabbit model of experimental endocarditis.
(This work was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 14 to 17 September 2003.)
MATERIALS AND METHODS
Bacterial strains.
Three MRSA strains (strains BCB8 and COL and a heterogeneous glycopeptide-intermediate S. aureus [hGISA] strain) and one methicillin-susceptible S. aureus strain (ATCC 29213) were used in this study. The BCB8 and hGISA strains were isolated from blood cultures. ATCC 29213 and COL were the reference strains.
Antibiotics.
Linezolid (research compound; Pharmacia Upjohn, Kalamazoo, Mich.), imipenem-cilastatin (clinical form; Merck Sharp & Dohme, Paris, France), and vancomycin and teicoplanin (clinical forms; Lilly, Saint Cloud, France) were provided by the manufacturers.
Medium.
Mueller-Hinton (MH) broth (Becton Dickinson, Le Pont de Clayes, France) supplemented with calcium (25 mg/liter) and magnesium (12.5 mg/liter) was used for susceptibility tests and killing curve experiments. Colony counts were determined with MH agar plates (Becton Dickinson).
Susceptibility testing.
The MICs of linezolid, vancomycin, teicoplanin, and imipenem for the four strains were determined in cation-supplemented MH broth by the microdilution technique (1, 29). Overnight MH broth cultures were used to prepare inocula of 105 CFU/ml. The MIC was defined as the lowest concentration of antimicrobial agent that prevented turbidity after 24 h of incubation at 37°C.
Checkerboard method.
The dynamic checkerboard method was performed in 96-well microtiter plates (Nalge Nunc International, Roskilde, Denmark) containing linezolid and imipenem at twofold dilutions dispensed in a checkerboard fashion (12). The final concentrations of both drugs (twofold dilutions) ranged from 1/8 to 8 times the MIC for linezolid and from 1/1,024 to 1 time the MIC for imipenem. Antibiotic dilutions were made in MH broth. Overnight cultures were used to yield a final inoculum of 5 × 106 to 1 × 107 CFU/ml. After inoculation and agitation, the microplates were incubated at 37°C for 24 h. Dilutions were made at 24 h by a micromethod with a multipipette. A total of 100 μl was removed from the first row of the microplate (MA) and placed in the first row of a second microplate (MD). Then, serial dilutions were performed in MD by taking 25 μl from the first row and placing it into 75 μl of diluent (MH broth) in the second row, and so on. The transfers were made by aspiration-compression with a multipipette. This operation was carried out for each row of MA such that a dilution microplate, MD, corresponded to each row. The contents of each microplate were then subcultured with a Steers multiple inoculator. The plates were incubated at 37°C for 24 h. Readings were then performed, and the number of bacteria was equal to the number of colonies observed multiplied by the final dilution coefficient. All results are expressed as log10 CFU per milliliter.
Time-kill curve studies.
For each strain, linezolid was studied at the MIC alone and in combination with imipenem at 1/512 and 1/32 the MIC. Time-kill curve studies were performed in MH broth in glass flasks with an inoculum of 5 × 106 to 1 × 107 CFU/ml in the presence of a single antibiotic or the combination of both antibiotics (31). A flask of inoculated MH broth with no antibiotic served as a control. The surviving bacteria were counted after 0, 3, 6, and 24 h of incubation at 37°C by subculturing 50-μl serial dilutions (in 0.9% sodium chloride) onto MH plates with a spiral plater (Spiral system; Interscience, Saint-Nom-La-Bretèche, France). The detection limit was 20 CFU/ml. A bactericidal effect was defined as a ≥3-log10 CFU/ml decrease after 24 h of incubation compared to the size of the initial inoculum. Synergism was defined as a decrease in the colony count of ≥2 log10 CFU/ml with the combination compared to the count obtained with the most active single drug. Antagonism was defined as an increase in the colony count of ≥2 log10 CFU/ml with the combination compared to the count obtained with the most active single antibiotic (24).
Endocarditis model.
The animals used for the endocarditis model were female New Zealand White rabbits (weight, 2.0 to 2.5 kg) housed in individual cages. They had free access to food and water. Studies with the experimental endocarditis model were performed as described previously (9, 33). All animal experimentation was approved by the Committee of Animal Ethics of the University of Nantes. To achieve valve injury, a polyethylene catheter was introduced into the left ventricle while the rabbits were under general anesthesia (intramuscular ketamine at 25 mg/kg of body weight). The catheter was left in place throughout the experiment. After 24 h of catheterization, each animal was inoculated intravenously with 1 ml of a bacterial solution (adjusted to 108 CFU/ml) of the COL or the hGISA MRSA strain (the two strains that were the most resistant to imipenem). Animals were randomly assigned to no treatment (controls), a continuous intravenous infusion of imipenem (15 and 100 mg/kg/day for animals infected with strains COL and hGISA, respectively), a linezolid dosing regimen that mimics the human dose of 10 mg/kg/12 h (intermittent dosing), or a combination of the two treatment regimens. To avoid stability problems with the continuous infusion of imipenem, the syringes were changed every 4 h. Control assays were performed at the end of the perfusion to validate the imipenem concentrations. A total dose of 70 mg/kg needed to be infused into the rabbit over a 12-h period in order to simulate the kinetics of a 10-mg/kg dose of linezolid in humans (i.e., 600 mg/12 h [the recommended dosing regimen]). Treatment was started 24 h after inoculation, and antibiotics were administered via the marginal ear vein. Animals were killed by administration of a 100-mg intravenous bolus of thiopental at the beginning of the treatment period (controls) or at the end of the 5-day treatment regimen. Aortic valve vegetations were excised; immediately placed on ice; and then weighed, homogenized in 0.5 ml of saline buffer, and plated on MH plates with a spiral plating system. Dilutions of 10−1, 10−2, and 10−4 were prepared to eliminate potential carryover effects. The viable counts after 24 h of incubation at 37°C were expressed as the mean ± standard deviation log10 CFU per gram of vegetation. The lower detection limit for this method is 1 CFU per 50 μl of undiluted vegetation homogenate.
Antibiotic concentrations in serum.
Blood samples were obtained through a catheter positioned in the median artery of the ear contralateral to the ear used for antibiotic infusion. For determination of the steady-state concentrations of imipenem, blood samples were taken at the end of the first day of treatment (i.e., after 24 h of perfusion). The serum samples were immediately mixed with an equal volume of a pH 6 stabilizing buffer, 2-(N-morpholino)ethanesulfonic acid, before they were frozen. All serum samples were frozen at −80°C until they were assayed. High-performance liquid chromatography was used to determine the concentrations of linezolid (lower detection limit, 0.1 mg/liter; coefficient of variation, <10%) (32) and imipenem (lower detection limit, 0.2 mg/liter; coefficient of variation, 4.6%) (26).
Statistics.
Statistical analysis was performed with StatView software (Abacus Concepts, Berkeley, Calif.). For each strain studied, analysis of variance was used to compare the effects between the different groups, followed by Scheffe's test to compare the treated groups two by two. A P value of ≤0.05 was considered significant.
RESULTS
Susceptibility testing.
The MICs for the S. aureus strains are summarized in Table 1. All strains were susceptible to linezolid. The MICs of vancomycin and teicoplanin were increased for the hGISA strain. The other strains were susceptible to glycopeptides.
TABLE 1.
MICs of study drugs for MRSA strains
| Antibiotic | MIC (mg/liter)
|
|||
|---|---|---|---|---|
| ATCC 29213 | BCB8 | COL | hGISA | |
| Linezolid | 2 | 2 | 2 | 1 |
| Vancomycin | 1 | 1 | 1 | 4 |
| Teicoplanin | 0.5 | 0.5 | 0.5 | 12 |
| Imipenem | 0.064 | 0.25 | 32 | 64 |
Dynamic checkerboard method.
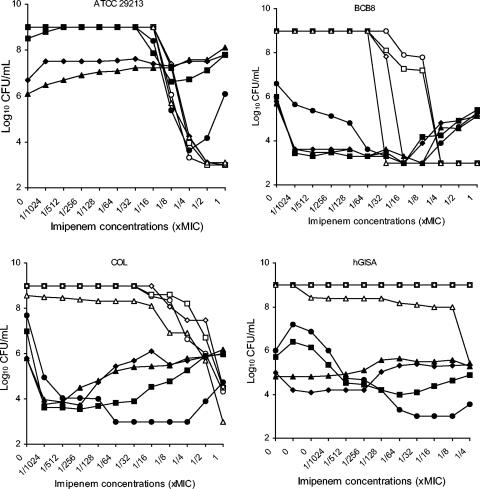
The dynamic checkerboard method was performed to evaluate the interaction of linezolid in combination with imipenem. The results for strains ATCC 29213, BCB8, COL, and hGISA at 24 h are shown in Fig. 1.
FIG. 1.
Results of dynamic checkerboard method at 24 h for strains ATCC 29213, BCB8, COL, and hGISA. The symbols for the different concentrations of linezolid are as follows: ○, no drug; □, 1/8 the MIC; ⋄, 1/4 the MIC; ▵, 1/2 the MIC; •, the MIC; ▪, 2 times the MIC; ♦, 4 times the MIC; ▴, 8 times the MIC.
Imipenem alone was active against the methicillin-susceptible S. aureus strain (ATCC 29213) at concentrations close to the MIC. The addition of increasing concentrations of linezolid decreased the antibacterial activity of imipenem, and antagonism was observed with imipenem concentrations of 1/8 to 1 time the MIC and linezolid concentrations from 1 to 8 times the MIC. For the MRSA strains, synergy was observed with linezolid (from the MIC) and low concentrations of imipenem. The use of higher concentrations of imipenem (i.e., concentrations close to the MIC) seemed to decrease the antibacterial activity of the combination.
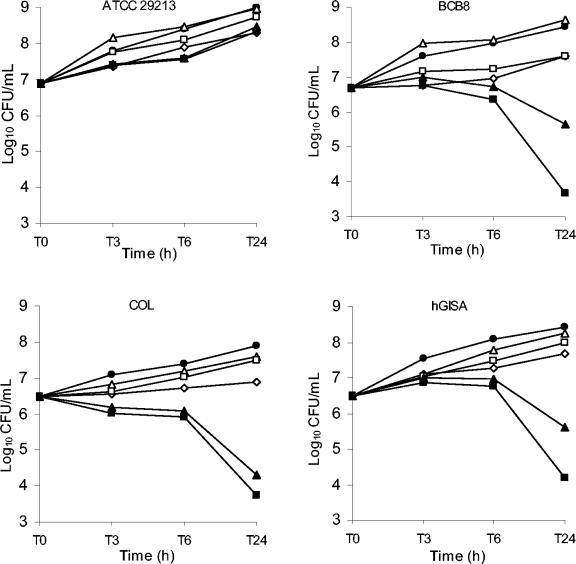
Time-kill studies.
The results of the time-kill studies are shown in Fig. 2. As expected from the results described above, no synergy was observed with linezolid and imipenem against methicillin-susceptible strain ATCC 29213. Linezolid (at the MIC) combined with low concentrations of imipenem (1/32 the MIC) showed synergy against all the MRSA strains tested.
FIG. 2.
Killing curves for linezolid alone and linezolid in combination with imipenem against ATCC 29213, BCB8, COL, and hGISA strains. •, control; ⋄, linezolid at the MIC; ▵, imipenem at 1/512 the MIC; □, imipenem at 1/32 the MIC; ▴, linezolid at the MIC plus imipenem at 1/512 the MIC; ▪, linezolid at the MIC plus imipenem at 1/32 the MIC.
Endocarditis model.
The results of the in vivo study are shown in Table 2. Linezolid significantly reduced the counts of both strains compared to those of the controls but failed to exhibit bactericidal activity when it was used alone, despite 5 days of treatment. The imipenem regimens (15 and 100 mg/kg/day) showed no or very low levels of activity against these MRSA strains. The combination exhibited bactericidal and synergistic activities against both MRSA strains, with a 5-log10 CFU/g decrease compared to the counts for the controls.
TABLE 2.
Bacterial titers in vegetations after 5 days of treatment
| Regimen | Mean ± SD log10 CFU/g of vegetation (no. of animals)
|
Imipenem concn (mg/liter) | |
|---|---|---|---|
| COL | hGISA | ||
| Control | 9.1 ± 0.7 (9) | 8.6 ± 0.4 (6) | |
| Linezolid (10 mg/kg/12 h)c | 6.4 ± 1.0 (8)a | 6.1 ± 0.4 (5)a | |
| Imipenem (15 mg/kg/day) | 9.4 ± 0.4 (5) | NDd | 0.45 ± 0.2 |
| Imipenem (100 mg/kg/day) | ND | 8.0 ± 0.3 (5) | 2.30 ± 0.9 |
| Linezolid + imipenem | 3.8 ± 0.9 (6)a,b | 3.7 ± 0.5 (5)a,b | |
P < 0.0001 versus controls.
P < 0.0001 versus linezolid and imipenem treatment by Scheffe's test after analysis of variance.
Simulated dose for humans.
ND, not done.
Antibiotic concentrations in serum.
After administration of a linezolid dose that simulated a 10-mg/kg dose in humans, the corresponding mean peak concentration, area under the curve, and half-life were 11.9 ± 1.1 mg/liter, 76.3 ± 5.9 mg · h/liter, and 2.7 ± 0.1 h, respectively, after administration of the first dose and 21.5 ± 1.3 mg/liter, 152.1 ± 9.2 mg · h/liter, and 3.4 ± 0.7 h, respectively, at day 5. For the imipenem-treated animals, the steady-state concentrations were 0.45 ± 0.2 and 2.30 ± 0.9 mg/liter for the 15- and 100-mg/kg/day regimens, respectively.
DISCUSSION
Linezolid plays an important role in the treatment of infections due to MRSA strains (46). Despite the reported low frequencies of mutation to linezolid in vitro, the development of resistance among both Enterococcus (3, 14, 21) and Staphylococcus (42, 45) species strains during linezolid therapy has been described previously. Moreover, nosocomial transmission of linezolid-resistant strains remains a possibility (34). Combination therapies that include linezolid could be used (i) to protect the ability of linezolid to be used in the future in particular and the oxazolidinone class of antimicrobial agents in general and (ii) to increase the early in vivo activity of the drug. Many in vitro studies with combinations that include linezolid have been published, but only a few studies have investigated the activities of linezolid in combination with other antimicrobial agents in vivo (6, 7, 20). Although those studies showed the efficacy of linezolid in combination with rifampin or gentamicin, the search for effective and bactericidal combinations (including linezolid) for the treatment of severe multiresistant S. aureus infections is ongoing.
The first part of this study evaluated the in vitro antibacterial activity of linezolid combined with imipenem. Two laboratory methods were used to determine the interactions between the drugs.
Time-kill curve experiments are frequently used to assess that activities of antimicrobial combinations in vitro; however, the number of antimicrobial concentrations and combinations that can be tested are limited. Therefore, we used the dynamic checkerboard method as a screen to determine the pertinent linezolid and imipenem concentrations to be tested by the time-kill curve method. We found that sub-MICs of imipenem in combination with linezolid showed the maximal bactericidal activity. The use of higher drug concentrations seemed to decrease the antibacterial activity of the combination, as was observed against BCB8 and COL strains (Fig. 1), suggesting a slight antagonism between the drugs under these conditions. Interestingly, the linezolid and imipenem combination results in an indifferent or antagonistic interaction against a methicillin-susceptible S. aureus strain (ATCC 29213). Although only one methicillin-susceptible strain was tested in this study, this observation suggests that the synergy observed against MRSA strains may be related to methicillin resistance. Methicillin resistance in S. aureus is mediated by a chromosomally located resistance determinant, mecA, which encodes a low-affinity penicillin-binding protein (PBP) named PBP 2a or PBP 2′ (13). Matsuda et al. (25) showed that in MRSA strains imipenem preferentially binds to PBP 4 and PBP 1 and then to PBP 3 and PBP 2. Subinhibitory concentrations of imipenem could interfere with only one PBP and not all PBPs, as demonstrated in a study with Escherichia coli (37). Satta et al. (37) showed with E. coli that at low concentrations of imipenem only one PBP (PBP 2) was saturated and that at concentrations exceeding the MIC all PBPs were saturated. The effective concentrations of imipenem in this study (i.e., sub-MICs of about 1/8 to 1/512 the MIC) suggest that the classical PBP-binding-mediated activity of imipenem is probably not involved in the synergy between the two antimicrobial agents. Indeed, subinhibitory concentrations of antibiotics could interfere with many critical processes, including phagocytosis (enhancement of bacterial susceptibility to host immune defenses), surface adherence, alteration of the bacterial cell wall, and toxin production. Several virulence-associated determinants of S. aureus are affected by low levels of different antibiotics in vitro (8). Studies are in progress to explore the mechanism of action involved in the synergy between linezolid and imipenem and to investigate the possible roles of PBPs and autolysins.
Among the potential antibiotics that can be combined with linezolid for the treatment of MRSA infections, imipenem is certainly not the most obvious choice because of its lack of activity against MRSA strains. Nevertheless, Garcia de la Maria et al. (11) reported on the efficacy of fosfomycin combined with imipenem against an MRSA strain in an experimental endocarditis model. Another study showed the efficacy of vancomycin in combination with imipenem against MRSA strains both in vitro and in vivo (41). Sweeney et al. (40) have investigated the in vitro activities of linezolid combined with 35 antimicrobial agents against staphylococci and enterococci using the checkerboard method and determination of the fractional inhibitory concentration. Although the combinations predominantly showed indifference against MRSA strains, linezolid combined with imipenem was synergistic against a vancomycin-resistant E. faecium strain and linezolid plus amoxicillin was synergistic against three MRSA strains. These results reinforce the interest in β-lactam antibiotics combined with linezolid.
In vitro determination of drug-drug interactions is always difficult. Because of the lack of a correlation between different methods for assessment of the activities of antibiotic combinations, in vivo experimental models are required to confirm the interactions observed in vitro. The experimental endocarditis model is particularly useful for testing the in vivo activities of new drugs or antibiotic combination regimens. Computer-controlled simulation of linezolid in rabbits at concentrations that mimic the kinetic profile of the drug in humans was used in the present study owing to the very short spontaneous half-life of the drug in rabbits (about 30 min) (5).
The aim of using continuous imipenem infusion instead of intermittent dosing in this study was to obtain an in vivo steady-state concentration that mimics the in vitro conditions so that synergy was observed as soon as possible (i.e., to achieve a target concentration of 1/32 the MIC for each strain). Practical problems that must be considered with this type of treatment include the stability of the drug at room temperature during the infusion. The continuous infusion of imipenem is not often used because of its instability (44). To avoid stability problems, imipenem syringes were changed every 4 h in our animal model.
The purpose of the second part of this study was to evaluate the in vivo therapeutic efficacy of the combination of linezolid plus imipenem compared to that of either antibiotic used alone. In a previous study with linezolid in a rabbit model of methicillin-resistant S. aureus endocarditis (19), we showed that linezolid significantly reduced the numbers of S. aureus cells in aortic valve vegetations but failed to exhibit a bactericidal effect, despite 5 days of treatment. The present study yielded the same results with linezolid therapy against both MRSA strains studied. Continuous infusion of imipenem showed no or a very low level of activity against both MRSA strains, confirming the failure of this agent alone against methicillin-resistant strains. The combination of linezolid plus imipenem exhibited bactericidal and synergistic activities against both MRSA strains, with at least a 4-log10 CFU/g decrease compared to the counts for the controls.
Although the continuous infusion of imipenem was effective here, the activities of other imipenem dosages in combination with linezolid against MRSA strains need to be evaluated, including intermittent dosing (i.e., every 6 to 8 h) and the standard dosage (i.e., 2 to 4 g/day). Assessment of the in vitro and in vivo activities of linezolid with other β-lactam antibiotics (oxacillin, cefamandole, etc.) should be of great interest (i) to determine if the synergy with β-lactams is a general phenomenon or one specific to imipenem and (ii) to help understand the mechanism of action of the synergy between linezolid and imipenem drugs.
Conclusion.
The present study demonstrates that linezolid in combination with subinhibitory concentrations of imipenem can be bactericidal against MRSA strains after 5 days of treatment. The concentrations of both antibiotics used are clinically achievable. Linezolid plus imipenem appears to be a promising combination for the treatment of severe MRSA infections and merits further investigation, especially to determine the mechanism of action involved in the synergistic interaction between these drugs. The clinical significance of these findings should be evaluated.
REFERENCES
- 1.Amsterdam, D. 1996. Susceptibility testing of antibiotics in liquid media, p. 52-111. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 2.Aoki, H., L. Ke, S. M. Poppe, T. J. Poel, E. A. Weaver, R.C. Gadwood, R. C. Thomas, D. L. Shinabarger, and M. C. Ganoza. 2002. Oxazolidinone antibiotics target the P site on Escherichia coli ribosomes. Antimicrob. Agents Chemother. 46:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassetti, M., P. A. Farrel, D. A. Callan, J. E. Topal, and L. M. Dembry. 2003. Emergence of linezolid-resistant Enterococcus faecium during treatment of enterococcal infections. Int. J. Antimicrob. Agents 21:593-594. [DOI] [PubMed] [Google Scholar]
- 4.Benquan, W., T. Yingchun, Z. Kouxing, Z. Tiantuo, Z. Jiaxing, and T. Shuqing. 2002. Staphylococcus heterogeneously resistant to vancomycin in China and antimicrobial activities of imipenem and vancomycin against it. J. Clin. Microbiol. 40:1109-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugnon, D., G. Potel, J. Caillon, D. Baron, H. B. Drugeon, P. Feigel, and M. F. Kergueris. 1998. In vivo simulation of human pharmacokinetics in the rabbit. Bull. Math. Biol. 60:545-567. [DOI] [PubMed] [Google Scholar]
- 6.Chiang, F. Y., and M. Climo. 2003. Efficacy of linezolid alone or in combination with vancomycin for treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3002-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dailey, C. F., P. J. Pagano, L.V. Buchanan, J.A. Paquette, J.V. Haas, and J. K. Gibson. 2003. Efficacy of linezolid plus rifampin in an experimental model of methicillin-susceptible Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 47:2655-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doss, S. A., G. S. Tillotson, and S. G. Amyes. 1993. Effect of subinhibitory concentrations of antibiotics on the virulence of Staphylococcus aureus. J. Appl. Bacteriol. 75:123-128. [DOI] [PubMed] [Google Scholar]
- 9.Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis. II. Survival of bacteria in endocardial vegetations. Br. J. Exp. Pathol. 53:50-53. [PMC free article] [PubMed] [Google Scholar]
- 10.Eliopoulos, G. M., and R. C. Moellering, Jr. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 11.Garcia de la Maria, C., F. Marco, J. M. Miro, Y. Armero, M. P. Jimenez-Alzate, M. E. Diaz, A. Moreno, et al. 2003. Efficacy of fosfomycin plus imipenem or ceftriazone combination in the treatment of experimental endocarditis due to methicillin resistant or glycopeptide intermediate resistant Staphylococcus aureus, abstr. B-1091, p. 58. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 12.Garrod, L. P., and P. M. Waterworth. 1962. Methods of testing combined antibiotic bactericidal action and the significance of the results. J. Clin. Pathol. 15:328-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgopapadakou, N. H., S. A. Smith, and D. P. Bonner. 1982. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific β-lactam antibiotics. Antimicrob. Agents Chemother. 22:172-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 15.Grif, K., M. P. Manfred, K. Pfaller, P. A. Miglioli, and F. Allerberger. 2001. In vitro activity of fosfomycin in combination with various antistaphylococcal substances. J. Antimicrob. Chemother. 48:209-217. [DOI] [PubMed] [Google Scholar]
- 16.Grohs, P., M. D. Kitzis, and L. Gutmann. 2003. In vitro bactericidal activities of linezolid in combination with vancomycin, gentamicin, ciprofloxacin, fusidic acid, and rifampin against Staphylococcus aureus. Antimicrob. Agents Chemother. 31:533-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 18.Jacqueline, C., J. Caillon, V. Le Mabecque, A. F. Miègeville, P. Y. Donnio, D. Bugnon, and G. Potel. 2003. In vitro activity of linezolid alone and in combination with gentamicin, vancomycin or rifampin against methicillin-resistant Staphylococcus aureus by time-kill curve methods. J. Antimicrob. Chemother. 51:857-864. [DOI] [PubMed] [Google Scholar]
- 19.Jacqueline, C., E. Batard, L. Perez, D. Boutoille, A. Hamel, J. Caillon, M. F. Kergueris, G. Potel, and D. Bugnon. 2002. In vivo efficacy of continuous infusion versus intermittent dosing of linezolid compared to vancomycin in a methicillin-resistant Staphylococcus aureus rabbit endocarditis model. Antimicrob. Agents Chemother. 46:3706-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacqueline, C., N. Asseray, E. Batard, V. Le Mabecque, M. F. Kergueris, L. Dube, D. Bugnon, G. Potel, and J. Caillon. 2004. In vivo efficacy of linezolid combined with gentamicin for treatment of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 24:393-396. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, A. P., L. Tysall, M. V. Stockdale, N. Woodford, M. E. Kaufmann, M. Warner, D. M. Livermore, F. Asboth, and F. J. Allerberger. 2002. Emerging linezolid-resistant Enterococcus faecalis and Enterococcus faecium isolated from two Austrian patients in the same intensive care unit. Eur. J. Clin. Microbiol. Infect. Dis. 21:751-754. [DOI] [PubMed] [Google Scholar]
- 22.Kaatz, G. W., and S. M. Seo. 1996. In vitro activities of oxazolidinone compounds U100592 and U100766 against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 40:799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le, T., and A. S. Bayer. 2003. Combination antibiotic therapy for infective endocarditis. Clin. Infect. Dis. 36:615-621. [DOI] [PubMed] [Google Scholar]
- 24.Lorian, V. 1991. Laboratory methods used to assess the activity of antimicrobial combinations, p. 434-444. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. The Williams & Wilkins Co., Baltimore, Md.
- 25.Matsuda, K., K. Nakamura, Y. Adachi, M. Inoue, and M. Kawakami. 1995. Autolysis of methicillin-resistant Staphylococcus aureus is involved in synergism between imipenem and cefotiam. Antimicrob. Agents Chemother. 39:2631-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers, C. M., and J. L. Blumer. 1984. Determination of imipenem and cilastatin in serum by high-pressure liquid chromatography. Antimicrob. Agents Chemother. 26:78-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadler, H. I., D. H. Pitkin, and W. Sheikh. 1989. The postantibiotic effect of meropenem and imipenem on selected bacteria. J. Antimicrob. Chemother. 24(Suppl. A):225-231. [DOI] [PubMed] [Google Scholar]
- 28.Nakazawa, H., Y. Kikuchi, T. Honda, T. Isago, and M. Nozaki. 2003. Enhancement of antimicrobial effects of various antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) by combination with fosfomycin. J. Infect. Chemother. 9:304-309. [DOI] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.Odenholt-Tornqvist, I., E. Lowdin, and O. Cars. 1997. Comparative in vitro pharmacodynamics of BO-2727, meropenem and imipenem against gram-positive and gram-negative bacteria. Clin. Microbiol. Infect. 3:73-81. [DOI] [PubMed] [Google Scholar]
- 31.Pearson, R. D., R. T. Steigbigel, H. T. Davis, and S. W. Chapmann. 1980. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 18:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng, G. W., R. P. Stryd, S. Murata, M. Igarashi, K. Chiba, H. Aoyama, M. Aoyama, T. Zenki, and N. Ozawa. 1999. Determination of linezolid in plasma by reversed-phase high-performance liquid chromatography. J. Pharm. Biomed. Anal. 20:65-73. [DOI] [PubMed] [Google Scholar]
- 33.Perlman, B. B., and L. R. Freedman. 1971. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J. Biol. Med. 44:206-213. [PMC free article] [PubMed] [Google Scholar]
- 34.Rahim, S., S. K. Pillai, H. S. Gold, L. Venkataraman, K. Inglima, and R. A. Press. 2003. Linezolid-resistant, vancomycin-resistant Enterococcus faecium infection in patients without prior exposure to linezolid. Clin. Infect. Dis. 36:146-148. [DOI] [PubMed] [Google Scholar]
- 35.Rochon-Edouard, S., M. Pestel-Caron, J. F. Lemeland, and F. Caron. 2000. In vitro synergistic effects of double and triple combinations of beta-lactams, vancomycin, and netilmicin against methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 44:3055-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rybak, M. J., D. M. Cappelletty, T. Moldovan, J. R. Aeschlimann, and G. W. Kaatz. 1998. Comparative in vitro activities and postantibiotic effects of the oxazolidinone compounds eperezolid (PNU-100592) and linezolid (PNU-100766) versus vancomycin against Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and Enterococcus faecium. Antimicrob. Agents Chemother. 42:721-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satta, G., G. Cornaglia, A. Mazzariol, G. Golini, S. Valisena, and R. Fontana. 1995. Target for bacteriostatic and bactericidal activities of β-lactam antibiotics against Escherichia coli resides in different penicillin-binding proteins. Antimicrob. Agents Chemother. 39:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shinabarger, D. L., K. R. Marotti, R. W. Murray, A. H. Lin, E. P. Melchior, S. M. Swaney, D. S. Dunyak, W. F. Demyan, and J. M. Buysse. 1997. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob. Agents Chemother. 41:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swaney, S. M., H. Aoki, M. C. Ganoza, and D. L. Shinabarger. 1998. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 42:3251-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweeney, M. T., and G. E. Zurenko. 2003. In vitro activities of linezolid combined with other antimicrobial agents against staphylococci, enterococci, pneumococci, and selected gram-negative organisms. Antimicrob. Agents Chemother. 47:1902-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Totsuka, K., M. Shiseki, K. Kikuchi, and Y. Matsui. 1999. Combined effects of vancomycin and imipenem against methicillin-resistant Staphylococcus aureus in vitro and in vivo. J. Antimicrob. Chemother. 44:455-460. [DOI] [PubMed] [Google Scholar]
- 42.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, and R. C. Moellering, Jr. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 43.Uete, T., and K. Matsuo. 1995. Synergistic enhancement of in vitro antimicrobial activity of imipenem and cefazolin, cephalothin, cefotiam, cefamandole or cefoperazone in combination against methicillin-sensitive and -resistant Staphylococcus aureus. Jpn. J. Antibiot. 48:402-408. [PubMed] [Google Scholar]
- 44.Viaene, E., H. Chanteux, H. Servais, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2002. Comparative stability studies of antipseudomonal β-lactams for potential administration through portable elastomeric pumps (home therapy for cystic fibrosis patients) and motor-operated syringes (intensive care units). Antimicrob. Agents Chemother. 46:2327-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson, P., J. A. Andrews, R. Charlesworth, R. Walesby, M. Singer, D. J. Farrell, and M. Robbins. 2003. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51:186-188. [DOI] [PubMed] [Google Scholar]
- 46.Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kilburn, S. E. Glickman, D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]