Abstract
Background
Obese ZSF-1 rats display many features of human type II diabetes including nephropathy (DN). The study aimed to further understand the relevance of this model to DN, for which glomerular filtration rate (GFR), renal fibrosis and several urinary/tissue biomarkers was followed over 24 weeks in ZSF-1 rats.
Methods
Intact/sham or uninephrectomized male and female ZSF-1 rats were studied. GFR was measured by transdermal clearance of fluorescein isothiocyanate-sinistrin. Urine was collected every 2–4 weeks for biomarker analysis. Renal tissue was examined histologically for fibrosis and for levels of inflammatory and fibrotic genes.
Results
Male obese ZSF-1 rats demonstrated metabolic syndrome and proteinuria. Female counterparts were hyperlipidemic with delayed proteinuria, but were not hyperglycemic. Kidney hyperfiltration was observed in male obese rats in weeks 2–4 after surgery, and subsequently declined to levels significantly lower than controls. Tubulointerstitial/glomerular fibrosis in male obese rats was significantly elevated by week 12 post surgery and continued to expand in the ensuing weeks, particularly in uninephrectomized rats. Female rats had less severe fibrosis. Except for epidermal growth factor which decreased, the levels of several key inflammatory, injury and fibrotic factors were elevated in both tissue (mRNA) and urine (protein) of male obese rats.
Conclusion
Male obese ZSF-1 rats represent an important DN model, manifesting key pathophysiological features including metabolic syndrome, proteinuria, progressive tubular and glomerular fibrosis, and transient hyperfiltration followed by progressive decline in renal function. Uninephrectomy significantly accelerated disease progression. Females were less severe in disease manifestation. Several urinary and tissue biomarkers were identified in the male obese rats that tracked with disease progression.
Key Words: Diabetic nephropathy, Diabetes, ZSF-1 rats, Glomerular filtration rate, Fibrosis, Urinary biomarkers
Introduction
Diabetes mellitus (DM) is becoming increasingly prevalent worldwide, with type 2 DM being the more predominant form in diabetic patients. Approximately 20–40% of patients with DM ultimately develop diabetic nephropathy (DN), which is the most common cause of chronic kidney disease [1,2,3,4]. However, current therapeutic strategies are far from being optimally effective as no available treatment successfully prevents or halts DN, and these patients often progress to end-stage renal disease (ESRD). Therefore, there is a growing unmet medical need to identify novel pharmacological approaches to effectively prevent DN or retard/stop its progression to ESRD.
DN is characterized by the accumulation of extracellular matrix leading to glomerulosclerosis and tubulointerstitial (TI) fibrosis that finally drives the progressive decline of renal function. However, a suitable preclinical animal model that mimics human DN has been lacking. Most animal models, particularly mouse models, do not manifest TI fibrosis and do not develop a progressive decline in renal function, although most show proteinuria/albuminuria and glomerular pathology [5,6,7,8,9]. The diabetic obese ZSF-1 rat is a relatively new animal model of type II DN that displays many clinical features of human disease [5,10,11]. ZSF-1 rats are bred by crossing lean female Zucker diabetic fatty (ZDF) rats with lean male spontaneously hypertensive heart failure rats (SHHRs) [10]. Both lean and obese animals inherit the gene for hypertension from the SHHR strain and have similarly elevated blood pressure, but only obese ZSF-1 rats develop hyperlipidemia, hyperglycemia and renal dysfunction [5,10,11]. Obese ZSF-1 rats at 20 weeks of age showed renal histologic changes consistent with early DN, including arteriolar thickening, tubular dilation and atrophy, glomerular basement membrane thickening and mesangial expansion [5]. Although ZSF-1 rats have been reported to share many of the features of human DN, the temporal progression of fibrosis and related changes to kidney function in non-anesthetized animals have not been characterized [5,10,11,12].
There has been significant emphasis on identifying urinary biomarkers in DN patients that may help stratify patient population, alert to early disease or disease progression, and/or indicate therapeutic efficacy. Currently, there are a number of novel candidate urinary biomarkers that show association with DN pathophysiology and may also have potential prognostic value for the early assessment of decline in renal function [13,14,15,16]. Measurement of these markers in animal models would aid translation between preclinical and clinical studies, facilitate mechanistic understanding of the disease, and contribute to the pharmacodynamic assessment of novel therapeutics. There are limited data on urinary biomarkers in the ZSF-1 model.
In this study, we further characterized the male ZSF-1 model by measuring the glomerular filtration rate (GFR) in unanesthetized rats, along with interstitial and glomerular fibrosis, and a variety of urinary and tissue biomarkers (including injury, inflammatory and fibrotic markers) longitudinally over a period of up to 24 weeks (starting at 8 weeks of age). As part of this characterization, these factors were compared between ZSF-1 animals that were intact or had a uninephrectomy (Unx) of the right kidney at week 8 of age. Additionally, as there is very little data on the progression of disease in female ZSF-1 rats, we also characterized differences and similarities between the genders as well.
Methods
Animals
All animal procedures were approved by AbbVie's Institutional Animal Care and Use Committee (IACUC) and were performed in accordance with IACUC guidelines. Obese ZSF-1 rats and lean littermates were bred by crossing female ZDF rats with male SHHRs at the Charles River Laboratories (Wilmington, Mass., USA). All rats were housed individually and fed a high carbohydrate diet (Purina 5008 rodent diet, Purina Mills, Mo., USA). At 8 weeks of age, animals underwent either a sham or a right Unx surgery. Rats that underwent a sham surgery and thus retained both kidneys were designated as 2K rats. Correspondingly, rats that underwent surgery for an Unx and had only one kidney during the study were designated as 1K rats.
Experimental Groups
We conducted 3 sets of experiments that measured multiple end points over the course of the study. Male obese ZSF-1 rats and the lean controls were characterized in one study over a period of 12 weeks (1K only) and in another study over a period of 24 weeks (1K and 2K). In both these studies, animals were 8 weeks of age at the start of the study. In the 12-week study, animals were sacrificed at different time points to examine the development of kidney fibrosis. In the third study, female ZSF-1 rats (1K only) were observed for 24 weeks starting at week 8 or 9 of age. In the 24-week studies (male and female), renal fibrosis was only measured at the end of the 24 weeks.
Urine and Blood Analysis
Urine samples were collected over a 24-hour period while animals were in metabolism cages once every 2–4 weeks from the start of the study. Blood samples were collected via tail vein for blood chemistry (glucose, triglyceride (TG) and cholesterol) at the end of each metabolism cage collection. Conventional urine chemistry (protein, glucose and creatinine) and blood chemistry indices were measured in fresh samples on Abbott Architect C16000 Chemistry Analyzer using standard clinical chemistry procedures (Abbott, Ill., USA). Additional aliquots of urine samples were stored at −80°C for subsequent analysis.
Most urinary biomarkers were analyzed using either the Bio-plex® 3D suspension array system (Bio-Rad, Hercules, Calif., USA) or the EnVision® Multilabel Reader (PerkinElmer, Waltham, Mass., USA). Clusterin, kidney injury molecule-1 (KIM-1), tissue inhibitor of metalloproteinase 1 (TIMP-1), vascular endothelial growth factor (VEGF), α1-acid glycoprotein (α1-AGP), cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), epidermal growth factor (EGF), tumor necrosis factor alpha (TNF-α), soluble TNF receptor type 1 (sTNFR1) and β2-microglobulin (β2-MG) were detected with MILLIPLEX MAP Rat Kidney Toxicity Magnetic Bead Panels (EMD Millipore, Billerica, Mass., USA). Transforming growth factor (TGF)-β1, TGF-β2 and TGF-β3 levels were detected using Bio-Plex Pro™ TGF-β assay (Bio-Rad, Hercules, Calif., USA). Collagen IV was measured using Rat Collagen Type IV ELISA Kits (MyBioSource, San Diego, Calif., USA). Liver-type fatty acid binding protein (L-FABP) was measured by using Mouse/Rat FABP1/L-FABP Immunoassay kits (R&D Systems, Minneapolis, Minn., USA). Manufacturers' procedures were followed for each type of assay kit. All urinary biomarkers were normalized by 24-hour urine volume and expressed as total amount per 24 h (i.e., urinary excretion rate).
Measurement of GFR
As previously described by Schock-Kusch et al. [17] and Cowley et al. [18], GFR was measured by measuring the subcutaneous clearance of fluorescein isothiocyanate (FITC)-sinistrin with a novel miniature device (NIC-kidney) from Mannheim Pharma & Diagnostics (Mannheim, Germany). FITC-sinistrin is a fluorescent-labeled pharmaceutical ingredient of a commercially available marker of GFR. The miniature device has an optical component, which consists of 2 emitting diodes that transcutaneously excite FITC-sinistrin at 480 nm and a photodiode to detect the emitted signal at 521 nm [17]. It also has a microprocessor for signal amplification/digitization and temporary data storage. The device was affixed to a depilated region of the back using a double-sided sticky patch and was further held in place with a piece of elastic dressing retainer (Tetra-Net, Ill., USA) surrounding the animal body. The NIC-kidney device was powered by a rechargeable battery that was held in the elastic dressing retainer during recording. After a resting baseline period of 2–5 min, a bolus of FITC-sinistrin (25–50 mg/kg, dissolved in saline) was injected via tail vein. Device mounting (including skin preparation) and tail vein injection were completed under a brief (<10 min) light anesthesia with 2% isoflurane. The excretion kinetics of FITC-sinistrin was recorded in conscious animals for 120 min as a decay of subcutaneous fluorescence of FITC-sinistrin with a sampling rate of 60 measurements per minute and an excitation time of 10 ms per measurement. Elimination half-life (t1/2) was determined using an established 1-compartment model and then the t1/2 was converted into GFR using an empirical formula as previously described [17,18].
TI Fibrosis
For evaluation of TI fibrosis, formalin-fixed kidney tissues were sectioned longitudinally at 5 μm thickness and then stained with a modified picrosirius red (PSR) staining in which tissue sections were pre-treated in 0.2% aqueous phosphomolybdic acid [19,20]. The PSR-stained sections were examined and 10× photomicrographs captured with a BX-51 fluorescence microscope (Olympus) using a TRITC filter set (excitation 545 nm, LP570 nm dichroic mirror, 610 nm suppression filters). This fluorescence method has been shown to be superior to bright-field techniques in distinguishing fine fibrous structures [20]. For each rat, 3 images from the outer cortex were captured and stored as TIFF files (one from each pole and one from the medial third of the organ). Image analysis was subsequently performed using the ImageSense software (Olympus). TI fibrotic percentage was defined as the percentage of positive stained area above an empirically derived detection threshold, but excluding the area occupied by glomeruli and blood vessels. The same parameters for microscopy and image analysis were uniformly applied to all images. The mean TI fibrosis percentage derived from the 3 images served as the value for each subject.
Glomerulosclerosis
To evaluate glomerulosclerosis, formalin-fixed paraffin-embedded kidney sections were cut at 5 μm thickness and stained with periodic acid-Schiff reagent. For each animal, at least 50 glomeruli from continuous fields were assessed. Each glomerulus was graded on a 0–4 scale, which represents the sclerotic area involving 0, 1–25, 26–50, 51–75 or >75% of the glomerulus. Scores for all of the glomeruli assessed were averaged and defined as the glomerulosclerosis index (GSI) for each animal [21,22].
Total Collagen Content
Total collagen content in the kidney was determined by hydroxyproline quantification (QuickZyme Biosciences). Briefly, cortical kidney tissue was hydrolyzed using 6 M HCl at 95°C for 20 h, and the diluted (1:10) supernatant was analyzed according to the manufacturer's instructions. Results are expressed as micrograms of hydroxyproline per milligram of kidney tissue.
Analysis of Gene Expression
Total RNA was isolated from kidney cortex using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, Calif., USA) according to the manufacturer's instruction. TaqMan real-time quantitative PCR was performed using the BioRad CFX384 Touch System (BioRad, Hercules, Calif., USA) by following the supplier's instruction for the assay kits. All TaqMan regents including primers were purchased from the Applied Biosystems (Foster City, Calif., USA). Hypoxanthine phosphoribosyltransferase I (HPRT1) mRNA was used as an invariant control (housekeeping gene).
Data Analysis
All data are expressed as mean ± SEM. Kidney weights were normalized by tibia length (expressed as gram per centimeter). Statistical and correlation analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc., Calif., USA). Group comparisons were conducted by 2-way analysis of variance followed by Bonferroni's multiple comparisons or Student's t test, as appropriate. Pearson's correlation analysis (single linear univariate) was performed to evaluate the correlation between GFR and the excretion rate of individual urinary biomarker that showed significant time-dependent changes. A p value of <0.05 was considered statistically significant.
Results
General Observations of ZSF-1 Rats
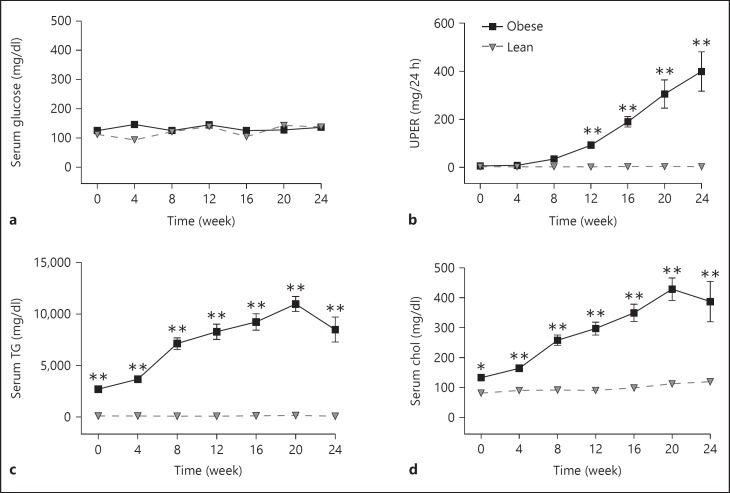
In the first (12-week) and second (24-week) ZSF-1 studies, the male obese rats showed similar metabolic phenotypes. The obese animals were diabetic, proteinuric and hyperlipidemic relative to the lean controls as shown in figure 1 for the 24-week study. Blood glucose for the 2K obese rats was significantly elevated during the course of the study beginning at week 4, but trended downward later in the study (fig. 1a). A similar glucose profile was observed for the 1K obese rats. Glucose levels were slightly lower in the 1K obese rats than in the 2K obese rats with the difference being statistically significant at 2 time points (weeks 4 and 12). Urinary protein excretion rate (UPER) continued to rise over the course of the study in both the 1K and 2K obese rats (fig. 1b), as did serum cholesterol and TGs (except for the last time point; fig. 1c, d). There was no difference in blood TG and cholesterol concentrations between 1K and 2K male obese rats. There was also no difference in UPER between 1K- and 2K-obese ZSF-1 rats at all the time points examined in this study, indicating that the remaining kidney in 1K-obese rats was leaking (2-fold) more protein relative to a single kidney in 2K-obese rats. Male lean ZSF-1 rats had little change in blood glucose, UPER and blood lipid (TG and cholesterol) levels during the 24-week longitudinal analysis. Body weight, food and water intake and urine output were all significantly higher in male obese rats than in male lean controls but there was little difference in these parameters between 1K- and 2K-obese rats (data not shown).
Fig. 1.
Blood glucose, UPER and blood lipids in male 1K and 2K ZSF-1 rats from the 24-week study. a Non-fasting blood glucose levels, b UPER, over 24-hour urine collection, c serum TGs and d serum cholesterol (Chol) were all elevated in the 1K- and 2K-obese rats. All panels share the same legend as shown in (b). Data are expressed as mean ± SEM (n = 5 per group). * p < 0.05 and ** p < 0.01 vs. respective lean rats; + p < 0.05 and ++ p < 0.01 for 1K- vs. 2K-obese rats.
The female 1K obese rats had some similarities to their male counterparts but also had very noticeable differences. Both the female obese and lean ZSF-1 rats showed no increase in blood glucose concentrations over the course of the 24-week study (fig. 2a). However, the female obese ZSF-1 rats showed time-dependent increases in blood lipids (fig. 2c, d), and the levels were comparable to the male obese ZSF-1 rats at similar time points. Proteinuria from obese female rats was significantly elevated versus the lean control (fig. 2b), but was delayed in onset and lower in levels relative to the male obese rats. The onset of proteinuria occurred between weeks 8 and 12, and reached 400 mg/day by week 24. In contrast, the onset of proteinuria in male 1K-obese rats was between weeks 4 and 6, and reached 800 mg/day by week 24. Female 1K-obese ZSF-1 rats had significantly lower body weight, food and water intake and urine output compared to age-matched male 1K-obese ZSF-1 rats although each of these parameters was still higher than in the female lean controls (data not shown).
Fig. 2.
Blood glucose, UPER, blood lipids in 1K female ZSF-1 rats from the 24-week study. a Non-fasting blood glucose levels was not different between obese and lean controls, b UPER increased over time in the obese female rats, c serum TGs and d serum cholesterol (Chol) were both elevated in the 1K-obese rats. All panels share the same legend as shown in (b). * p < 0.05 and ** p < 0.01 vs. lean rats.
Kidney Fibrosis
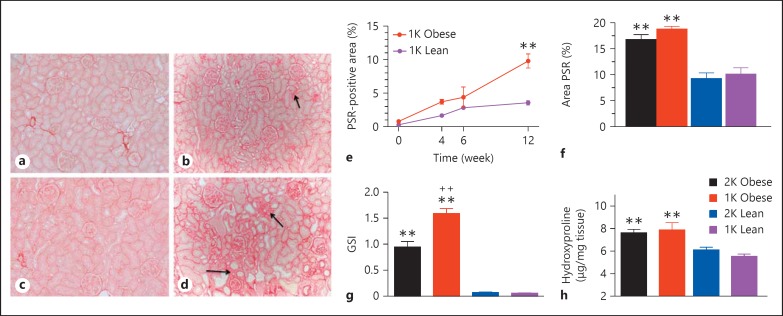
The progression of kidney fibrosis was examined at different time points in the male 1K 12-week study, and at the 24-week mark for 1K and 2K animals in the 24-week study (fig. 3). As shown in figure 3, both 1K-obese and 1K-lean rats did not show positive PSR staining in the TI area at baseline immediately after Unx surgery (fig. 3a, c, and e). By week 12 after Unx, the percentage of positive PSR staining in the TI area increased in 1K-obese rats to 9.8% and was significantly greater than the 1K-lean ZSF-1 rats (3.6% area; fig. 3b, e). In the 24-week study, the percentage of positive PSR staining in the TI area increased to 18.8 and 16.8% for 1K- and 2K-obese ZSF-1 rats, respectively, which was significantly greater than in the related lean controls (10.1 and 9.2%, respectively; fig. 3d, f). There was no difference between the 1K and 2K animals.
Fig. 3.
Fibrotic changes in kidneys of male ZSF-1 rats. PSR staining of kidney tissues from 1K-lean rats at baseline (a) and 24 weeks after surgery (b). Representative PSR staining of kidney tissues from 1K-obese rats at baseline (c) and 24 weeks after surgery (d). Representative fibrotic areas in the TI are marked with arrows in (b) and (d). e Time-dependent changes in percentile PSR-positive area in the TI space of kidneys from the 12-week study with 1K ZSF-1 rats. f Percentage of PSR positive area in the TI space from the 24-week study demonstrated elevation in the 1K- and 2K-obese rats. g Increased GSI in 1K- and 2K-obese rats relative to lean controls from the 24-week study. h Hydroxyproline content of renal cortex was also elevated in the obese rats from the 24-week study, which was normalized by tissue weight. All bar graphs share the legend as shown in (h). ** p < 0.01 vs. respective lean rats; ++ p < 0.01 for 1K- vs. 2K-obese rats.
Glomerulosclerosis was only examined in the 24-week study. The GSI significantly increased in the kidneys of both 1K- and 2K-male obese ZSF-1 rats compared to male lean controls (fig. 3g). Glomerulosclerosis in the 1K animals was significantly elevated versus in the 2K-obese animals (score of 1.6 vs. 0.96).
Hydroxyproline content (a measure of total collagen in tissue) was significantly increased in both male 1K- and 2K-obese ZSF-1 rats versus their respective lean controls in the 24-week study (fig. 3h). Hydroxyproline content was not different between 1K and 2K animals in either obese or lean groups. However, the Unx significantly increased the weight of the remaining kidney relative to the 2K-obese rats and the 2K-lean controls (1K- vs. 2K-obese rats: 0.84 ± 0.02 vs. 0.61 ± 0.03 g/cm, p < 0.01; 1K vs. 2K lean rats: 0.55 ± 0.02 vs. 0.37 ± 0.01 g/cm, p < 0.01).
Interstitial kidney fibrosis and GSI was measured in female 1K ZSF-1 rats at 2 time points, weeks 16 and 24. The percent PSR-positive area increased significantly in 1K-female obese rats at both weeks 16 and 24, respectively, compared to the 1K-female lean controls (week 16: obese vs. leans, 7.7 ± 0.6 vs. 3.5 ± 0.3%, p < 0.01; week 24: 8.5 ± 0.6 vs. 2.6 ± 0.3%, p < 0.01). Although there was a significant increase in PSR-positive area in the female obese ZSF-1 rats, it was 2.2-fold lower compared to the 1K-male obese ZSF-1 rats at week 24. The GSI also increased significantly in 1K-female obese ZSF-1 rats at both time points compared to the 1K-female lean rats (week 16: obese vs. leans, 0.327 ± 0.055 vs. 0.028 ± 0.002, p < 0.01; week 24: 0.728 ± 0.267 vs. 0.025 ± 0.011, p < 0.01).
Time-Dependent Changes of GFR
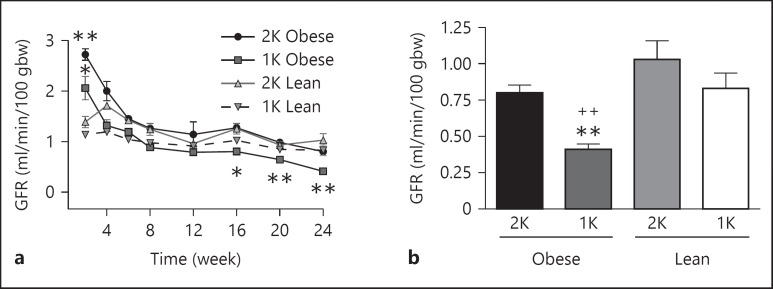
GFR was assessed with a novel method that measures the subcutaneous elimination of FITC-sinistrin, which is exclusively filtered by the kidney (Schock-Kusch et al. [17]). The GFR was significantly elevated 2–3-fold in both 1K- and 2K-male obese ZSF-1 rats compared to the respective lean control rats at week 2 (fig. 4a), indicating hyperfiltration at early time points. The GFR subsequently declined over time in 1K-obese rats and was significantly lower on weeks 16, 20 and 24 than the 1K-lean controls (fig. 4a, b) demonstrating a time-dependent decline in overall renal function. It was also noted that renal function decline was enhanced in 1K-obese ZSF-1 rats compared to the 2K-obese rats over the course of the study. Although the GFR of 2K-obese ZSF-1 rats at week 24 was 22.3% lower than the 2K-lean control animals at this time point (fig. 4b), the difference was not significant.
Fig. 4.
GFR in male ZSF-1 rats. a Time-course of GFR in 1K and 2K ZSF-1 rats over 24 weeks after surgery. b Bar graph to facilitate comparison of GFR at week 24, which demonstrated a decline in 1K-obese animals relative to the 1K-lean and 2K-obese rats. * p < 0.05, ** p < 0.01 vs. respective lean control rats; ++ p < 0.01 vs. 2K-obese rats.
The measured GFR was significantly lower in female obese 1K-ZSF-1 rats compared to female lean 1K controls (0.69 vs. 0.89 ml/min/100 grams of body weight (gbw), p < 0.05) at week 24, but was still higher than age-matched male obese 1K-ZSF-1 rats (0.41 ml/min/100 gbw, p < 0.01). GFR was not different between age-matched female and male 1K lean ZSF-1 rats (0.83 vs. 0.89 ml/min/100 gbw).
Changes in Urinary Biomarkers and Correlation with GFR
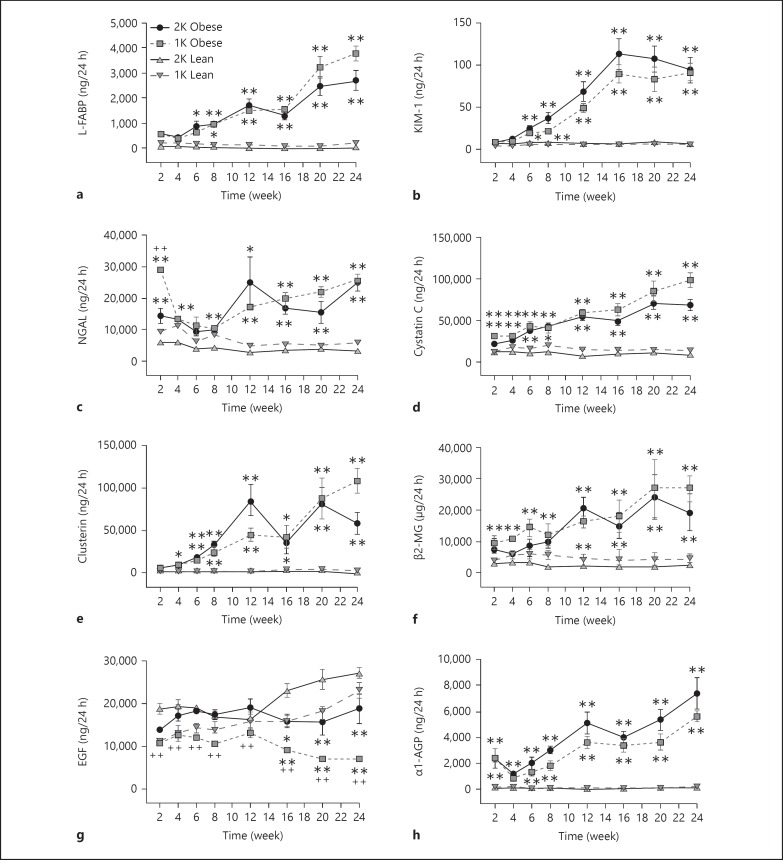
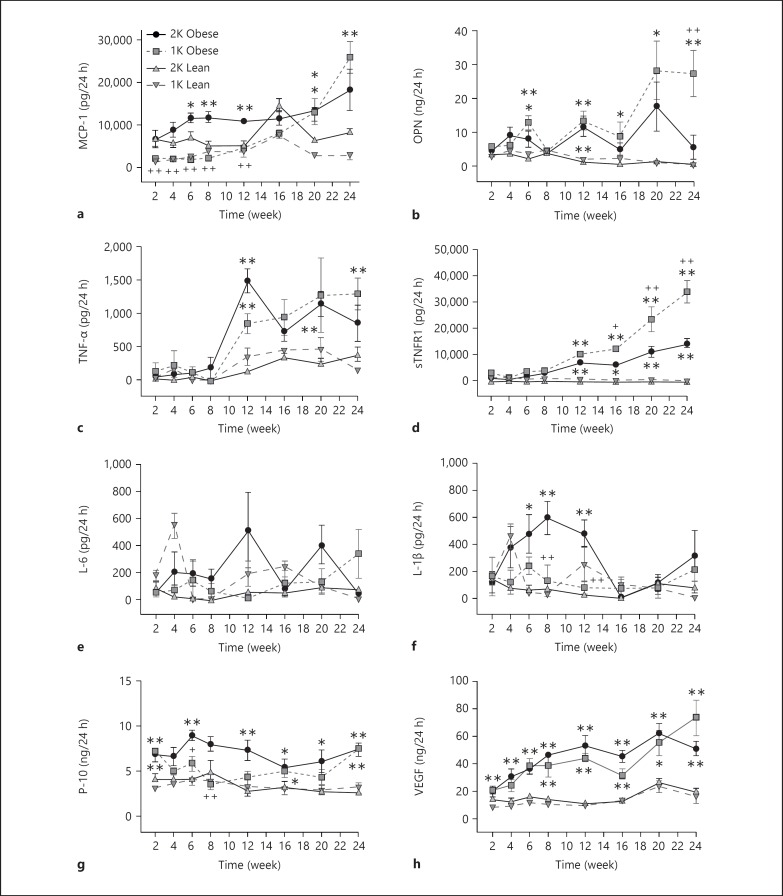
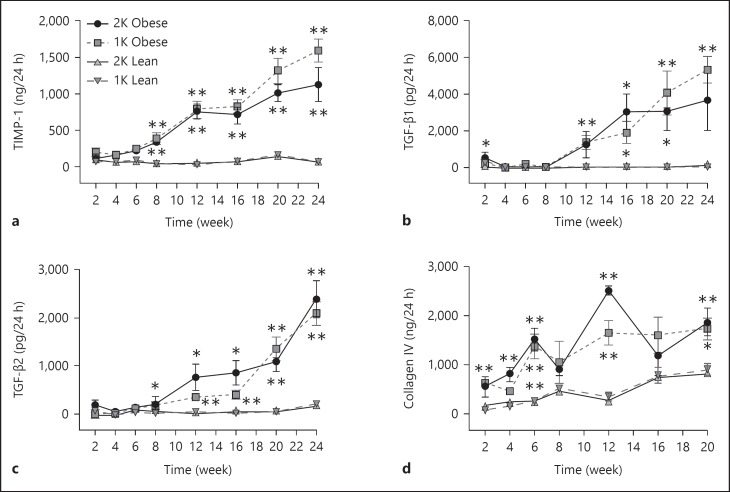
In addition to urinary protein, we also measured 21 other biomarkers in urine samples from male ZSF-1 rats over the 24-week study. Female rats were not studied for this end point. The markers were divided into 3 main categories including (1) injury markers (L-FABP, KIM-1, NGAL, cystatin C, clusterin, β2-MG, α1-AGP, EGF; fig. 5), (2) inflammatory markers (monocyte chemoattractant protein (MCP-1), osteopontin (OPN), interleukin (IL)-6, IL-1β, TNF-α, sTNFR1, VEGF, interferon-gamma-inducible 10 kD protein (IP-10); fig. 6) and (3) fibrotic markers (TIMP-1, collagen IV, TGF-β1, TGF-β2, and TGF-β3; fig. 7). There were no consistent changes in the levels of any urinary biomarker from male lean control rats (1K or 2K) over the course of the study. In contrast, in male obese ZSF-1 rats (1K or 2K), all but 2 of the urinary biomarkers showed a significant increase in the excretion rate at some point over the 24-week observation period in comparison to lean controls (fig. 5, 6, 7). Most markers continued to increase their urinary levels over the course of the study, but IL-1β (2K) and TGF-β3 (1K and 2K) increased at earlier time points before returning to normative levels, and IP-10 (1K and 2K) levels were sporadic. The elevation of urinary excretion rate for a few biomarkers (i.e., collagen IV, NGAL, cystatin C, β2-MG, α1-AGP, VEGF) in the male obese rats was observed as early as 2 weeks after Unx. The majority of these markers were kidney injury-related markers. It was also interesting to note that the urinary excretion rate of NGAL displayed a U-shaped time course (in particular for 1K-obese rats). Fibrosis markers tended to increase later in the model. Perhaps the greatest increase over time was observed from clusterin, which increased more than 58-fold relative to the age-matched male lean rats by the end of the observation period. For the 2 markers that did not increase in levels, IL-6 did not change at all and EGF decreased its rate over time for both 1K- and 2K-obese rats compared to the respective lean controls (fig. 5g, 6e).
Fig. 5.
a-h Time-dependent changes of kidney injury-related urinary biomarkers in male ZSF-1 rats. All panels share the same legend as shown in (a). * p < 0.05, ** p < 0.01 vs. respective lean controls; ++ p < 0.01 vs. 2K-obese rats.
Fig. 6.
a-h Time-dependent changes of inflammation-related urinary biomarkers in male ZSF-1 rats. All panels share the same figure legends as shown in (a). * p < 0.05 and ** p < 0.01 vs. respective lean controls; + p < 0.05 and ++ p < 0.01 vs. 2K-obese rats.
Fig. 7.
a-d Time-dependent changes of fibrosis-related urinary markers in ZSF-1 rats. All panels share the same legends as shown in (a). * p < 0.05 and ** p < 0.01 vs. respective lean controls.
There was little difference in the total urinary excretion rate for the majority of measured markers when comparing 1K- to 2K-male obese ZSF-1 rats. Three markers (IL-1β, IP-10 and MCP-1) were significantly lower in 1K versus 2K rats at early-to-middle time points, but not later in the model (fig. 6). OPN was higher in 1K than in 2K rats only at the last time point. The excretion rate of sTNFR1 from 1K-obese rats gradually separated from 2K rats starting at week 16 and continued to separate for the remaining time of the study (fig. 6d). The urinary excretion of EGF was lower in both 1K- and 2K-obese rats relative to their lean controls but at the later time points, the levels in the 1K-obese rats were significantly lower than in the 2K-obese rats. It should be noted that the differences between 1K and 2K rats are likely amplified when the urinary excretion rate is corrected by the number of kidneys, indicating that the remaining kidney in 1K-obese rats was producing/excreting more injury and/or pathological factors than a single kidney in the 2K-obese rats.
It is also interesting to compare the time courses of proteinuria against the 6 tubular epithelial markers measured in this study. However, we found no consistent pattern across these markers. Significant elevation was observed in male obese ZSF-1 rats at 6 weeks after surgery for proteinuria, L-FABP and KIM-1. NGAL and cystatin C rose as early as 2 weeks after surgery while clusterin rose at week 4. Significant changes for EGF were not shown until week 16 after surgery.
Table 1 summarizes all the correlations between GFR and the measured urinary biomarkers, including urinary protein levels from the 24-week study. Except for EGF (with a positive slope), all correlation slopes were negative for both 1K- and 2K-obese rats. The correlations between GFR and urinary levels were not significant for OPN and TGF-β1 in 2K-obese rats, but all others were. In both 1K- and 2K-obese male rats, proteinuria (UPER) demonstrated the greatest correlation with GFR.
Table 1.
Correlation of urinary markers with GFR in male obese ZSF-1 rats
| Urinary markers | 1K-obese rats |
2K-obese rats |
||||
|---|---|---|---|---|---|---|
| correlation slope | R2 value | p value | correlation slope | R2 value | p value | |
| Protein | –0.76 | 0.58 | <0.0001 | –0.72 | 0.52 | <0.0001 |
| L-FABP | –0.71 | 0.50 | <0.0001 | –0.59 | 0.35 | 0.0003 |
| KIM-1 | –0.64 | 0.41 | <0.0001 | –0.61 | 0.37 | 0.0003 |
| Cystatin C | –0.67 | 0.45 | <0.0001 | –0.63 | 0.39 | <0.0001 |
| Clusterin | –0.66 | 0.43 | <0.0001 | –0.57 | 0.33 | 0.0008 |
| β2-MG | –0.63 | 0.40 | <0.0001 | –0.54 | 0.29 | 0.0012 |
| α1-AGP | –0.54 | 0.30 | 0.0004 | –0.47 | 0.22 | 0.0053 |
| VEGF | –0.57 | 0.32 | 0.0002 | –0.69 | 0.47 | <0.0001 |
| MCP-1 | –0.59 | 0.35 | <0.0001 | –0.45 | 0.21 | 0.0079 |
| OPN | –0.48 | 0.23 | 0.0023 | –0.13 | 0.02 | 0.48 (ns) |
| TNF-α | –0.48 | 0.23 | 0.0025 | –0.50 | 0.25 | 0.0033 |
| sTNFR1 | –0.55 | 0.30 | 0.0009 | –0.63 | 0.40 | <0.0001 |
| TIMP-1 | –0.71 | 0.51 | <0.0001 | –0.68 | 0.46 | <0.0001 |
| Collagen IV | –0.44 | 0.19 | 0.0103 | –0.54 | 0.29 | 0.0030 |
| TGF-α1 | –0.60 | 0.36 | <0.0001 | –0.34 | 0.12 | 0.051 (ns) |
| TGF-α2 | –0.66 | 0.43 | <0.0001 | –0.55 | 0.30 | 0.0013 |
| EGF | 0.43 | 0.18 | 0.0077 | –0.46 | 0.212 | 0.0065 |
ns = Not significant.
Gene Expression Profile
The mRNA expression levels for various markers at week 24 in renal tissue were normalized to the housekeeping gene, HPRT, and comparisons of male 1K-obese versus 1K-lean rats, male 2K-obese versus 2K-lean rats and male 1K- versus 2K-obese rats are shown in table 2. The included genes represent fibrotic (TGF-β1, TIMP-1, collagens and PAI-1) and inflammatory pathways (MCP-1, TNF-α, IL-6, OPN and IP-10). The expression of all genes (except for VEGF) examined in the obese animals was significantly increased relative to the lean controls and ranged from 1.8-fold (IP-10 and collagen 4α1) to 61-fold (2K OPN). There were no differences between the 1K- and 2K-obese male rats in the renal expression of these genes at the 24-week time point.
Table 2.
mRNA expression levels of multiple markers in kidney tissues of male ZSF-1 rats
| Markers | Fold difference | ||
|---|---|---|---|
| 1K-obese vs. 1K-lean | 2K-obese vs. 2K-lean | 1K- vs. 2K-obese | |
| MCP-1 | 10** | 15** | 0.9 |
| IL-6 | 4.9** | 6.8** | 0.9 |
| IP-10 | 1.8** | 2.4** | 1.1 |
| TNF-α | 2.5** | 2.5** | 1.2 |
| sTNFR1 | 2.5** | 2.0** | 1.0 |
| TIMP-1 | 7.9** | 10** | 1.2 |
| OPN | 30** | 61** | 1.2 |
| TGF-α1 | 2.8** | 2.7** | 1.3 |
| VEGF | 0.4** | 0.64* | 0.8 |
| EGF | 0.12** | 0.27** | 0.5++ |
| Collagen 4α1 | 1.8** | 2.4** | 1.0 |
| Collagen 1α1 | 3.1** | 5.1** | 1.1 |
| Collagen 3α1 | 3.2** | 4.8** | 1.2 |
| PAI-1 | 7.2** | 10** | 1.1 |
1K and 2K obese show increased fold change relative to lean controls. EGF was lower in 1K than 2K obese rats.
p < 0.05,
p < 0.01 for obese vs. lean rats;
p < 0.01 for 1K- vs. 2K-obese rats.
Discussion
The current study further demonstrates that the male obese ZSF-1 model shares many characteristics of human DN and is a valuable model in learning about the disease and possibly evaluating therapeutics. The longitudinal characterization of the model demonstrates the progression of several clinical features of type II DN such as obesity, hyperglycemia, hyperlipidemia, proteinuria, kidney fibrosis (glomerular and TI) and decline in renal function. Many of these features are consistent with previous reports [5,10,11]. We expanded on these findings by (1) demonstrating that a Unx accelerates and enhances some of these pathophysiological features, (2) demonstrating a decline in GFR in unanesthetized 1K animals, (3) demonstrating relevant and progressive changes of key urinary biomarkers of renal disease and (4) demonstrating a less severe disease phenotype, relative to males, in female obese ZSF-1 rats.
This was the first study on renal function that measured GFR in unanesthetized ZSF-1 rats. The newly developed GFR measurement technique [17] demonstrated a transient hyperfiltration period in both 1K- and 2K-obese rats, followed by a normalization period and then a progressive drop, particularly in the 1K rats, in the latter weeks of the study. Hyperfiltration is an early pathophysiological feature that is considered a risk factor in the progressive development of DN [14,23]. The decline of renal function in the male obese ZSF-1 rats was more pronounced in the nephrectomized animals demonstrating significantly lower GFR than the lean controls by week 16 of the study and dropping even lower as the study continued to the 24-week mark, which represents 32 weeks of age for the animals. Clearly, the nephrectomy accelerated this decline. GFR in male obese 2K-ZSF-1 rats also showed a time-dependent decline during the 24-week study, but the terminal GFR value at the final week was only slightly lower than that in the male 2K-lean control rats. A similar lack of functional decline with 32-week-old 2K ZSF-1 animals has been reported [12], but other groups have seen a more significant decline in their measure of GFR [10,11]. The inconsistent findings between the studies could be related to different assessment methods, animal state (anesthetized or unanesthetized) and linked to the last point, type of anesthetic agent used. Nonetheless, extending the period of observation in the current study would have likely revealed a decline in GFR in the 2K-obese animals as both the Kuo et al. [12] and Tofovic et al. [10] demonstrated a significant decline in 2K-obese animals by ages of 40 and 46 weeks, respectively.
A key aspect of the ZSF-1 model that differentiates it from other rodent models of DN, particularly mice, is the development of significant TI fibrosis. Most mouse models do not develop this pathology [6,7,8]. In 1K-obese ZSF-1 rats, fibrosis rose to 9.8% of the interstitial space by 12 weeks after the nephrectomy, which was significantly higher than the male lean ZSF-1 controls at that time point, and by week 24, it had progressed to 18.8% of the area. Interstitial fibrosis was virtually non-existent in animals examined right after the nephrectomy. The area of TI fibrosis was slightly lower (16.8%) in the 2K-obese ZSF-1 group compared to the 1K-obese rats at the 24-week point of the study. A relationship between the development of TI fibrosis and a decline in GFR and/or patient survival has been controversial with studies presenting evidence on either side of this putative linkage [24,25,26,27], but a recent large scale study with DN patients demonstrated a strong link between interstitial fibrosis and renal outcome independent of other clinical features [28]. In the same study, glomerular lesions were also associated with renal outcome and correlated with the development of interstitial fibrosis. This link was apparent in the 1K-obese rats as there were significant increases in glomerular and TI fibrosis accompanied by a decline in GFR. However, while there was a significant increase in TI fibrosis and glomerulosclerosis in the 2K-obese ZSF-1 rats, these were not accompanied by a significant decline in GFR compared to lean controls. By week 24, the 2K-obese animals had a GFR 22.3% lower than their lean counterparts, and like TI fibrosis, an extension of the current study beyond 24 weeks may tip the glomerular lesions toward the severity observed in the 1K rats and trigger a significant decline in GFR (as observed by other groups studying 2K ZSF-1 rats at later time points).
Urinary biomarkers have the potential to be a simple non-invasive way to monitor the development and progression of renal disease and are an area of very active research for DN [13,14,16]. Proteinuria has always been an important urinary biomarker in the diagnosis and monitoring of nephropathy [15,16,29]. Interest in identifying additional urinary markers of disease pathology has been growing, and identifying similar markers in preclinical models would be an important translational step. The markers in the current study were selected based on relevance to renal cell injury, inflammation or fibrosis. Each of the markers associated with renal injury, except for EGF, progressively increased over the 24-week period, and there was little difference between 1K- and 2K-male obese ZSF-1 rats. The elevated urinary excretion for most renal injury markers examined in this study is consistent with higher levels observed in diabetic patients, although not all of the biomarkers studied here have been examined in large scale longitudinal clinical cohorts [14,30]. The time-dependent increase in urinary excretion rate for cystatin C, β2-MG, clusterin and KIM-1 are also in agreement with data from ZDF rats [31]. The atypical U-shaped time course for NGAL urinary excretion rate was also observed in ZDF rats [31]. KIM-1 and NGAL are widely considered to be sensitive biomarkers to detect acute kidney injury. Recent studies have shown that both KIM-1 and NGAL could be potential biomarkers for progression of chronic kidney diseases including DN [13,30,32]. EGF is a marker for tubular epithelial cell injury, and its urinary excretion rate decreased over the course of the study; the decrease was more substantial in the 1K-obese ZSF-1 rats. Urinary excretion rate of EGF has been reported to decrease over time in ZDF rats [31], as well as in patients with DN [33,34]. The decreased urinary excretion rate of EGF appears to be related to a similar decrease in mRNA levels of EGF in renal tissue for both 1K- and 2K-obese rats, and similar to the urinary content, the tissue expression of EGF mRNA was lower in 1K- compared to 2K-obese rats.
There was some variance in the urinary markers of inflammation in terms of time course, differences between 1K- and 2K-obese animals, connection to tissue mRNA expression and relevance. The expression of kidney tissue mRNA for most of these markers was elevated and was not different between 1K and 2K animals at the 24-week mark. Urinary IL-6 levels did not change over the course of the study despite significant increases in tissue expression of mRNA. Urinary MCP-1 and IL-1β rose early in 2K- but not 1K-obese rats, and only MCP-1 continued to increase for both groups of rats. OPN increased over the course of the study with fluctuations, mainly in the 1K-obese rats. IP-10 was slightly, but significantly, elevated over the 24 weeks of observation in 2K-obese rats, while the levels in 1K rats were variable. TNF-α mediates its pro-inflammatory effects via 2 receptor isoforms, TNF receptor 1 (TNFR1) and TNFR2, which are both membrane-bound and also exist in soluble form [35]. A time-dependent increase in urinary excretion of TNF-α and soluble TNFR1 was observed in obese ZSF-1 rats. Patients with DN have elevated serum and urinary concentrations of TNF-α [30]. Increased serum levels of both TNFR1 and TNFR2 have been shown to correlate with GFR in diabetic patients and have the potential as predictors of progressive renal disease in diabetes [36]. Structural kidney damage in patients with type 2 diabetes is associated with TNF-α activity and specifically with plasma concentrations of soluble TNFR1 [37]. Our current study further supports the potential pathophysiological role of TNF-α pathway in the development of diabetic kidney damage and dysfunction. VEGF is a potent cytokine that increases angiogenesis and endothelial permeability. Urinary excretion of VEGF has been reported to be elevated in patients with type II DN [38]. The urinary excretion of VEGF was also elevated in obese ZSF-1 rats in the current study. However, the mRNA level of VEGF in kidneys of obese ZSF-1 rats was decreased. Further studies are needed to understand atypical time courses for certain biomarkers and the disconnection between urinary excretion rate and tissue gene expression levels.
We measured 4 urinary biomarkers of kidney fibrosis in this study. They all showed progressive elevations over the 24-week observation in obese ZSF-1 rats, except for collagen IV in 2K ZSF-1 rats which dropped at later time points from its peak at week 12. There were no significant differences for these biomarkers between 1K- and 2K-obese animals. The major increases in excretion rate for TIMP-1, TGF-β1 and TGF-β2 typically first occurred in the 8–12 week time frame and most likely corresponded to the increase in observed tissue fibrosis. Urinary collagen IV had elevated levels earlier than the other fibrotic markers, and has been associated with disease severity in DN patients [39]. The quick rise here may imply that collagen IV can be a maker of early fibrotic disease and may be associated with injury in the model at this time point. The urinary excretion of TIMP-1 was highly time-dependent in the current study. Its excretion was also shown to be elevated in ZDF rats but had little change from weeks 12 to 25 of age [31]. Urine excretion of TIMP-1 was reported to be significantly increased in patients with renal diseases including diabetic kidney disease. In the study by Horstrup et al. [40], it was also shown that urinary concentrations (normalized by urinary creatinine) of TIMP-1 increased with progressive reduction in renal function (measured as creatinine clearance). The increased urinary excretion rate of the 3 fibrotic markers (TIMP-1, TGF-β1 and collagen IV) was also supported by corresponding elevations in gene expression. Tissue gene expression level of other fibrotic markers (collagen 1 and 3, PAI-1) was also shown to be elevated in both 1K- and 2K-obese ZSF-1 rats at the end of the 24-week study.
Significant correlation with renal function (GFR) decline was shown for 17 biomarkers in male 1K-obese ZSF-1 rats and 15 markers in male 2K-obese ZSF-1 rats. The negative correlation between GFR decline and urinary excretion rate has been previously reported in humans with DN for a number of biomarkers such as proteinuria, MCP-1, TIMP-1, NGAL and TGF-β1 [16,25,32,40]. Proteinuria/albuminuria still offers the highest value in clinical practice in terms of its utility in the diagnosis of DN and the predictive potential of renal functional decline although many novel urinary biomarkers are still under intensive clinical investigation. In our study, the progressive increase of UPER also showed the best inverse correlation with the progressive decline of GFR although it was not the biomarker that showed the earliest elevation in urinary excretion rate. Among the measured urinary biomarkers in our study, TIMP-1 showed the second best inverse correlation with GFR decline in both 1K and 2K ZSF-1 rats. Interestingly, Nielsen et al. [32] showed that urinary NGAL correlated positively with glomerular hyperfiltration early in the clinical course of diabetes. We also observed significant elevation of urinary NGAL secretion during the early time points when hyperfiltration was observed. Overall, the values of novel urinary biomarkers in predicting progressive (fast vs. slow) kidney function loss remains to be established in large-scale, prospective longitudinal clinical studies.
Female obese ZSF-1 rats demonstrated a less intense DN phenotype relative to their male counterparts. Hyperlipidemia in female 1K-obese ZSF-1 rats was slightly higher than that in male obese ZSF-1 rats, but the female rats were not hyperglycemic. Proteinuria was evident in the female rats, but was delayed and less severe compared to age-matched male 1K-obese ZSF-1 rats. Renal fibrosis and functional impairment was also less severe in female obese ZSF-1 rats in comparison to the male counterparts. In the absence of diabetes, the renal damage in female obese ZSF-1 rats might be associated with lipid nephrotoxicity as suggested in the literature [41]. Further studies are required to understand why female ZSF-1 rats do not develop diabetes but still develop renal damage and also to explore the other differences that exist between the genders. Across all ages in humans, the incidence and rate of progression of most non-diabetic renal diseases are markedly higher in men compared with age-matched women [42]. However, in individuals with diabetes, differences (if any) in the incidence and progression of related renal disease between the sexes has not been fully established [43].
In summary, male obese ZSF-1 rats represent an important model to examine the mechanisms and pathways relevant to DN and to evaluate potential therapeutics for this disease. The model manifests key pathophysiological features such as metabolic syndrome, advancing proteinuria, progressive TI fibrosis, glomerulosclerosis, transient hyperfiltration followed by a progressive decline in renal function, and the engagement of key pathways associated with renal injury, inflammation and fibrosis. Additionally, the current data indicate that renal mass reduction (Unx) can significantly accelerate disease progression, and thus represents a useful procedure to potentially shorten study duration in the evaluation of novel therapeutics. The female counterpart of the strain displayed some of the key features of this disease, but was delayed in its manifestation and tended to be less severe. Importantly, from a translational perspective, several urinary biomarkers were identified in the male obese ZSF-1 rats that tracked with disease progression and were linked to the pathophysiological expression of key mRNA markers in the diseased renal tissue. This new urinary biomarker data set demonstrated a profile similar to human DN for markers like proteinuria, L-FABP, NGAL, KIM-1, TIMP-1, MCP-1, TGF-β1, TNF-α and delineated novel markers (e.g., sTNFR1, VEGF, cystatin C and EGF) for further exploration. Those biomarkers with robust changes may have value in helping monitor efficacy in preclinical and clinical studies.
Disclosure Statement
All authors are employees of AbbVie. The design, study conduct and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review and approval of the publication.
References
- 1.Packham DK, Alves TP, Dwyer JP, Atkins R, de Zeeuw D, Cooper M, Shahinfar S, Lewis JB, Lambers Heerspink HJ. Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: results from the DIAMETRIC (diabetes mellitus treatment for renal insufficiency consortium) database. Am J Kidney Dis. 2012;59:75–83. doi: 10.1053/j.ajkd.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Himmelfarb J, Tuttle KR. New therapies for diabetic kidney disease. N Engl J Med. 2013;369:2549–2550. doi: 10.1056/NEJMe1313104. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Fernandez B, Ortiz A, Gomez-Guerrero C, Egido J. Therapeutic approaches to diabetic nephropathy – beyond the RAS. Nat Rev Nephrol. 2014;10:325–346. doi: 10.1038/nrneph.2014.74. [DOI] [PubMed] [Google Scholar]
- 4.Gosmanov AR, Wall BM, Gosmanova EO. Diagnosis and treatment of diabetic kidney disease. Am J Med Sci. 2014;347:406–413. doi: 10.1097/MAJ.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 5.Prabhakar S, Starnes J, Shi S, Lonis B, Tran R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J Am Soc Nephrol. 2007;18:2945–2952. doi: 10.1681/ASN.2006080895. [DOI] [PubMed] [Google Scholar]
- 6.Kaur M, Bedi O, Sachdeva S, Reddy BV, Kumar P. Rodent animal models: from mild to advanced stages of diabetic nephropathy. Inflammopharmacology. 2014;22:279–293. doi: 10.1007/s10787-014-0215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters V, Schmitt CP. Murine models of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2012;120:191–193. doi: 10.1055/s-0032-1304569. [DOI] [PubMed] [Google Scholar]
- 8.Betz B, Conway BR. Recent advances in animal models of diabetic nephropathy. Nephron Exp Nephrol. 2014;126:191–195. doi: 10.1159/000363300. [DOI] [PubMed] [Google Scholar]
- 9.Heinonen SE, Genové G, Bengtsson E, Hübschle T, Åkesson L, Hiss K, Benardeau A, Ylä-Herttuala S, Jönsson-Rylander AC, Gomez MF. Animal models of diabetic macrovascular complications: key players in the development of new therapeutic approaches. J Diabetes Res. 2015;2015:404085. doi: 10.1155/2015/404085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tofovic SP, Kusaka H, Kost CK, Jr, Bastacky S. Renal function and structure in diabetic, hypertensive, obese ZDFxSHHF-hybrid rats. Ren Fail. 2000;22:387–406. doi: 10.1081/jdi-100100882. [DOI] [PubMed] [Google Scholar]
- 11.Bilan VP, Salah EM, Bastacky S, Jones HB, Mayers RM, Zinker B, Poucher SM, Tofovic SP. Diabetic nephropathy and long-term treatment effects of rosiglitazone and enalapril in obese ZSF1 rats. J Endocrinol. 2011;210:293–308. doi: 10.1530/JOE-11-0122. [DOI] [PubMed] [Google Scholar]
- 12.Kuo J, Lee CW, Qian HS, Fryer R. Renal structure-function relationship in the obese ZSF1 rat model of diabetic nephropathy. FASEB J. 2015;29((1 suppl)):959.5. [Google Scholar]
- 13.Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80:806–821. doi: 10.1038/ki.2011.198. [DOI] [PubMed] [Google Scholar]
- 14.Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis. 2014;63(2 suppl 2):S39–S62. doi: 10.1053/j.ajkd.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 15.Cravedi P, Remuzzi G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br J Clin Pharmacol. 2013;76:516–523. doi: 10.1111/bcp.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verhave JC, Bouchard J, Goupil R, Pichette V, Brachemi S, Madore F, Troyanov S. Clinical value of inflammatory urinary biomarkers in overt diabetic nephropathy: a prospective study. Diabetes Res Clin Pract. 2013;101:333–340. doi: 10.1016/j.diabres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Schock-Kusch D, Xie Q, Shulhevich Y, Hesser J, Stsepankou D, Sadick M, Koenig S, Hoecklin F, Pill J, Gretz N. Transcutaneous assessment of renal function in conscious rats with a device for measuring FITC-sinistrin disappearance curves. Kidney Int. 2011;79:1254–1258. doi: 10.1038/ki.2011.31. [DOI] [PubMed] [Google Scholar]
- 18.Cowley AW, Jr, Ryan RP, Kurth T, Skelton MM, Schock-Kusch D, Gretz N. Progression of glomerular filtration rate reduction determined in conscious Dahl salt-sensitive hypertensive rats. Hypertension. 2013;62:85–90. doi: 10.1161/HYPERTENSIONAHA.113.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolber PC, Spach MS. Picrosirius red staining of cardiac muscle following phosphomolybdic acid treatment. Stain Tech. 1987;62:23–26. doi: 10.3109/10520298709107961. [DOI] [PubMed] [Google Scholar]
- 20.Dolber PC, Spach MS. Conventional and confocal fluorescence microscopy of collagen fibers in the heart. J Histochem Cytochem. 1993;41:465–469. doi: 10.1177/41.3.7679127. [DOI] [PubMed] [Google Scholar]
- 21.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16:1013–1023. doi: 10.1681/ASN.2004080720. [DOI] [PubMed] [Google Scholar]
- 22.El Nahas AM, Bassett AH, Cope GH, Le Carpentier JE. Role of growth hormone in the development of experimental renal scarring. Kidney Int. 1991;40:29–34. doi: 10.1038/ki.1991.175. [DOI] [PubMed] [Google Scholar]
- 23.Breyer JA. Diabetic nephropathy in insulin-dependent patients. Am J Kidney Dis. 1992;20:533–547. doi: 10.1016/s0272-6386(12)70215-9. [DOI] [PubMed] [Google Scholar]
- 24.Ruggenenti P, Gambara V, Perna A, Bertani T, Remuzzi G. The nephropathy of non-insuline-dependent diabetes: predictors of outcome relative to diverse patterns of renal injury. J Am Soc Nephrol. 1998;9:2336–2343. doi: 10.1681/ASN.V9122336. [DOI] [PubMed] [Google Scholar]
- 25.Christensen PK, Larsen S, Horn T, Olsen S, Parving HH. Renal function and structure in albuminuric type 2 diabetic patients without retinopathy. Nephrol Dial Transplant. 2001;16:2337–2347. doi: 10.1093/ndt/16.12.2337. [DOI] [PubMed] [Google Scholar]
- 26.Okada T, Nagao T, Matsumoto H, Nagaoka Y, Wada T, Nakao T. Histological predictors for renal prognosis in diabetic nephropathy in diabetes mellitus type 2 patients with overt proteinuria. Nephrology (Carlton) 2012;17:68–75. doi: 10.1111/j.1440-1797.2011.01525.x. [DOI] [PubMed] [Google Scholar]
- 27.Lewis A, Steadman R, Manley P, Craig K, de la Motte C, Hascall V, Phillips AO. Diabetic nephropathy, inflammation, hyaluronan and interstitial fibrosis. Histol Histopathol. 2008;23:731–739. doi: 10.14670/HH-23.731. [DOI] [PubMed] [Google Scholar]
- 28.An Y, Xu F, Le W, Ge Y, Zhou M, Chen H, Zeng C, Zhang H, Liu Z. Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol Dial Transplant. 2015;30:257–266. doi: 10.1093/ndt/gfu250. [DOI] [PubMed] [Google Scholar]
- 29.Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74:22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 30.Currie G, Mckay G, Delles C. Biomarkers in diabetic nephropathy: present and future. World J Diabetes. 2014;5:763–776. doi: 10.4239/wjd.v5.i6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Togashi Y, Miyamoto Y. Urinary cystatin C as a biomarker for diabetic nephropathy and its immunohistochemical localization in kidney in Zucker diabetic fatty (ZDF) rats. Exp Toxicol Pathol. 2013;65:615–622. doi: 10.1016/j.etp.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen SE, Reinhard H, Zdunek D, Hess G, Gutiérrez OM, Wolf M, Parving HH, Jacobsen PK, Rossing P. Tubular markers are associated with decline in kidney function in proteinuric type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97:71–76. doi: 10.1016/j.diabres.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Mathiesen ER, Nexø E, Hommel E, Parving HH. Reduced urinary excretion of epidermal growth factor in incipient and overt diabetic nephropathy. Diabet Med. 1989;6:121–126. doi: 10.1111/j.1464-5491.1989.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 34.Dagogo-Jack S, Marshall SM, Kendall-Taylor P, Alberti KG. Urinary excretion of human epidermal growth factor in the various stages of diabetic nephropathy. Clin Endocrinol (Oxf) 1989;31:167–173. doi: 10.1111/j.1365-2265.1989.tb01239.x. [DOI] [PubMed] [Google Scholar]
- 35.Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR. Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases. Am J Nephrol. 2012;36:261–270. doi: 10.1159/000342333. [DOI] [PubMed] [Google Scholar]
- 36.Gohda T, Tomino Y. Novel biomarkers for the progression of diabetic nephropathy: soluble TNF receptors. Curr Diab Rep. 2013;13:560–566. doi: 10.1007/s11892-013-0385-9. [DOI] [PubMed] [Google Scholar]
- 37.Fernández-Real JM, Vendrell J, García I, Ricart W, Vallès M. Structural damage in diabetic nephropathy is associated with TNF-α system activity. Acta Diabetol. 2012;49:301–305. doi: 10.1007/s00592-011-0349-y. [DOI] [PubMed] [Google Scholar]
- 38.Kim NH, Kim KB, Kim DL, Kim SG, Choi KM, Baik SH, Choi DS, Kang YS, Han SY, Han KH, Ji YH, Cha DR. Plasma and urinary vascular endothelial growth factor and diabetic nephropathy in type 2 diabetes mellitus. Diabet Med. 2004;21:545–551. doi: 10.1111/j.1464-5491.2004.01200.x. [DOI] [PubMed] [Google Scholar]
- 39.Cawood TJ, Bashir M, Brady J, Murray B, Murray PT, O'Shea D. Urinary collagen IV and πGST: potential biomarkers for detecting localized kidney injury in diabetes – a pilot study. Am J Nephrol. 2010;32:219–225. doi: 10.1159/000317531. [DOI] [PubMed] [Google Scholar]
- 40.Hörstrup JH, Gehrmann M, Schneider B, Plöger A, Froese P, Schirop T, Kampf D, Frei U, Neumann R, Eckardt KU. Elevation of serum and urine levels of TIMP-1 and tenascin in patients with renal disease. Nephrol Dial Transplant. 2002;17:1005–1013. doi: 10.1093/ndt/17.6.1005. [DOI] [PubMed] [Google Scholar]
- 41.Gyebi L, Soltani Z, Reisin E. Lipid nephrotoxicity: new concept for an old disease. Curr Hypertens Rep. 2012;14:177–181. doi: 10.1007/s11906-012-0250-2. [DOI] [PubMed] [Google Scholar]
- 42.Neugarten J. Gender and the progression of renal disease. J Am Soc Nephrol. 2002;13:2807–2809. doi: 10.1097/01.asn.0000035846.89753.d4. [DOI] [PubMed] [Google Scholar]
- 43.Maric C, Sullivan S. Estrogens and the diabetic kidney. Gend Med. 2008;5((suppl A)):S103–S113. [Google Scholar]