Abstract
Background:
Emergence agitation (EA) after sevoflurane anesthesia is common in children during recovery from general anesthesia and may result in postoperative complications. This study investigated safety and effectiveness of intranasal dexmedetomidine in reducing the incidence and severity of EA.
Methods:
This prospective, randomized double-blinded controlled trial included 86 patients scheduled for the tonsillectomy and/or adenoidectomy under general anesthesia with sevoflurane. They were randomly allocated into two groups. Group D received intranasal dexmedetomidine at 1 μg/kg, and Group C received intranasal saline 0.9% after the induction of general anesthesia. Four-point agitation scale and Face, Legs, Activity, Cry and Consolability (FLACC) scale for pain assessment were measured at six time points (after extubation, leaving the operating room, on arrival to postanesthesia care unit [PACU], 10, 20, and 30 min after arrival in PACU). Extubation, emergence, and discharge times were recorded in addition to any adverse effects.
Results:
There was a significant difference in the incidence of EA between Groups D and C (6.98% and 58%, respectively, with P = 0.001). The median four-point agitation scales and the median scores of FLACC pain scales of Group D were significantly lower than those of Group C at the all six time points with P < 0.05. Extubation, emergence, and discharge times were comparable in both groups, and none of the subjects reported any adverse effects.
Conclusion:
This study demonstrates that a 1 μg/kg dose of intranasal dexmedetomidine administered after the induction of anesthesia reduces post-sevoflurane incidence and severity of EA in children undergone tonsillectomy and/or adenoidectomy with no adverse effects and smooth recovery profile.
Keywords: Dexmedetomidine, emergence agitation, sevoflurane, tonsillectomy and adenoidectomy
Introduction
Emergence agitation (EA) in pediatrics is defined as a postoperative negative behavior that may be accompanied by symptoms as combative movements, excitability, thrashing, disorientation, and inconsolable crying.[1] The definite cause and pathophysiology of EA are not fully elucidated but risk factors include preschool age, preoperative anxiety, postoperative pain, nausea, vomiting, otolaryngology procedures, and inhalational anesthetics specially sevoflurane. Due to its low blood/gas partition coefficient (0.68) and weak airway irritation, sevoflurane is the most popular anesthetic used for children.[2] However, it is associated with higher incidence of (EA) (up to 80%) and this incidence is not related to the duration of exposure and the dose of sevoflurane.[3] Multiple drugs and techniques used to control this problem as benzodiazepines, propofol, fentanyl, and α-2 agonists to improve quality of recovery profile in pediatric age group.[4,5] Among these drugs dexmedetomidine, the dextroenantiomer of medetomidine; the methylated derivative of etomidine is a highly specific α-2 adrenoceptor agonist and has sedative and analgesic properties without significant respiratory depression at the clinically approved dosage.[6,7]
There is now an increasing evidence to support the use of dexmedetomidine as a premedication, sedative, anesthetic adjunct, and for EA management in pediatric age group[8,9] for nonpainful[10] and painful procedures[11] despite the lack of the United States’ Food and Drug Administration approval for use in this age group. Intranasally administered dexmedetomidine could be tolerated safely in the pediatric age group for sedation in high doses up to 4 μg/kg.[12,13] The objective of the current study was to determine the influence of intranasally administered dexmedetomidine on EA incidence and severity in children undergoing tonsillectomy and/or adenoidectomy after sevoflurane anesthesia.
Methods
This prospective, randomized controlled double-blinded clinical trial was conducted between November 2015 and March 2016. The study obtained an approval from the local Institution Research and Ethics Committee and registered at The Pan African Clinical Trials Registry (www.pactr.org) by the identification number of registry (PACTR201604001572340). Informed written consent was obtained from the parents of all the children. The study included 86 patients ASA I and II physical status, and their ages ranged between 3 and 7-years. These patients were scheduled for an elective tonsillectomy and/or adenoidectomy under general anesthesia with sevoflurane. Patients with obstructive sleep apnea, mental retardation or developmental delay, chest, cardiac or neurological diseases, known allergy or hypersensitivity to dexmedetomidine, and patients receiving medications known to interact with dexmedetomidine such as furosemide, lorazepam, and diphenhydramine were excluded from the study. All patients were admitted to hospital on the morning of surgery. They waited in pediatric preanesthesia holding area with their parents. They received no premedication and moved to the operating room (OR) accompanied by one of their parents. The parent was permitted to be present during the induction of anesthesia. All patients fasted 6 h for solids and 2 h for clear fluids. Patients were randomly allocated using a computerized random number generator into two groups. Group D (Study group) received intranasal dexmedetomidine (Precedex; Hospira Inc., Lake Forest, IL, USA) at 1 μg/kg after induction of general anesthesia. Intranasal dexmedetomidine was prepared from the 100 μg/ml parenteral preparation in a 1-ml syringe (with 0.9% saline added to make a final volume of 1 ml.), 0.5 ml installed in each nostril. Group C (control group) received intranasal saline 0.9% after induction of general anesthesia, 0.5 ml in each nostril. Dose calculation, drug preparation, and administration were done by attending anesthesiologists who were not involved or had not participated in this trial. The observers and data collectors were blinded to the study drug given also. General anesthesia induction was done by gradual increase of sevoflurane concentration to a maximum of 6 Vol. % in 100% oxygen (6 L/min) via facemask. An intravenous (IV) catheter was inserted after the loss of eyelash reflex, then dexamethasone at a dose of 0.3 mg/kg given, and the airway was secured with oral endotracheal tube after an adequate depth of anesthesia reached with fentanyl 1 μg/kg and cis-atracurium 0.1 mg/kg. Electrocardiogram, oxygen saturation (SpO2), mean arterial pressure, heart rate (HR), end-tidal CO2 concentration (EtCO2), and end-tidal sevoflurane concentration were monitored continuously. Sevoflurane concentration was maintained at 2–4 Vol. % in 50% oxygen air mixture (2 L/min) after intubation, then adjusted according to the patient's response to provide a stable HR, blood pressure (BP), and SpO2 with pressure-controlled ventilation, inspiratory pressure, and respiratory rate adjusted to maintain EtCO2 between 35 and 45 mmHg. All patients received acetaminophen (200 mg) suppository after induction of general anesthesia. During surgery, the surgeon infiltrated the operative site by 1% lidocaine with epinephrine (1:100,000) for pain and bleeding control (1 ml in each tonsillar bed). At the end of surgery, sevoflurane was discontinued, reversal of neuromuscular block facilitated by neostigmine bromide 20 μg/kg with atropine sulfate 20 μg/kg, and endotracheal tube was removed in lateral decubitus when the patients met the criteria of extubation (return of gag reflex, facial grimace, and purposeful motor movements). The time between the insertion and removal of the mouth gag recorded as the duration of surgery, and the time from sevoflurane mask induction till the extubation time was recorded as the duration of anesthesia. The time to extubation defined as the time from the end of surgery to tracheal extubation and the emergence time defined as the time of first response to command or eye opening on command after extubation were also recorded. On admission to postanesthesia care unit (PACU), the patients were monitored for HR, noninvasive BP, SpO2, and respiratory rate continuously for 30 min by anesthesia nurses who were blinded to groups’ allocation. The primary outcome of this trial was the incidence of EA (highest score) which was assessed at six time points (after extubation, leaving the OR, on arrival to PACU, 10, 20, and 30 min after arrival in PACU) by four-point agitation scale[14] [Table 1].
Table 1.
Four-point scale for the assessment of emergence agitation[14]

Agitation scores of 3 and 4 were defined as an agitation episode and treated by nalbuphine (0.1 mg/kg) as a rescue therapy to control agitation episodes. The total dose of nalbuphine was calculated and compared for significance between both groups.
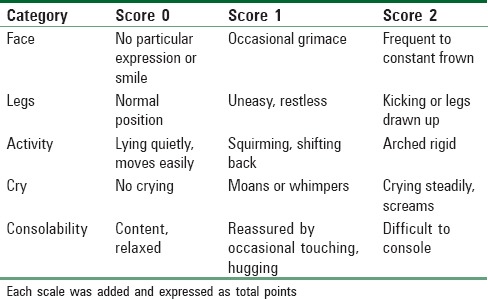
Pain assessment was done using Face, Legs, Activity, Cry, and Consolability (FLACC) scale[15] [Table 2] at the same six time points (after extubation, on leaving the OR, on arrival to PACU, 10, 20, and 30 min after arrival in PACU). Nalbuphine as a rescue analgesic at a dose of 0.1 mg/kg was given if FLACC scores ≥5. The four-point agitation score and FLACC scale score assessment were done by anesthesiologists blinded to groups’ allocation. In addition, time to discharge from PACU, defined as time started from patient's arrival to PACU till modified Aldrete score[16] ≥9, and the incidence of adverse events (nausea, vomiting, somnolence, apnea, desaturation, hypotension, and bradycardia) were recorded. Ondansetron (0.15 mg/kg) was given to control nausea and vomiting if any and total dose was calculated and compared for significance between both groups. Bradycardia (≤60 bpm) treated by atropine 20 μg/kg, and hypotension (≤20% of baseline reading) treated by ephedrine 5 mg increments. Patients were transferred to the ward after being fully conscious with stable vital signs for 30 min, and the absence of bleeding, pain, nausea or vomiting.
Table 2.
Face, Legs, Activity, Cry, and Consolability scale score for pain assessment[15]
Statistical methods and analysis
Based on the results of Aono et al.,[14] a sample size of 36 children per study group was estimated to have an 80% power (α= 0.05, two-tailed) and to detect a difference of 30% in the incidence of EA (primary outcome). Forty-three patients were included in each group to account for possible dropouts. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 16 (SPSS Inc., Chicago, IL, USA). Comparison of quantitative variables between the study groups was done using unpaired Student's t-test when the data were normally distributed and Mann–Whitney rank sum (when indicated). For comparing categorical data, Chi-square test was performed, and Fisher's exact test was used when appropriate. Continuous variables are presented as mean ± standard deviation, ordinal data presented as median (interquartile range [range]), and categorical data are presented as numbers and frequencies. P ≤ 0.05 was considered statistically significant.
Results
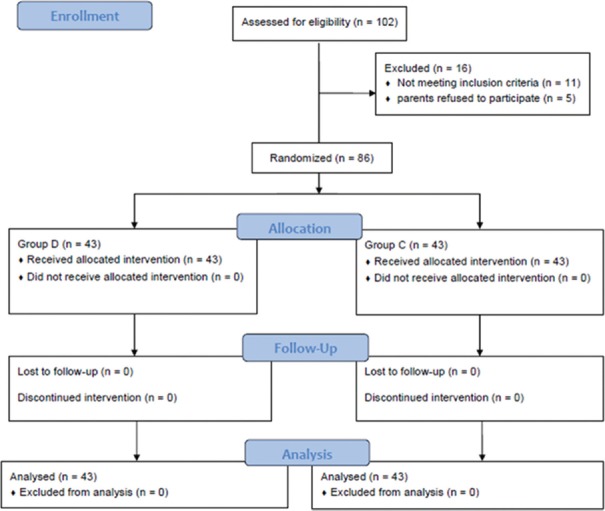
From the 102 patients assessed for eligibility, 11 patients did not meet the inclusion criteria and five patients did not participate in the study since their parents refused. So, the remaining 86 patients were enrolled in the study [Figure 1].
Figure 1.
Consort flowchart showing the number of patients at each phase of the study
Demographic characteristics, duration of surgery, duration of anesthesia, and type of surgery were comparable in both groups [Table 3]. There was no statistically significant difference between the two groups regarding extubation, emergence, and discharge times with a P > 0.05 [Table 4].
Table 3.
Demographic characteristics and intraoperative parameters
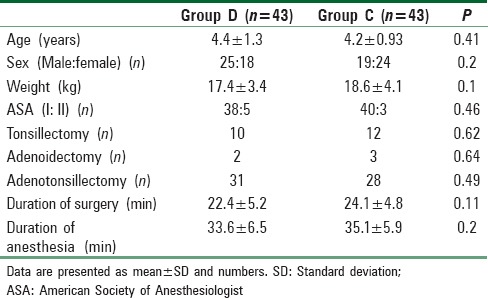
Table 4.
Recovery profile in operating room and postanesthesia care unit
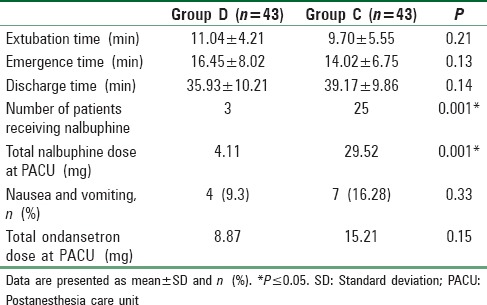
Postoperative nalbuphine dose consumption as a rescue medication for agitation episodes and pain was significantly higher in Group C compared with Group D with a P = 0.001 [Table 4].
There was statistically insignificant difference between the two groups with regard to nausea, vomiting, and total dose of ondansetron used during emergence or in the PACU with a P > 0.05 [Table 4]. There were no complications as somnolence, apnea, desaturation, hypotension, and bradycardia reported during emergence or in PACU in both groups before discharge to the ward.
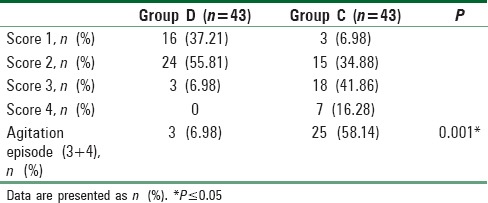
The incidence of EA was significantly lower in children allocated to Group D (three participants, 6.98%) compared to those allocated to Group C (25 participants, 58.14%) with a P = 0.001 [Table 5].
Table 5.
Incidence of agitation
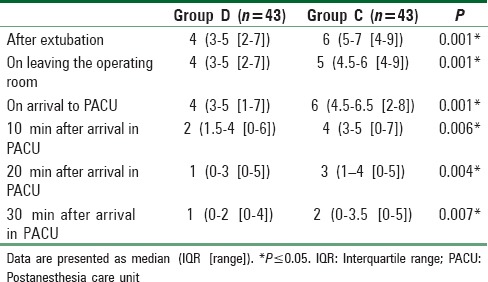
The median agitation scales score of Group D were significantly lower than those of Group C at all time points with a P < 0.001 [Table 6] and also the median FLACC scales score of Group D were significantly lower than those of Group C at all time points with a P < 0.01 [Table 7].
Table 6.
Severity of agitation at six points of time
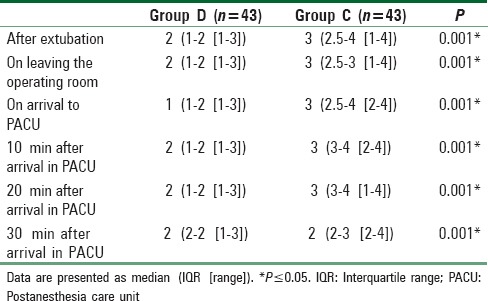
Table 7.
The scoring system for pain scale at six points of time
Discussion
Confirming the previous trials,[1,17,18,19] this trial emphasized that dexmedetomidine markedly decreases the incidence of EA and curtail its severity in children after sevoflurane anesthesia without complications and with smooth postoperative recovery course.
The intranasal route of administration was characterized by being noninvasive with relatively delayed onset (30–45 min), extended duration of action although short elimination half-life (1.8–3 h),[20,21] and with no or little adverse effects in comparison to IV route, its safety and efficacy documented in various studies in comparison with other drugs and placebo when used as a premedication, anesthetic adjunct, or for the management of EA.[20,22]
In their trial to detect the appropriate dose of dexmedetomidine for the prevention of EA in children undergone tonsillectomies or adenoidectomies after desflurane anesthesia, Kim et al. reported that IV dose of 0.25 μg/kg and 0.38 μg/kg could prevent EA in 50% and 95%, respectively.[23] They have used five-point scale for EA scoring different that we have used in our trial, and their suggested smaller doses for EA prevention could be explained by the fact that desflurane may induce EA with shorter duration than sevoflurane.[23]
In our study, we used a dose of 1 μg/kg for the prevention of EA post-sevoflurane for children undergone tonsillectomy and/or adenoidectomy. Akin et al. used the same dose and route of administration in comparison to intranasal midazolam, their primary end-point was satisfactory mask induction which was significantly better in midazolam group, and they were unable to find statistically significant difference in incidence and severity of EA between groups when administration of the drugs was 45–60 min before induction. In contrary, our trial was powered primarily to detect the preventive effect of dexmedetomidine on incidence and severity of EA. Nevertheless, Akin et al. reported that the number of children requiring postoperative analgesia was significantly lower in children allocated to the dexmedetomidine group, and these data go in line with our findings.[24] In the same context, Guler et al. reported that an IV bolus of 0.5 μg/kg of dexmedetomidine given 5 min before the end of surgery could significantly decrease incidence and severity of EA in children undergone adenotonsillectomy with sevoflurane.[18] In line with our findings, Olutoye et al. reported that there was no statistically significant difference in the analgesic effects of dexmedetomidine 0.5 μg/kg and morphine 50 μg/kg in children underwent tonsillectomies and adenoidectomies, and they added that postoperative sedation and incidence of nausea and vomiting were less with dexmedetomidine.[25] In our trial, we used the FLACC scale to assess pain score, and it worked efficiently in nonverbal pediatric patients, and it was easily estimated in OR and PACU. The previously mentioned trials used other pain scales such as objective pain score,[18,24] children and infants postoperative pain scale (CHIPPS),[17] Wong–Baker FACES pain rating scale,[23] and the Children's Hospital of Eastern Ontario Pain score.[25] The pain after this type of surgical procedures could be severe and this may increase the incidence and aggravate the severity of EA, in addition, assessment of pain could be mistaken as an agitation episode and vice versa. Furthermore, most of the children with high-FLACC scale score ≥5 had also high agitation score of 3 or 4 and require nalbuphine rescue dose. The clinical and statistical significant difference of nalbuphine use in control group emphasized both the sedative and the analgesic properties of dexmedetomidine which are also used for sedation and analgesia. The comparable recovery times between both groups could be attributed to the sedative effects of dexmedetomidine in Group D and on the other hand excess use of nalbuphine in Group C [Table 4].
There was relative delay of anesthesia time over surgery time in both groups; this delay could be attributed mainly to the period from removing mouse gag till extubation (extubation time). Although the extubation time was longer in Group D than in Group C by about 2 min (mean difference), it was statistically insignificant difference [Table 3]. Of note, this relative delay was comparable between both groups [Table 4]. There was no statistically significant difference in the incidence of nausea and vomiting between both groups and also there were no reported cases of perioperative hypotension, bradycardia, apnea, desaturation, and excessive somnolence reflected high safety profile of the drug even with higher doses (2 μg/kg) as those used by Yuen et al. for better sedation and parent separation.[12] In this trial, we choose to administer the drug intranasally after the induction of anesthesia to compensate for delayed onset (30–45 min) to procedure lasting for 25–45 min with peak effect at 90–105 min after administration.[26] Although the anesthesia time is not matched perfectly in this trial (33.6 ± 6.5 and 35.1 ± 5.9 in Groups D and C, respectively) [Table 3] with the reported onset time of intranasal dexmedetomidine (30–45 min),[26] it is fairly acceptable in clinical practice. The role of dexmedetomidine in the reduction of incidence and severity of EA in children after sevoflurane anesthesia is documented with different dose regimens, timing, techniques, and route of administration in two meta-analyses done by Gyanesh et al. and Sun et al.[8,9] As a limitation to this study, we use one milliliter syringes dripping for intranasal drug installation due to lack of intranasal drug delivery systems as an atomizer and nasal spray which improve drug absorption, hasten onset time, and optimize bioavailability instead we use 1-mL syringe dripping. In addition, we did not measure serum concentration of dexmedetomidine repeatedly after intranasal installation, and future studies are needed to elucidate detailed pharmacokinetics and pharmacodynamics of the drug in pediatric age group via the intranasal route.
Conclusion
This study demonstrates that a dose of 1 μg/kg intranasal dexmedetomidine administered after the induction of anesthesia reduces post-sevoflurane incidence and severity of EA in children undergone tonsillectomy and/or adenoidectomy with no adverse effects and smooth postoperative course.
Financial support and sponsorship
Authors would like to acknowledge the financial support provided by Benha University Hospital and Fayoum University Hospital.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors would like to acknowledge all members of the Departments of Anaesthesia of Benha and Fayoum universities hospitals, especially to heads of the two departments for their valuable supports
References
- 1.Mountain BW, Smithson L, Cramolini M, Wyatt TH, Newman M. Dexmedetomidine as a pediatric anesthetic premedication to reduce anxiety and to deter emergence delirium. AANA J. 2011;79:219–24. [PubMed] [Google Scholar]
- 2.Vlajkovic GP, Sindjelic RP. Emergence delirium in children: Many questions, few answers. Anesth Analg. 2007;104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 3.Kuratani N, Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: A meta-analysis of randomized controlled trials. Anesthesiology. 2008;109:225–32. doi: 10.1097/ALN.0b013e31817f5c18. [DOI] [PubMed] [Google Scholar]
- 4.Yassin H, Boules M. Comparative study between the effect of propofol and fentanyl on the incidence and severity of emergence agitation after sevoflurane anesthesia in pediatrics. Ain Shams J Anesthesiol. 2015;8:529–34. [Google Scholar]
- 5.Patel A, Davidson M, Tran MC, Quraishi H, Schoenberg C, Sant M, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg. 2010;111:1004–10. doi: 10.1213/ANE.0b013e3181ee82fa. [DOI] [PubMed] [Google Scholar]
- 6.Tobias JD. Dexmedetomidine: Applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:115–31. doi: 10.1097/01.PCC.0000257100.31779.41. [DOI] [PubMed] [Google Scholar]
- 7.Abdelhamid AM, Mahmoud A, Abdelhaq MM, Yasin HM, Bayoumi A. Dexmedetomidine as an additive to local anesthetics compared with intravenous dexmedetomidine in peribulbar block for cataract surgery. Saudi J Anaesth. 2016;10:50–4. doi: 10.4103/1658-354X.169475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyanesh P, Haldar R, Srivastava D, Agrawal PM, Tiwari AK, Singh PK, et al. Meta-analysis of dexmedetomidine on emergence agitation and recovery profiles in children after sevoflurane anesthesia: Different administration and different dosage. Anaesthesia. 2014;24:12–8. [Google Scholar]
- 9.Sun L, Guo R. Dexmedetomidine for preventing sevoflurane-related emergence agitation in children: A meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand. 2014;58:642–50. doi: 10.1111/aas.12292. [DOI] [PubMed] [Google Scholar]
- 10.Miller JW, Divanovic AA, Hossain MM, Mahmoud MA, Loepke AW. Dosing and efficacy of intranasal dexmedetomidine sedation for pediatric transthoracic echocardiography: A retrospective study. Can J Anaesth. 2016;63:834–41. doi: 10.1007/s12630-016-0617-y. [DOI] [PubMed] [Google Scholar]
- 11.Sheta SA, Al-Sarheed MA, Abdelhalim AA. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: A double-blinded randomized controlled trial. Paediatr Anaesth. 2014;24:181–9. doi: 10.1111/pan.12287. [DOI] [PubMed] [Google Scholar]
- 12.Yuen VM, Hui TW, Irwin MG, Yao TJ, Chan L, Wong GL, et al. A randomised comparison of two intranasal dexmedetomidine doses for premedication in children. Anaesthesia. 2012;67:1210–6. doi: 10.1111/j.1365-2044.2012.07309.x. [DOI] [PubMed] [Google Scholar]
- 13.Tug A, Hanci A, Turk HS, Aybey F, Isil CT, Sayin P, et al. Comparison of two different intranasal doses of dexmedetomidine in children for magnetic resonance imaging sedation. Paediatr Drugs. 2015;17:479–85. doi: 10.1007/s40272-015-0145-1. [DOI] [PubMed] [Google Scholar]
- 14.Aono J, Ueda W, Mamiya K, Takimoto E, Manabe M. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology. 1997;87:1298–300. doi: 10.1097/00000542-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23:293–7. [PubMed] [Google Scholar]
- 16.Aldrete JA. Modifications to the postanesthesia score for use in ambulatory surgery. J Perianesth Nurs. 1998;13:148–55. doi: 10.1016/s1089-9472(98)80044-0. [DOI] [PubMed] [Google Scholar]
- 17.Sato M, Shirakami G, Tazuke-Nishimura M, Matsuura S, Tanimoto K, Fukuda K. Effect of single-dose dexmedetomidine on emergence agitation and recovery profiles after sevoflurane anesthesia in pediatric ambulatory surgery. J Anesth. 2010;24:675–82. doi: 10.1007/s00540-010-0976-4. [DOI] [PubMed] [Google Scholar]
- 18.Guler G, Akin A, Tosun Z, Ors S, Esmaoglu A, Boyaci A. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. 2005;15:762–6. doi: 10.1111/j.1460-9592.2004.01541.x. [DOI] [PubMed] [Google Scholar]
- 19.Boku A, Hanamoto H, Oyamaguchi A, Inoue M, Morimoto Y, Niwa H. Effectiveness of dexmedetomidine for emergence agitation in infants undergoing palatoplasty: A randomized controlled trial. Braz J Anesthesiol. 2016;66:37–43. doi: 10.1016/j.bjane.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Chrysostomou C, Schulman SR, Herrera Castellanos M, Cofer BE, Mitra S, da Rocha MG, et al. A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J Pediatr. 2014;164:276–82.e1-3. doi: 10.1016/j.jpeds.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Vilo S, Rautiainen P, Kaisti K, Aantaa R, Scheinin M, Manner T, et al. Pharmacokinetics of intravenous dexmedetomidine in children under 11 yr of age. Br J Anaesth. 2008;100:697–700. doi: 10.1093/bja/aen070. [DOI] [PubMed] [Google Scholar]
- 22.Mekitarian Filho E, Robinson F, de Carvalho WB, Gilio AE, Mason KP. Intranasal dexmedetomidine for sedation for pediatric computed tomography imaging. J Pediatr. 2015;166:1313–5.e1. doi: 10.1016/j.jpeds.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Byon HJ, Kim JE, Park YH, Lee JH, Kim JT. Appropriate dose of dexmedetomidine for the prevention of emergence agitation after desflurane anesthesia for tonsillectomy or adenoidectomy in children: Up and down sequential allocation. BMC Anesthesiol. 2015;15:79. doi: 10.1186/s12871-015-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akin A, Bayram A, Esmaoglu A, Tosun Z, Aksu R, Altuntas R, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth. 2012;22:871–6. doi: 10.1111/j.1460-9592.2012.03802.x. [DOI] [PubMed] [Google Scholar]
- 25.Olutoye O, Kim T, Giannoni C, Stayer S. Dexmedetomidine as an analgesic for pediatric tonsillectomy and adenoidectomy. Paediatr Anaesth. 2007;17:1007–8. doi: 10.1111/j.1460-9592.2007.02234.x. [DOI] [PubMed] [Google Scholar]
- 26.Iirola T, Vilo S, Manner T, Aantaa R, Lahtinen M, Scheinin M, et al. Bioavailability of dexmedetomidine after intranasal administration. Eur J Clin Pharmacol. 2011;67:825–31. doi: 10.1007/s00228-011-1002-y. [DOI] [PubMed] [Google Scholar]